Back to Journals » Infection and Drug Resistance » Volume 16
Malaria Risk Perception and Preventive Behaviors Among Elementary School Students, Southwest Ethiopia. Generalized Structural Equation Model
Authors Deressa A , Gamachu M , Birhanu A , Mamo Ayana G , Raru TB , Negash B, Merga BT , Regassa LD , Ababulgu FA
Received 27 April 2023
Accepted for publication 29 June 2023
Published 13 July 2023 Volume 2023:16 Pages 4579—4592
DOI https://doi.org/10.2147/IDR.S415376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Alemayehu Deressa,1 Mulugeta Gamachu,2,3 Abdi Birhanu,2 Galana Mamo Ayana,1 Temam Beshir Raru,1 Belay Negash,1 Bedasa Taye Merga,1 Lemma Demissei Regassa,1 Fira Abamecha Ababulgu4
1School of Public Health, CHMS, Haramaya University, Harar, Ethiopia; 2School of Medicine, CHMS, Haramaya University, Harar, Ethiopia; 3Departments of Public Health, Rift Valley University, Harar, Ethiopia; 4Department of Health, Behavior, and Society, Faculty of Public Health Institute of Health, Jimma University, Jimma, Ethiopia
Correspondence: Alemayehu Deressa, School of Public Health,Haramaya University, P. O. Box: 235, Harar, Harari, Ethiopia, Tel +251917841709, Fax +251256668081, Email [email protected] Mulugeta Gamachu, School of Medicine, CHMS, Haramaya University, Harar, Ethiopia, Tel +251917032032, Email [email protected]
Background: In 2020, more than three billion of the world’s population were the risk of being infected with malaria and four out of five deaths were from the African population. However, information is scarce on the association between risk perceptions and malaria prevention behaviors in resource-limited countries, particularly Ethiopia. Therefore, this study aimed to assess malaria risk perceptions and preventive behaviors.
Methods: A cross-sectional study design was conducted among 401 elementary school students in Jimma zone, Oromia, Ethiopia, from April 2 to June 8, 2020. Data were collected through interviews using a semi-structured questionnaire. The data were entered into Epi-data 4.6 and analyzed using STATA version 14.2. The descriptive statistics were presented using frequency and percentages. A Cronbach’s α coefficient of 0.7 or higher was used to assess the reliability of each domain. The Generalized Structural Equation Model (GSEM) was employed to examine the relationships and prediction of explanatory variables with risk perception and preventive behaviors of malaria. The model with a lower information criterion was taken as a better-fitting model. Finally, the statistically significant model effects were declared at a P-value of less than 0.05 at a confidence interval of 95%.
Results: This study showed that having knowledge about malaria had an indirect positive effect on malaria preventive behavior (β = 1.29, 95% CI 0.11 to 2.47), and had a positive total effect on the preventive behavior (β = 2.99, 95% CI 0.08 to 2.67). Besides, an increased knowledge level had a direct positive effect on malaria risk perceptions (β = 0.08, 95% CI 0.01 to 0.14), and malaria risk perception had a direct positive effect on malaria preventive behavior (β = 1.21, 95% CI 0.10 to 2.31).
Conclusion and Recommendation: This study demonstrated that having knowledge about malaria had a direct and indirect association with malaria preventive behavior. An increased level of knowledge had a direct positive effect on malaria risk perceptions. Moreover, malaria risk perception had a direct positive effect on malaria preventive behavior. Therefore, malaria prevention-targeted interventions, behavior change, and knowledge enhancing communication should be enhanced or scaled up to contribute to prompt treatment and progress toward the elimination of malaria.
Keywords: malaria, risk perception, health-seeking behaviors, factors, Ethiopia
Introduction
The number of malaria cases worldwide was estimated to be 247 million in 2021, up from 245 million in 2020, and 234 million of those cases were thought to be in African regions, making up about 95% of all cases worldwide.1 The Sustainable Development Goals (SDGs) of malaria elimination by 2030 was formally implemented in 2015 and indicated the elimination of malaria is intrinsically linked to most of the sustainable development goals.2 As the guiding principles, the World Health Organization (WHO) forwarded a framework called “Global Technical Strategy (GTS)” to guide the malaria elimination strategy from 2016 to 2030 and targets to decrease new malaria cases and malaria-induced death by a minimum of 90%.3 As part of this program, the utilization of long-lasting insecticide-treated nets (LLINs) and residual spray in the interior wall of the home is the most recommended malaria prevention method. Among those prevention measures including early detection and treatment could bring changes in reducing malaria cases in Africa.4–6
In Africa, Ethiopia is one of the countries where malaria species such as Plasmodium vivax and P. falciparum are co-incident.7 Despite the efforts that the Ethiopian government has made, malaria is still one of the top ten diseases that lead to morbidity and mortality in the country.8 In addition, the country’s achievements in combating malaria are challenged by resisting the insecticides, newly emerging vectors, health ill effects of irrigation and hydropower containers, and dynamic climatic conditions.9–11 The individuals’ risk perception of infection can influence individuals’ behavior. Having a low score for risk perception leads to the implementation of low preventive measures12 and also affirmed that Personal Protective Behaviors (PPBs) are highly expected to provide an effective prevention capacity for vector-borne infections like malaria.13
The world is at risk of the disease and malaria is a leading cause of morbidity and mortality in much of the developing world.14 The most malaria cases were in the African Region (92%) and accounted for 93% of all malaria deaths.15 In Ethiopia there were about 1,530,739 confirmed malaria cases with 356 reported deaths due to malaria with the pooled prevalence of 13.61%.16,17 Therefore, to diminish the burden of malaria, schools are the natural hub for community services to promote healthy practices18 such as in sharing key characteristics like preventive behavior, experience, and cultural background that makes them a more credible source of information.19,20 In Jimma zone they are considering Peer Learning and Education Approach (PLEA) programs that had been conducted in primary schools which mainly focused on individuals being educated on use of insecticide nets (ITNS), appropriate and timely seeking care for malaria, appropriate use of quality anti-malaria drugs, acceptance of insecticide residual spray (IRS), and draining of potential breeding sources in the villages.
Studies showed that community and school-related levels of prevention and control measures are proven methods for effectively tackling malaria. Particularly, the engagement of students, and the school community, which is crucial for malaria prevention practices.21 Individual factors like perception, level of knowledge, and skills towards malaria prevention programs are determinants of the prevention programs. Self-efficacy, school environment, perceived community support, and perceived fear of malaria were determining factors for malaria risks and prevention.22–24 In addition, the study revealed that perception-related individual susceptibility, perceived malarial severity, perceived prevention, treatment costs, response efficacy, and preventive behaviors could affect the level of malarial risk perception.25 Generally, since risk perception and preventive behaviors have a synergistic effect on infection prevention, assessing these issues from students’ perspectives could have important inferences in the era of disease elimination, particularly for malaria. Even if reports have revealed that school children are significantly getting infected by malaria,26 what factors directly or indirectly affect malaria risk perception and preventive behaviors of the school children are not explored very well in Ethiopia. Therefore, this study aimed to assess the malaria risk perception and preventive behaviors among elementary students in the Jimma zone by using a generalized structural equation model that showed which factors directly and indirectly affect malaria risk perception and preventive actions among students.
Methods and Materials
Study Design, Area, and Period
A cross-sectional study design was conducted from April 2 to June 8, 2020, among trained peer educators of primary students (6–8 grade) in Jimma zone of Shebe-Sombo, limmu-Kossa and Gera districts (Figure 1) where pilots of peer learning education intervention about malaria prevention and control were implemented. Jimma zone is one of the administrative zones of Oromia regional states, which is located around 352 km away from Addis Ababa, the capital city of Ethiopia. The zone covers an area of 199,316.18 km2. The total population was 2,770,329 out of which about 89.1% of the populations are living in rural areas and 10.9% are living in urban areas. The zone generally lies at an altitude range between 1000 and 3500 meters above sea level.27 It has a relatively cool tropical monsoon climate, a long annual wet season, and a daily temperature of between 24 °C and 27 °C. The zone consists of 23 districts and 17 (73%) of the villages (kebeles) are malaria endemic areas and 85% of the population of the zone lives in malaria risk areas. In terms of stratification of risks, 41%, 32% and 27% of the Kebeles are at high, medium, and low risk of malaria transmission respectively.28
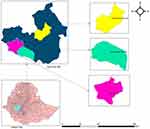 |
Figure 1 Geographical map of study area on risk perception and preventive behaviors among elementary school students in southwestern Ethiopia, 2020. |
Study Population
A large-scale school-based social and behavior change communication intervention to prevent malaria; an innovative and participatory approach entitled; “PLEA-Malaria” was implemented in 75 rural primary schools in the Jimma zone. Malaria prevention and control training was given to selected students who were sent to teach their peers in class and community members through the process called peer learning and education approach. Around 8842 peer educators in 75 primary schools trained on malaria prevention and control and have been undertaking malaria communication interventions both at schools and community levels in intervention areas. Accordingly, all trained peer educator’s students who were learning grade 6–8 in Jimma zone in 75 primary schools were source populations. All selected trained peer educators’ students who were learning grade 6–8 in Jimma zone were the study population.
Sample Size Determination
The sample size was calculated using two approaches the first one was using a single population proportion approach: 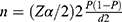 , Where:- n = desired sample size, p = 0.5, proportion of malaria risk perception of trained peer educators which indicates the maximum variability of the study population and gives maximum sample size was considered, since there were no previous studies in Ethiopia that can especially help to address our objectives, z - confidence interval is 95%, d = desired precision (%) – 5% = 0.05, using the formula, the sample size become n = 384. Since the source population is less than 10,000, the population correction formula was used:
, Where:- n = desired sample size, p = 0.5, proportion of malaria risk perception of trained peer educators which indicates the maximum variability of the study population and gives maximum sample size was considered, since there were no previous studies in Ethiopia that can especially help to address our objectives, z - confidence interval is 95%, d = desired precision (%) – 5% = 0.05, using the formula, the sample size become n = 384. Since the source population is less than 10,000, the population correction formula was used: 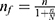 ,
, 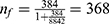 , Where, nf = the final sample size, n = initial sample size (384), N; Source population all trained peer educator students = 8842. In addition, 10% non-response was added. Finally, the calculated sample size becomes 404 trained peer educator students.
, Where, nf = the final sample size, n = initial sample size (384), N; Source population all trained peer educator students = 8842. In addition, 10% non-response was added. Finally, the calculated sample size becomes 404 trained peer educator students.
The second approach was using a structural equation model (SEM). Sample size calculation in a structural equation model depends on the complexity of the model. A general rule of thumb is that the minimum sample size should be 5~20 times the number of parameters to be estimated.29 In the model we suspected that we had 22 parameters to be estimated. According to the afore mentioned rule, the minimum sample size required for malaria risk perception was 220 (10*22). Then adding a 10% non-response rate, the sample size becomes 242. Since the sample size calculated by using SEM was smaller than the sample size calculated using the single population proportion approach, 404 was taken as the final total sample size for the current study. Therefore, the total sample size required for this study was 404.
Sampling Procedure
The participants for this study were trained peer educator students in the Jimma zone of five districts which included 75 primary schools. A total population size of at least 8842 class representatives and 1–5 social network leaders have had training from school focal teachers in the 75 implementation schools. An expected number of 118 trained peer educators per school were found. Considering the representatives three districts were randomly selected. From the three districts, 45 primary schools were included in the intervention (N = 3*15* = 45 ~ 5310 trained peer educators). Fifteen schools from each district realized a representative of 30% and we included 5 schools in each district, using the same distribution. Therefore, a sample of 404 is drawn from 15 schools; approximately 27 peer educators were selected from each school. Finally, 27 peer educators; including 9 each in 6th, 7th, and 8th grade were selected by using lottery methods (Supplementary 1 Supplementary Figure 1). Since the schools were closed due to COVID-19 during data collection, after taking a complete list of trained peer educator students from each school as a sampling frame, house-to-house data collection was conducted taking in to account the preventive measures of COVID-19.
Data Collection Tool and Quality Control
A semi-structured questionnaire (see Supplementary 2) was developed after reviewing available relevant literature and used to collect information on sociodemographic factors, malaria related knowledge, malaria related peer learning and education practice, malaria preventive behavior, treatment seeking behavior, and malaria risk perception. A pretest was conducted on 5% of the sample size and data was collected by trained health extension workers and teachers with health professional supervisors. Double data entry was made by two data clerks to maintain the consistency of the data by comparing the two separately entered data.
Operational Definition and Measurement
Malaria related knowledge: Multidimensional questions were used to measure comprehensive malaria knowledge related to the cause of malaria, sign and symptoms, prevention measures, vulnerable groups, and malaria biting vector behavior knowledge. Yes = 1/No = 0 and multiple questions were used. Correct answers were counted down and the higher scores showed a higher knowledge level.
Malaria preventive behaviors: Any efforts undertaken by peer educators including sleeping under LLINs last night, indoor residual spray use, using mosquito repellant, appropriate anti malaria drug use and health seeking for those with a fever.
Behavioral measures: Type of participation on malaria prevention behaviors and time of care seeking was measured in “days”.
Malaria risk perception: Perceived susceptibility to malaria infection defined individuals’ perception vulnerability to malaria based on their daily experiences about the presence of malaria, and individuals who caught malaria in the neighborhood or in the family. Perceived severity explores student perception of the bad consequences resulting from malaria in causing pain, death, and interruption with their daily work, such as schooling, and its impact on their academic performances. Weighted scores of susceptibilities and severity summed up and divided by two to create weighted score of risk perception about malaria.
Data Processing
Each filled questionnaire was checked visually for completeness and consistency. Data were entered into Epi-data 4.6 then exported to STATA 14. After the data were entered and cleaned, it was checked again systematically for its completeness and consistency across individual responses, and then re-cataloging was performed to make data suitable for analysis.
Model Building and Analysis
Descriptive statistics and summary statistics were presented using text, figures, and tables. Reliability was also assessed for each domain of malaria risk perception using the Cronbach’s α coefficient and values of 0.7 or higher were considered satisfactory. The score of each domain of malaria risk perception was obtained by summation of their corresponding items for each participant. Generalized Structural Equation Modeling (GSEM)30 was used to examine relationships and predict among socio-demographic factors, malaria preventive behavior, treatment seeking behavior, and malaria risk perception domains for each group. Continuous, count and dichotomous variables were analyzed with Gaussian family with identity link function, Poisson with log link function and binomial with logit link function. Malaria risk perception was a latent variable which constituted items with dichotomous response; their measurement model was analyzed with binomial families with logit link function.
An over identified model with minimum information standards was retained. A final model was selected based on statistical significance of path coefficient, the theoretical meaningfulness of the relationship and minimum information conditions. Diagrammatically, the effect of each exogenous variable on the respective dependent variable was indicated by the path coefficient along with a single headed arrow, and the correlation between measurement errors (residual errors that reflect the unexplained variation in the observable endogenous variables due to all unmeasured causes) was indicated by double arrows. Statistically significant results were assumed for P < 0.05 at a confidence interval of 95%.
Results
Socio Demographic Characteristics
A total of 401 students participated in the study, resulting in a response rate of 99.2%. Of all respondents, 242 (60.3%) were male and229 (57.1%) were 15–19 years of age. Mean age was 15.59 (±SD 2.24) years. The majority of study participants, 84.8% and 73.6% were rural residents and followers of the Muslim religion, respectively. About a fifth of respondents received health training other than malaria. Most respondents, 344 (85.8%), were Oromo (Table 1). The average family size of a respondent was 6.75 (±SD 2.134).
 |
Table 1 Sociodemographic Characteristics of the Participants, Jimma Zone, Southwest Ethiopia, 2020 |
Malaria Related Knowledge
The research shows that almost all (395 (98.5%)) respondents had heard of malaria. The mean score of trained peer students for knowledge related to essential malaria action, was 0.6474 (SD = 0.1753). More than half of the students, 212 (52.9%) were scored as knowledgable regarding EMA, above the mean. Most respondents (346, 86.0%) reported that fever was the main symptom of malaria and 6 (1.5%) did not know. Almost all (372, 92.8%) respondents reported mosquito bites were the cause of malaria and 5 (1.2%) did not know. Almost all of respondents (377, 94.0%) reported that sleeping under a mosquito net can protect individuals from malaria and less than five respondents did not know. Most respondents knew pregnant women and children under three years of age were more at risk than others (342, 85.3%) and 17 (4.2%) did not know about risk groups. From misconceptions about the causes of malaria, drinking dirty water (82, 20.4%), getting soaked with rain (78, 19.5%), cold and changed weather (73, 18.2%), shaking the hands of a person with malaria (40, 10.0%) and eating sugarcane (29, 7.2%) were cited as the causes of malaria (Table 2).
 |
Table 2 Frequency of the Respondents’ Knowledge About Malaria Signs and Symptoms, Risks of Transmission, and Prevention Methods in Jimma Zone, Southwest, Ethiopia, 2020 |
Malaria Related Peer Learning and Education Practice in School
Three hundred and forty-one (85.0%) students reported having done peer teaching in schools for the past two years and 124 (30.9%) of them conducted them in a schedule of every two weeks. But, during study periods peer education was conducted among only 156 (38.9%) of them and the majority of 245 (61.1%) reported they did not conductpeer education in their school during study periods (Table 3).
 |
Table 3 Peer Education Conducted in Two Years Past and Currently Conducted Schedule in Schools in Jimma Zone, Ethiopia, 2020 |
Malaria Preventive Behavior, Treatment Seeking Behavior, and Malaria Risk Perception
The mean score of malaria risk perception of respondents was 18.75 (SD=3.06). Many respondents (364, 91.9%) of households have recommended mosquito nets but only 224 (56.6%) of them slept under a net the previous night of data collection day. From respondent’s 36 (8.8%) had been sick from fever and 32 (88.9) of them sought advice or treatment for the fever (Table 4).
 |
Table 4 Malaria Preventive Behavior, Treatment-Seeking Behavior, and Malaria Risk Perception of Peer Educators’ Students in Schools in Jimma Zone, Ethiopia, 2020 |
Factors Associated with Malaria Risk Perception
The final model is depicted in figure one, all of the path coefficients were statistically significant at an alpha level of 0.05. This model includes both structural components (relationships among latent or observable variables) and measurement components (relationships among latent variables and their items).
As we can see from Figure 2 the model had included seven exogenous variables (age, residence, sex, knowledge, grade point average (GPA), severity, and susceptibility), one mediator variable malaria risk perception (MRP), and five endogenous variables (bed net utilization, mosquito repellant use, indoor residual spray, treatment seeking, and anti malaria drug use).
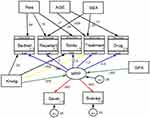 |
Figure 2 GSEM for factor associated with risk perception and preventive behaviors among elementary school students in southwest Ethiopia, 2020. |
Generalized Structural Equation Model (GSEM) for factors associated with malaria risk perception of peer educators’ students in school in Jimma zone, Ethiopia, 2020. Having a knowledge about malaria had an indirect positive effect on treatment seeking behavior (β = 1.29, 95% CI 0.11 to 2.47) and it also had a positive total effect on treatment seeking behavior (β = 2.99, 95% CI 0.08 to 2.67]. Increased knowledge level also had a direct positive effect on malaria risk perceptions (β = 0.08, 95% CI 0.01 to 0.14). Malaria risk perception also had a direct positive effect on preventive behavior (treatment seeking behavior) (β = 1.21, 95% CI 0.10 to 2.31) (Table 5).
 |
Table 5 Direct, Indirect, and the Total Effect of Socio-Demographical, and Malaria Preventive Behavior Domains on Malaria Risk Perceptions (Derived from GSEM). |
Discussions
This study aimed to assess the malaria risk perceptions and preventive behaviors among elementary school students in Jimma town, Ethiopia. Accordingly, the study revealed that having a good knowledge about malaria had an indirect and direct positive effect on treatment seeking behavior which is one of the malaria preventive behavior domains. Those with good knowledge about malaria had an increased health seeking behavior. This finding is supported byprevious studies conducted among South Asians,31 Sudanese,32 and another study from Ethiopia supporting the association between knowledge about malaria and preventive measures such as usual use of bed nets.33 This implies those with knowledge about malaria including its sign and symptoms, transmission ways and prevention methods are also knowledgeable about its treatment and where to seek treatment.34,35
We found that increased knowledge level about malaria had a direct positive effect on malaria risk perceptions. In line with our findings other studies also showed the effect of knowledge about malaria on risk perception from west Ethiopia,36 Cameroon,37 Nigeria,38 and Iran.39 This is explained by the fact the risk perception is influenced by exposure to different information and knowledge about health problems. In addition, the reason could be because people who attained better knowledge levels might have been offered malaria-oriented lessons at schools and even the people who have a better education level can read different materials in different languages and they can easily understand the messages from the written materials that focuses on the malaria risk prevention measures. This finding reaffirmed that knowledge about health problems is the most important tool that enables people to make the right decision for themselves and their families in fighting against infectious diseases, including malaria.39,40 This study implies that being knowledgeable about malaria can enhance malaria preventive practices in a very feasible way through peer education at the school level, which may play a great role in malaria eradication programs at the national and international levels since the disease is targeted to be eradicated globally. Furthermore, according to the Ethiopian context, school-aged populations from rural areas of the country rarely had exposure to media. Thus, peer education may be the only feasible access to malaria-related information for primary school students.
The study also showed that the direct positive effect of malaria risk perception on malaria preventive behavior. This finding is in line with the report of a study in Ethiopia that those with perceived susceptibility and severity had sought healthy preventive behavior against malaria infection36,41 along with a study in rural Nigeria.39 This might be because performing certain health behavior is directly linked to the perceived threat, the perceived benefits and barriers of the suggested behavior change, the self-efficacy and the cues to action.42 The positive perception towards health conditions may have a positive relationship with health care seeking behaviors.43 Thus, this study implies health seeking behaviors can be enhanced through promoting positive perceptions against the health event. In addition, the successful implementation of peer learning education at school on perceived susceptibility and severity of malaria would be enormous in increasing preventive action such as bed net utilization, appropriate anti-malaria drug use, disposal of stagnant water from around the home, and periodic utilization of indoor residual spray within students’ families, which has a key role in reducing malaria morbidity and mortality.
Limitation of the Study
Studying has certain limitations. The nature of the cross-sectional study design makes it difficult to establish causation between related factors and outcome variables. Also, recall bias can be one of the study’s limitations and interviewer bias may also arise. Data is collected from participants’ self-reports and can be compared with social expectation bias.
Conclusion and Recommendation
This study demonstrated that having knowledge about malaria had a direct and indirect association with malaria prevention behavior. An increased level of knowledge had a direct positive effect on malaria risk perceptions. In addition, this study showed that the malaria risk perception had a direct positive effect on malaria prevention behavior. Therefore, malaria prevention-targeted interventions, behavior change, and knowledge enhancing communication should be enhanced or scaled up to contribute prompt treatment in the progress toward elimination of malaria.
Data Sharing Statement
The data sets used for this study are available from the corresponding authors on reasonable request.
Ethics Statement
The study was carried out in accordance with the Helsinki Declaration, and ethical approval was obtained from the Institutional Health Research Ethics Review Committee (IHRERC) of Jimma University, Institute of Health. Support letters from the Institute of Health were submitted to the selected district health office then to each school, where the study was conducted. After getting all permission letters from the responsible body, and informed voluntary, written, consent was signed by study participants. Confidentiality was maintained by using codes instead of the participant’s name. Participants were also informed that they have full right to refuse participation or withdraw any time from the research.
Acknowledgment
The authors express their deepest gratitude to Jimma University and those involved in the study, as well as to the interviewees, data collectors, and researchers, as well as to the district health office administrators for their constructive support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported financially by Jimma University. However, the funding agency had no role in the collection, analysis, and interpretation of the data as well as the writing-up of the manuscript.
Disclosure
The authors have no competing interests to declare for this study.
References
1. World Health Organization. World malaria report 2022; 2022. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022.
2. End Malaria Malaria and sustainable development goals; 2022. Available from: https://endmalaria.org/malaria-sustainable-development-goals.
3. World Health Organization. Global Technical Strategy for Malaria 2016–2030. World Health Organization; 2015.
4. Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. doi:10.1038/nature15535
5. Atieli HE, Zhou G, Afrane Y, et al. Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasit Vectors. 2011;4(1):1–10. doi:10.1186/1756-3305-4-113
6. World Health Organization. World Malaria Report 2015. World Health Organization; 2016.
7. Ketema T, Bacha K, Getahun K, Portillo HA, Bassat Q. Plasmodium vivax epidemiology in Ethiopia 2000–2020: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(9):e0009781. doi:10.1371/journal.pntd.0009781
8. FMOH. National Malaria Elimination Program (NMEP) – Ethiopia; 2020. Available from: https://www.moh.gov.et/site/initiatives-4-col/.
9. Tadesse FG, Ashine T, Teka H, et al. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg Infect Dis. 2021;27(2):603. doi:10.3201/eid2702.200019
10. Yalew AW. Achievements, gaps, and emerging challenges in controlling malaria in Ethiopia. Policy brief. Front Trop Dis. 2022;2. doi:10.3389/fitd.2021.771030
11. Lo E, Hemming-Schroeder E, Yewhalaw D, et al. Transmission dynamics of co-endemic Plasmodium vivax and P. falciparum in Ethiopia and prevalence of antimalarial resistant genotypes. PLoS Negl Trop Dis. 2017;11(7):e0005806. doi:10.1371/journal.pntd.0005806
12. Aerts C, Revilla M, Duval L, et al. Understanding the role of disease knowledge and risk perception in shaping preventive behavior for selected vector-borne diseases in Guyana. PLoS Negl Trop Dis. 2020;14(4):e0008149. doi:10.1371/journal.pntd.0008149
13. Omodior O, Luetke MC, Nelson EJ. Mosquito-borne infectious disease, risk-perceptions, and personal protective behavior among U.S. international travelers. Prev Med Rep. 2018;12:336–342. doi:10.1016/j.pmedr.2018.10.018
14. World Health Organization. Malaria atlas project; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/.
15. World Health Organization. World Malaria Report. Geneva: World Health Organization; 2018.
16. CDC. Ethiopia malaria operational plan FY 2019- President’s Malaria. Plos One. 2019;2019:1–71.
17. Kendie FA, Hailegebriel WKT, Nibret Semegn E, Ferede MW. Prevalence of malaria among adults in Ethiopia: a systematic review and meta-analysis. J Trop Med. 2021;2021:8863002. doi:10.1155/2021/8863002
18. Eisenstein C, Zamperoni V, Humphrey N, et al. Evaluating the peer education project in secondary schools. J Public Ment Health. 2019;18(1):58–65. doi:10.1108/JPMH-07-2018-0048
19. Malenga T, Kabaghe AN, Manda-Taylor L, et al. Malaria control in rural Malawi: implementing peer health education for behaviour change. Global Health. 2017;13(1):1–10. doi:10.1186/s12992-017-0309-6
20. Dobbie F, Purves R, McKell J, et al. Implementation of a peer-led school based smoking prevention programme: a mixed methods process evaluation. BMC Public Health. 2019;19(1):1–9. doi:10.1186/s12889-019-7112-7
21. Kebede AL, Alemayehu G, Sudhakar M, Birhanu D, Birhanu Z. School-based social and behavior change communication (SBCC) advances community exposure to malaria messages, acceptance, and preventive practices in Ethiopia: a pre-posttest study. PLoS One. 2020;15(6):e0235189. doi:10.1371/journal.pone.0235189
22. Abamecha F, Midaksa G, Sudhakar M, et al. Acceptability and feasibility of the school-engaged social and behavior change communication approach on malaria prevention in Ethiopia: implications for engagement, empowerment, and retention (EER) of education sectors in malaria elimination efforts. BMC Public Health. 2021;21(1):1909. doi:10.1186/s12889-021-11995-z
23. Aragie TB. Knowledge of malaria prevention and control methods and associated factors among rural households in west Belessa district, north west Ethiopia, 2019. BMC Public Health. 2020;20(1):1275. doi:10.1186/s12889-020-09332-x
24. Laver SM, Wetzels J, Behrens RH. Knowledge of malaria, risk perception, and compliance with prophylaxis and personal and environmental preventive measures in travelers exiting Zimbabwe from Harare and Victoria Falls International Airport. J Travel Med. 2006;8(6):298–303. doi:10.2310/7060.2001.23975
25. Ghahremani L, Faryabi R, Kaveh MH. Effect of health education based on the protection motivation theory on malaria preventive behaviors in rural households of Kerman, Iran. Int J Prev Med. 2014;5(4):463–471.
26. Abdishu M, Gobena T, Damena M, Abdi H, Birhanu A. Determinants of malaria morbidity among school-aged children living in East Hararghe Zone, Oromia, Ethiopia: a community-based case–control study. Pediatr Health Med Therap. 2022;13:183. doi:10.2147/PHMT.S347621
27. Service ES. Ethiopia statistics service; 2007. Available from: https://www.statsethiopia.gov.et.
28. Office JH. Jimma Zone Health Department (Ethiopia); 2020. Available from: https://www.jimazonehealth.gov.et.
29. Lei P-W, Wu Q. Introduction to structural equation modeling: issues and practical considerations. Educ Measure. 2007;26:33–43.
30. Lei PW, Wu Q. Introduction to structural equation modeling: issues and practical considerations. Educ Measure. 2007;26(3):33–43. doi:10.1111/j.1745-3992.2007.00099.x
31. Joshi MS, Lalvani A. ‘Home from home’: risk perceptions, malaria and the use of chemoprophylaxis among UK South Asians. Ethn Health. 2010;15(4):365–375. doi:10.1080/13557851003729098
32. Hasabo EA, Khalid RI, Mustafa GE, et al. Treatment-seeking behaviour, awareness and preventive practice toward malaria in Abu Ushar, Gezira state, Sudan: a household survey experience from a rural area. Malar J. 2022;21(1):182. doi:10.1186/s12936-022-04207-5
33. Jimma D, Tasfaye G, Deressa W, Woyessa A, Kebede D, Alamirew D. Baseline survey for the implementation of insecticide treated mosquito nets in malaria control in Ethiopia. Ethiop J Health Dev. 2005;19(1):16–23. doi:10.4314/ejhd.v19i1.9966
34. Yasuoka J, Kikuchi K, Nanishi K, et al. Malaria knowledge, preventive actions, and treatment-seeking behavior among ethnic minorities in Ratanakiri Province, Cambodia: a community-based cross-sectional survey. BMC Public Health. 2018;18(1):1206. doi:10.1186/s12889-018-6123-0
35. Nganda RY, Drakeley C, Reyburn H, Marchant T. Knowledge of malaria influences the use of insecticide treated nets but not intermittent presumptive treatment by pregnant women in Tanzania. Malar J. 2004;3(1):42. doi:10.1186/1475-2875-3-42
36. Mitiku I, Assefa A. Caregivers’ perception of malaria and treatment-seeking behaviour for under five children in Mandura District, West Ethiopia: a cross-sectional study. Malar J. 2017;16(1):144. doi:10.1186/s12936-017-1798-8
37. Kimbi HK, Nkesa SB, Ndamukong-Nyanga JL, Sumbele IUN, Atashili J, Atanga MBS. Knowledge and perceptions towards malaria prevention among vulnerable groups in the Buea Health District, Cameroon. BMC Public Health. 2014;14(1):883. doi:10.1186/1471-2458-14-883
38. Adedotun A, Morenikeji O, Odaibo A. Knowledge, attitudes and practices about malaria in an urban community in south-western Nigeria. J Vector Borne Dis. 2010;47(3):155–159.
39. Hanafi-Bojd A, Vatandoost H, Oshaghi M, et al. Knowledge, attitudes and practices regarding malaria control in an endemic area of southern Iran. Southeast Asian J Trop Med Public Health. 2011;42(3):491.
40. Erhun W, Agbani E, Adesanya S. Malaria prevention: knowledge, attitude and practice in a Southwestern Nigerian community. Afri J Biomed Res. 2005;8(1):25–29.
41. Dida N, Darega B, Abebe A. Treatment-seeking behavior and associated factors among malaria suspected patients in Bale Zone, Southeast Ethiopia: institution-based cross-sectional study. J Fam Med. 2015;2(1):5.
42. Champion VL, Skinner CS. The health belief model. Health Behav Health Educ. 2008;4:45–65.
43. Moscoso-Porras MG, Alvarado GF. Association between perceived discrimination and healthcare–seeking behavior in people with a disability. Disabil Health J. 2018;11(1):93–98. doi:10.1016/j.dhjo.2017.04.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
