Back to Journals » Journal of Inflammation Research » Volume 16
Macrophage Heterogeneity and Its Impact on Myocardial Ischemia-Reperfusion Injury: An Integrative Review
Authors Xu S , Xu C, Xu J , Zhang K, Zhang H
Received 13 September 2023
Accepted for publication 30 November 2023
Published 7 December 2023 Volume 2023:16 Pages 5971—5987
DOI https://doi.org/10.2147/JIR.S436560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Shuwan Xu,1,2,* Cong Xu,1,* Jiahua Xu,1 Kun Zhang,2 Huanji Zhang1
1Cardiovascular Department, the Eighth Affiliated Hospital of Sun Yat-Sen University, Sun Yat-Sen University, Shenzhen, Guangdong, People’s Republic of China; 2Department of Cardiology, Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University, Sun Yat-Sen University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huanji Zhang, Cardiovascular Department, The Eighth Affiliated Hospital of Sun Yat-Sen University, No. 3025 Shennan Middle Road, Shenzhen, 518033, People’s Republic of China, Tel +8613688808979, Email [email protected] Kun Zhang, Department of Cardiology, Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University, 107 Yanjiang West Road, Guangzhou, 510120, People’s Republic of China, Tel +8613450390268, Email [email protected]
Abstract: The coronary reperfusion following acute myocardial infarction can paradoxically trigger myocardial ischemia-reperfusion (IR) injury. This complex phenomenon involves the intricate interplay of different subsets of macrophages. These macrophages are crucial players in the post-infarction inflammatory response and subsequent myocardial anti-inflammatory repair. However, their diverse functions can lead to both beneficial and detrimental effects. On one hand, these macrophages play a crucial role in orchestrating the inflammatory response, aiding in the clearance of cellular debris and initiating tissue repair mechanisms. On the other hand, their excessive infiltration and activation can contribute to the perpetuation of the inflammatory cascade, leading to additional myocardial injury and adverse cardiac remodeling. Multiple mechanisms contribute to the IR injury mediated by macrophages, including oxidative stress, apoptosis, and autophagy. These processes further exacerbate the damage to the already vulnerable myocardial tissue. To address this delicate balance, therapeutic strategies aiming to target and modulate macrophage polarization and function are being explored. By fine-tuning the immune inflammatory response, such interventions hold promise in mitigating post-infarction myocardial injury and fostering a more favorable environment for myocardial healing and recovery. Through advancements in this area of research, potential anti-inflammatory interventions may pave the way for improved clinical outcomes and better management of patients after acute myocardial infarction.
Keywords: ischemia-reperfusion injury, macrophages, inflammatory cascade, cardiac remodeling, oxidative stress
Introduction
Acute myocardial infarction (AMI), one of the leading causes of cardiovascular-related mortality worldwide, results from the prolonged ischemic death of myocardial cells. AMI is often accompanied by progressive deterioration of cardiac pumping function, and the formation of myocardial scars leads to adverse cardiac remodeling and heart failure.1 Early reperfusion therapy, from initial thrombolytic drugs to catheter-based percutaneous coronary intervention (PCI), has been the primary approach to salvage ischemic myocardium and reduce early mortality in AMI patients.2 However, while reperfusion therapy effectively reduces the loss of contractile myocardial muscle mass, paradoxically, it triggers aggravated myocardial injury, induces sterile inflammation, and increases the infarct size, collectively known as ischemia-reperfusion (IR) injury.2 This process involves multiple cells and signaling pathways, including immune cell-induced inflammatory cascades, reactive oxygen and nitrogen species-mediated myocardial oxidative stress, and mitochondrial calcium imbalance in cardiomyocytes.3–5 Macrophages, being a crucial element of the innate immune system, have a vital regulatory function in the inflammatory response and IR injury subsequent to myocardial infarction. This review summarizes several pathophysiological mechanisms by which macrophages are involved in myocardial IR injury and emphasizes the crucial roles of different macrophage subtypes in mediating inflammation and resolution. Given that various drugs and intervention therapies tested in preclinical models have shown limited translation into clinical benefits for AMI patients, developing targeted therapeutic strategies to modulate macrophage polarization and function offers promising therapeutic potential. The use of biomimetic delivery systems targeting macrophage polarization and function, such as mesenchymal stromal cells (MSCs)-derived extracellular vesicles, provides new opportunities for targeted immunomodulation and anti-inflammatory interventions. In conclusion, understanding the intricate involvement of macrophages in the pathophysiology of IR injury and inflammation after myocardial infarction is critical for developing effective therapeutic approaches. Targeted modulation of different macrophage subtypes could offer promising avenues for attenuating the inflammatory response and promoting cardiac repair. Biomimetic delivery systems and MSC-derived extracellular vesicles represent potential novel therapies for specifically regulating immune responses and anti-inflammatory interventions in the context of myocardial infarction. Further research and clinical studies are warranted to validate their efficacy and safety in AMI patients.
Overview of Ischemia-Reperfusion Injury
Myocardial ischemia commonly occurs on the basis of coronary atherosclerosis and is triggered by coronary artery occlusion or redistribution of blood flow away from a specific vascular territory.6 Myocardial infarction is characterized by irreversible damage to myocardial cells caused by sustained ischemia, leading to myocardial necrosis in the corresponding perfusion area.7 Necrotic myocardium contributes to the formation of a mechanically weak region, where scar deposition occurs to prevent cardiac rupture and progressive functional deterioration. However, excessive remodeling of the infarcted area and remote myocardium can affect ventricular size and function, leading to heart failure or potential life-threatening arrhythmias.8 Myocardial infarction is typically attributed to the rupture, erosion, and subsequent thrombus formation overlying a lipid plaque, resulting in sudden coronary artery occlusion.9,10 This type of myocardial infarction is defined as Type I MI. In contrast, Type II MI, which is unrelated to plaque rupture, occurs due to myocardial oxygen supply-demand imbalance, such as endothelial dysfunction, coronary artery spasm, or arrhythmias.7
Timely and effective reperfusion following acute myocardial infarction is the only way to salvage ischemic myocardium and reduce the loss of contractile myocardial muscle mass. Pharmacological thrombolysis, PCI, or surgical coronary artery bypass grafting (CABG) can restore early blood flow reconstruction, effectively limit the infarct size, and greatly improve early survival rates in patients with acute MI.2 However, while reperfusion therapy can rescue dying myocardial cells, it paradoxically triggers exacerbation of myocardial injury known as IR injury, leading to an increase in the infarct size by 25%-40%.11
IR injury is a complex process involving multiple factors, such as metabolic factors, inflammatory responses, oxidative stress, and microvascular obstruction. The immune response plays a central role in this injury, marked by the recruitment and activation of immune cells associated with both the innate and adaptive immune systems.12 Innate inflammatory cells express pattern recognition receptors (PRRs) such as Toll-like receptors, NOD-like receptors, C-type lectin receptors, and RIG-1-like receptors.13 These receptors, upon binding to danger signals released by ischemic and necrotic myocardial cells known as damage-associated molecular patterns (DAMPs), activate IR, leading to the activation of signaling mediators and the production of pro-inflammatory mediators, thereby inducing tissue inflammation.
The inflammatory response in myocardial cells during the ischemic phase is already induced, but the restoration of blood flow and oxygen supply can further activate inflammatory signaling pathways.14 The local or systemic inflammatory response caused by this immune reaction can exacerbate the damage from coronary artery occlusion and become a significant factor constraining patient prognosis.15 As a result of revascularization therapy, myocardial IR injury is inevitable, yet the existing treatment modalities have not demonstrated ideal therapeutic outcomes. Over the past decade, numerous large-scale clinical trials aimed at reducing reperfusion injury have been conducted: the POST and DANAMI trials assessed the impact of ischemic preconditioning on myocardial no-reflow,16,17 the CIRCUS trial investigated the role of cyclosporine, and the IMMEDIATE trial studied the effects of intravenous glucose-insulin-potassium.18,19 None of these treatments were able to effectively attenuate myocardial injury caused by IR. Additionally, despite promising results from a large number of preclinical studies demonstrating the efficacy of mechanical or pharmacological interventions in reducing infarct size, translating these findings into clinical benefit in acute myocardial infarction patients has proven challenging.2 Therefore, a comprehensive understanding of the underlying immunopathological mechanisms is crucial for the treatment of reperfusion injury.
The Role of Macrophages in Post-Myocardial Infarction
Classification and Their Roles of Resident Macrophages Following Myocardial Infarction
Macrophages are distributed throughout various tissues in the body and primarily play a role in phagocytosing pathogens and cellular debris, as well as activating other immune cells to respond to pathogens.20 Cardiac tissue resident macrophages are a specific population of macrophages located between cardiomyocytes, endothelial cells, and fibroblasts, taking on a spindle-shaped morphology.21 They originate from the yolk sac during embryonic development and are closely associated with the development of the myocardial wall vasculature, regulating blood supply and drainage.22 Resident macrophages in cardiac tissue are widely distributed and contribute to self-renewal through phagocytosis, participate in maintaining electrical conduction, and are involved in the clearance of bacteria and apoptotic cells, playing a crucial role in cardiac homeostasis and injury repair.21,23,24
Under homeostatic conditions, macrophages in the myocardium exhibit diverse functional subtypes. Tissue-resident cardiac macrophages can be categorized into two subpopulations: CCR2− and CCR2+.25–27 Within the resident macrophage community, CCR2− macrophages uphold the cell population through cellular proliferation, whereas their CCR2+ counterparts primarily sustain themselves by recruiting monocytes from the circulation and undergoing autonomous self-renewal processes.28 After myocardial cell death, the tissue-resident CCR2+ macrophages become activated and facilitate the recruitment of monocytes through a MYD88-dependent mechanism. This activation results in the release of monocyte chemoattractant proteins (MCPs) and the mobilization of monocytes.29 In the ischemic region, there is an approximate 60% reduction in the quantity of CCR2− resident macrophages, with cell numbers gradually increasing through localized proliferation.30 Meanwhile, within the cardiac muscle, the resident macrophage subpopulation undergoes substantial replacement by recruited CCR2+ Ly6Chigh monocytes and macrophages derived from CCR2+ monocytes.30 Furthermore, CCR2+ macrophages play a role in regulating the differentiation of monocytes into inflammatory monocyte-derived macrophage populations. They also facilitate the rapid infiltration of inflammatory monocytes and macrophages into the affected area.
In the context of cardiovascular disease, the mobilization and infiltration of peripheral monocytes into diseased tissues are predominantly viewed as maladaptive responses, contributing to various conditions such as infarct expansion, left ventricular systolic dysfunction, left ventricular dilatation, and the progression of atherosclerotic plaques.31,32 Studies have found that monocyte-derived macrophages express high levels of pro-inflammatory genes in the stressed heart,25,33 and inhibiting monocyte recruitment by interrupting MCP1 and CCR2 signaling can reduce excessive inflammation and confer protective effects in mouse models of myocardial infarction and atherosclerosis.32,34 Hence, inhibiting the activation of tissue-resident CCR2+ macrophages or neutralizing the effector cytokines they produce could potentially offer benefits following myocardial infarction. Intriguingly, previous studies have indicated that the relative abundance of CCR2+ macrophages in tissues may increase with age. Consequently, in the aging heart, there could be an upsurge in excessive inflammatory responses following myocardial injury, possibly explaining why aging is linked to heightened inflammation and poorer clinical outcomes after myocardial infarction.35 Thus, therapeutic approaches targeting tissue-resident CCR2+ macrophages may be more effective in elderly individuals.
Involvement of Macrophages in Post-Myocardial Infarction Repair
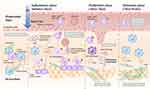
The repair process of injured myocardium following myocardial infarction involves three overlapping phases: the inflammatory phase, proliferative phase, and maturation phase.36 The immune response plays a critical role in regulating cardiac repair throughout this process, as shown in Figure 1. In the early stages of injury, necrotic myocardial cells release DAMPs into the extracellular space, including mitochondrial DNA fragments, Ca2+, high mobility group box 1 protein (HMBGB1), and others. PRRs on innate immune cells recognize DAMPs, triggering the release of chemotactic factors and pro-inflammatory cytokines, leading to sterile inflammation and recruitment/activation of monocytes and macrophages.37 Phagocytosis and efferocytosis are the main functions of macrophages during the inflammatory phase following infarction, primarily responsible for extracellular matrix degradation and engulfment of dead cells. This process peaks around 3 days after the injury occurs. As macrophages phagocytose apoptotic cells and activate anti-inflammatory cascades, the reparative phase of infarct healing commences, characterized by immune cell-mediated anti-inflammatory signaling activation, fibroblast proliferation, and deposition of granulation tissue. Macrophages can modulate their own secretion of pro-fibrotic and pro-angiogenic factors (Figure 2) based on changing levels of cytokines in the surrounding environment, thus regulating infarct healing, scar maturation, and maintaining oxygen and nutrient supply to the granulation tissue.38 Additionally, neutrophils, monocytes, endothelial cells, and myofibroblasts contribute to the suppression and resolution of the inflammatory response.39 The proliferative phase gradually transitions into the maturation phase after approximately 10 days and can persist for several months. During this stage, extracellular matrix remodeling occurs, compensatory hypertrophy in the non-infarcted area takes place to accommodate the hemodynamic load, and significant structural and functional changes occur in the ventricle.8 Macrophages, as versatile cells of the innate immune system, play an indispensable role in the entire process of injury repair following myocardial infarction. They contribute to the initial inflammatory response and actively participate in wound healing mechanisms. Different subtypes of macrophages synergistically regulate various pathological and physiological processes, including phagocytosis, modulation of inflammatory responses, fibroblast activation, and extracellular matrix remodeling.38
In the homeostatic state, cardiac resident macrophages are divided into two subpopulations, CCR2+ and CCR2-. Following the infarction, dying cardiomyocytes release damage-associated molecular patterns (DAMPs), which are recognized by pattern recognition receptors (PRRs) on neighboring cells, initiating the inflammatory phase. Endothelial cells express adhesion molecules, and macrophages release pro-inflammatory cytokines and chemokines. DAMPs and pro-inflammatory cytokines lead to the rapid recruitment of inflammatory monocytes and neutrophils to the infarct area, where they are responsible for extracellular matrix degradation and phagocytosis of necrotic cells. This process reaches its peak three days after injury. As macrophages engulf apoptotic cells, an anti-inflammatory cascade is activated, and the infarcted tissue repair enters the proliferative phase. Monocytes differentiate into reparative macrophages that produce TGFβ, IL-10, and VEGF, mediating collagen synthesis in fibroblasts, inflammation resolution, and angiogenesis, respectively. The proliferative phase gradually transitions to the maturation phase after approximately ten days and continues for several months. During this stage, monocyte recruitment ceases, the extracellular matrix undergoes remodeling, and scar formation takes place.
Crosstalk Between Macrophages and Surrounding Cells
In the intricate tapestry of the heart, diverse cell types such as cardiomyocytes, fibroblasts, immune cells, vascular endothelial cells, and more coexist.40 Beyond fulfilling their individual roles, these cells intricately engage in crosstalk, a dynamic interplay that proves pivotal in maintaining cardiac equilibrium and shaping the course of myocardial infarction.
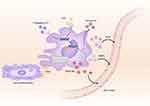
Efferocytosis is the process by which macrophages engage in phagocytic endocytosis to process and degrade apoptotic cells. This process is essential for the resolution of inflammation and tissue repair following myocardial infarction. In the aftermath of myocardial infarction, a substantial number of dying myocardial cells necessitate multiple, rapid engulfment events by macrophages to clear cellular components originating from the deceased cells.41 Ge et al42 discovered that the specific expression deficiency of the gene Lgmn in resident cardiac macrophages leads to the accumulation of apoptotic myocardial cells and a reduced efferocytosis index in the border area. Furthermore, Lgmn deficiency can enhance the infiltration of MHC-IIhighCCR2+ macrophages and the recruitment of MHC-IIlowCCR2+ monocytes by downregulating anti-inflammatory mediators (IL-10 and TGF-β) and upregulating pro-inflammatory mediators (IL-1β, TNF-α, etc.). The intricate dance between macrophages and myocardial cells involves specific efferocytosis receptors on the macrophage membrane that regulate their interaction. In a state of balance, myocardial cells release subcellular particles containing faulty mitochondria. Nearby resident macrophages recognize and eliminate these particles through the MerTk receptor, preventing the activation of inflammatory pathways and autophagy. After myocardial infarction, macrophages employ the MerTk receptor to identify and clear deceased myocardial cells, averting secondary necrosis and further inflammatory responses.43–45 The integrin-associated protein CD47 experiences an increase in expression in myocardial cells during myocardial infarction. This heightened expression disrupts macrophage efferocytosis through the CD47-SIRPα axis. The introduction of CD47 antibodies can counteract this effect, enhancing the efferocytosis of dead myocardial cells.46 Furthermore, cannabinoid receptors, including type1 (CB1) and type2 (CB2), are widespread in various cell types such as macrophages, myocardial cells, and neutrophils. Recent studies indicate that both CB1 and CB2 play roles in protecting against myocardial injury, with CB2 taking a lead in shielding the heart from IR injury.47 In a mouse model of IR injury, the selective CB2 agonist JWH-133 demonstrates robust anti-inflammatory effects, including the restriction of infarct size and the promotion of survival among stressed myocardial cells.48 In the early phases following myocardial infarction, reparative fibrosis is crucial for preventing ventricular wall rupture. However, an excessive fibrotic response in the infarcted area becomes a potential catalyst for the onset of heart failure.49 Macrophages recruited to the site of injury play a key role in this process by expressing renin and angiotensin-converting enzyme (ACE), leading to the generation of angiotensin II. This activation of angiotensin II, in turn, stimulates TGF-β1, promoting the recruitment of myofibroblasts. Moreover, macrophage-produced Angiotensinogen II binds to angiotensin type 1 receptors on myofibroblasts, inducing the expression of TGF-β1 and matrix proteins, thereby fostering tissue repair and the formation of scar tissue.50
Following myocardial infarction, the immune system takes charge of orchestrating the inflammatory response, tissue repair, and remodeling processes, crucially influencing the extent of myocardial injury and disease progression.51 In the aftermath of myocardial infarction, a diverse array of immune cells is recruited to the infarcted area driven by DAMPs and various cytokines. In the inflammatory phase, neutrophils recruit Ly6Chigh monocytes/macrophages in a manner dependent on angiotensin II. Moreover, neutrophils in the damaged area stimulate the repair-oriented polarization of macrophages and the release of VEGF-A by upregulating annexin A1, thereby playing a role in mediating vascular regeneration.52 As the repair phase unfolds, Ly6Clow macrophages produce matrix metalloproteinase-12 (MMP-12), diminishing levels of neutrophil chemotactic factors to restrain neutrophil infiltration and promote the healing of wounds.53 Within the T lymphocyte population, CD4+ T lymphocytes, especially the helper subset, engage in dynamic interactions with macrophages post-myocardial infarction. In addition to the T1 (secreting INF-γ and TNF) and T2 (secreting IL-4 and IL-13) phenotypes, post-MI CD4+ T cells can be classified into “effector” (Tef; Foxp3−) and “regulatory” (Treg; Foxp3+) subsets. T1 cells drive the polarization of pro-inflammatory macrophages, while T2 and Treg cells foster the polarization of anti-inflammatory macrophages.54 Post-MI, Treg cells stimulate the transcription of CX3CR1 and TGF-β1 in macrophages through the production of IL-35, thereby promoting the survival of Ly6Clow macrophages and the deposition of extracellular matrix. In the realm of granulocytes, the interplay between eosinophils and macrophages after myocardial infarction should not be overlooked. The absence of eosinophils results in increased expression of pro-inflammatory mediators such as IL-18, CCL5, and TNF-α in the infarcted area, inhibiting the polarization of macrophages towards an anti-inflammatory phenotype.55 Additionally, the secretion of IL-5 by macrophages induces eosinophil aggregation and, through the IL-4/STAT6 axis, facilitates the recovery of heart function.56
Abundant evidence indicates the collective involvement of various cell types in the inflammatory response post-MI, highlighting their therapeutic potential in mitigating heart IR injury. Strategies targeting the crosstalk between different cells and macrophages may offer specific and crucial pathways for the prevention and treatment of MI/RI.
Macrophage Polarization and the Roles of Different Subsets
The rapid increase in the number of monocytes and macrophages in ischemic myocardium relies primarily on recruitment rather than local expansion.57 After myocardial infarction, monocytes are rapidly mobilized from the bone marrow or released from the spleen and migrate to the infarcted area, where they differentiate into macrophages or dendritic cells to trigger an immune response.58 Macrophages exhibit significant heterogeneity and plasticity, and subpopulations of macrophages with different functions and origins can have either protective or pathological roles.59 Monocytes in the infarct zone can differentiate into pro-inflammatory (M1) and reparative (M2) macrophages. M1 macrophages express TNF-α, iNOS, IL-1β, and IL-6, promoting a robust pro-inflammatory response and contributing to myocardial IR injury. Conversely, M2 macrophages express IL-10, arginase-1, and arginase-2 instead of iNOS. This leads to arginine depletion and production of polyamines and proline (instead of nitric oxide). These factors play essential roles in cell differentiation and collagen synthesis, respectively, supporting tissue repair and the resolution of inflammation.8,60 Both M1 and M2 macrophages coordinate their actions to participate in the cardiac repair process following myocardial infarction.
M1 macrophages are rapidly recruited to the infarcted area in the early stages of myocardial infarction, and through the TGF-β1/Smad3/MMP pathway, they stimulate fibroblasts to release extracellular matrix, thereby activating myocardial fibrosis.61 As mentioned earlier, the activation of the anti-inflammatory cascade is triggered by the uptake of apoptotic cells and matrix fragments by M1 macrophages, which counteracts the inflammatory response in the infarcted area and reduces adverse myocardial remodeling. During the proliferative phase of the repair process, the dominant subpopulation of macrophages shifts from M1 to M2. M2 macrophages, induced by IL-4/IL-13/IL-10, promote cardiac remodeling through the production of TGF-β and VEGF, which mediate scar formation and angiogenesis.41 In the maturation phase, infiltrating macrophages transition to a phenotype that promotes inflammation resolution, accelerating cardiac fibrosis and facilitating injury repair. Although early inflammatory activation is a necessary event for transitioning to the later reparative program, excessive infiltration of inflammatory macrophages can aggravate myocardial injury and remodeling after myocardial infarction by releasing pro-inflammatory cytokines, cytotoxic mediators, and reactive oxygen species (ROS).8 Therefore, strict control of the recruitment of inflammatory macrophages and timely regulation of their transition to a reparative phenotype (M2) are crucial for ensuring tissue healing, preventing excessive inflammatory response, and avoiding adverse remodeling and systolic dysfunction.8
It is worth mentioning that the heterogeneity of macrophages may highly depend on microenvironmental cues, including extracellular vesicles (EVs).62 Previous studies have demonstrated an elevation in the release of EVs, particularly ischemia/reperfusion-induced cardiac EVs (IR-EVs), in the heart during IR injury. These IR-EVs have been found to enhance pro-inflammatory, chemotactic, and phagocytic functions in macrophages, thereby mediating M1 polarization and exacerbating IR-induced cardiac injury and dysfunction.63 Furthermore, IR-EVs, as inflammatory mediators, not only promote local cardiac inflammation but also increase the infiltration of immune cells in multiple organs, creating a pro-inflammatory environment in distant organs. Inhibiting the generation and release of EVs has been found to alleviate IR-induced cardiac inflammation and injury and reduce systemic inflammation in IR mice.63 Indeed, given the detrimental effects of excessive M1 macrophage activation in myocardial I/R injury, leveraging the EV pathway to promote early and extensive infiltration of M2 macrophages could be a promising therapeutic strategy. Maintaining a proper balance between M1 and M2 macrophages may help attenuate the pro-inflammatory response and promote tissue repair, ultimately providing beneficial outcomes in the management of myocardial I/R injury.
Furthermore, the regulation of PRR expression holds promising therapeutic prospects in modulating macrophage differentiation and alleviating IR injury. For instance, the E3 ubiquitin ligase Peli1, a regulatory protein in the TLR signaling pathway, is activated in macrophages following IR injury. Loss of Peli1 in macrophages inhibits IRF5 nuclear translocation, suppressing M1 polarization of macrophages and reducing myocardial IR injury.64 Cross-signaling between AXL and TLR4 in cardiac macrophages guides glycolytic metabolism and promotes the secretion of pro-inflammatory IL-1β, leading to increased cardiac inflammation. Selective small molecule AXL inhibitors effectively improve cardiac healing.65 Dectin-1, a member of the C-type lectin receptor family, exacerbates myocardial injury in inflammatory macrophages through the release of pro-inflammatory cytokines and mediation of neutrophil infiltration. Dectin-1 antibodies mediate significant improvements in cardiac function and reduce M1 macrophage polarization.66 Therefore, drug development targeting these types of targets will provide more options for IR treatment.
Mechanisms of Macrophage Involvement in Myocardial Ischemia-Reperfusion Injury
Oxidative Stress During Ischemia-Reperfusion Injury
The decreased oxygen supply resulting from ischemia directly leads to a reduction in mitochondrial oxidative phosphorylation, and myocardial cell metabolism shifts from aerobic to anaerobic metabolism.67 Reperfusion restores oxygen and nutrient supply to prevent ischemia-induced cell death and supports cellular metabolism while removing residual byproducts of cellular metabolism. However, paradoxically, the burst of free radicals and inflammatory reactions may induce more severe tissue damage. Oxidative stress becomes a major factor contributing to cell death and tissue injury in IR injury.68
ROS are the primary types of free radicals involved in myocardial reperfusion injury through multiple pathways. They contribute to the opening of the mitochondrial permeability transition pore, act as chemoattractants for neutrophils, and mediate dysfunction of the sarcoplasmic reticulum. These processes collectively lead to tissue damage and worsen the outcome of myocardial reperfusion injury.69,70 ROS can also cause lipid peroxidation of the myocardial cell membrane, intracellular calcium overload, enzyme denaturation, and DNA oxidation damage, ultimately leading to myocardial cell apoptosis.71 In the myocardium injured by ischemia-reperfusion (IR), recruited and activated macrophages act as reservoirs, generating a substantial amount of ROS and releasing them into the microenvironment. These ROS directly exert cytotoxic effects on myocardial cells, contributing to tissue damage and exacerbating the injury caused by IR.72,73 During the reperfusion phase, the excessive burden of ROS leads to excessive autophagy in myocardial cells, which has adverse effects on myocardial cells.74 Therefore, the removal of excessive ROS in the myocardial microenvironment holds great potential in IR therapy. Antioxidants have been shown to have cardioprotective effects to some extent in animal models and several clinical trials.75 For example, p-coumaric acid (p-CA), which contains a hydroxyl group, provides hydrogen atoms to scavenge ROS, terminates the free radical chain reaction, and forms stable products by electron transfer through a phenyl ring and a double bond side chain.76 p-CA inhibits the polarization of M1 macrophages and promotes the polarization of M2 macrophages by increasing the expression of IDO in vitro and in the hearts of MI/R mice, thereby alleviating IR injury.76
Furthermore, NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome inhibitors have demonstrated significant therapeutic effects in combating ROS and mitigating oxidative stress in myocardial cells. In a study by Xu et al77 using donor-heart rats to investigate IR injury in hearts donated after circulatory death (DCD), it was found that intravenous administration of the NLRP3 inhibitor MCC950 in rats could reduce the inflammatory response and oxidative stress levels in DCD hearts preserved with normothermic ex vivo heart perfusion, effectively countering cardiac IR injury. Research by Sun et al78 revealed that MCC950 treatment markedly reduced the myocardial infarct area, alleviated pathological changes in myocardial tissue, increased left ventricular development pressure, and improved the levels of the maximum rise/decrease rate of left ventricular pressure. This treatment not only prevented myocardial oxidative damage but also inhibited the formation of NLRP3 inflammasomes. In another innovative approach, Cheng et al79 designed a novel drug delivery system known as negatively charged surface MMP9 hydrolytic microspheres (NMM) and incorporated MCC950 into NMM (NMM-m). The released MCC effectively inhibited the activity of NLRP3 inflammasomes, thereby suppressing the secretion of inflammatory factors in granulocytes. This intervention not only prevented early inflammatory damage but also contributed to the improvement of heart function.
Furthermore, the excessive production of ROS is closely associated with elevated histone acetylation levels. Notably, the histone acetyltransferase KAT8 (also known as MOF) promotes the deactivation (trans-activation) of the ROS-synthesizing enzyme NADPH oxidase (NOX) in macrophages following IR injury. As a consequence, this dysregulation leads to an abnormal increase in ROS production and accumulation, contributing to the detrimental effects of IR injury.80 Therefore, downregulation of MOF in macrophages may help inhibit ROS generation from upstream sources. The transcriptional regulator MKL1 promotes heart IR injury by recruiting MOF to trans-activate NOX genes in macrophages. Each individual component of small-molecule compounds (CCG-1423, MG149, and GKT137831) that inhibit the MKL1-MOF-NOX axis can effectively alleviate myocardial IR injury in mice.75
Nuclear factor erythroid 2-related factor (Nrf2) is a key transcription factor that acts as an oxidative stress sensor and protects cells from oxidative damage.81 Under normal conditions, it remains inactive by binding to the inhibitor Kelch-like ECH-associated protein 1 (Keap1). However, during excessive oxidative stress, Nrf2 can be released from Keap1 and translocate to the cell nucleus, where it gradually accumulates. Subsequently, Nrf2 associates with genes containing antioxidant response elements (ARE), which play a role in mitigating oxidative damage and maintaining cellular redox homeostasis. As a result, the transcription of a range of cell-protective, antioxidant, and anti-inflammatory protein-encoding genes is activated, including HO-1 and NQO1,82 which helps alleviate oxidative stress damage. The Nrf2/Keap1/ARE signaling system is considered a major cellular defense mechanism against oxidative and exogenous stress.83 Activation of the Keap1/Nrf2 axis can inhibit ROS damage and provide protective effects against cardiac IR injury.84
Pyroptosis-Mediated Ischemia-Reperfusion Injury
NLRP3 inflammasome activation has long been recognized as a crucial catalyst for pyroptosis. I/R can induce the activation of the NLRP3 inflammasome. In simple terms, nod-like receptors located in the cytoplasm directly or via ASC recruit pro-Caspase-1 within the cells, forming a multiprotein complex. As the local concentration of pro-Caspase-1 increases, it undergoes self-cleavage, generating active Caspase-1, which then cleaves Gasdermin D into GSDMD terminus.85,86 Gasdermin pores formed by the previously cleaved N-terminal of GSDMD mediate the release of mature IL-1β, leading to cell membrane swelling, rupture, and typical lytic cell death.87 This process is defined as pyroptosis. Pyroptosis is a newly discovered programmed cell death mode that occurs in various tissues. In addition to causing cell death, pyroptosis also triggers excessive inflammatory damage.88 GSDMD serves as the executor of pyroptosis, and previous studies have shown that the specific deletion of GSDMD in cardiomyocytes reduces myocardial infarct size and improves post-myocardial infarction cardiac function.89,90 However, its role is relatively limited, suggesting the involvement of other cell-mediated pyroptosis in myocardial infarction. The expression level of GSDMD is relatively high in immune cells such as monocytes and macrophages.91,92 Ye et al93 found that GSDMD is mainly expressed in infiltrated macrophages in the infarcted area. GSDMD-deficient mice exhibit reduced release of inflammatory cytokines, decreased neutrophil infiltration, significantly reduced infarct size, and attenuated IR injury, highlighting the potential therapeutic role of targeted modulation of GSDMD in myocardial IR injury.
Autophagy-Mediated Ischemia-Reperfusion Injury
Autophagy is a dynamic process that entails the formation of autophagosomes, which subsequently fuse with lysosomes.94 Unfolded proteins and damaged organelles are engulfed by double-membrane vesicles called autophagosomes and subsequently delivered to lysosomes for degradation. Dysregulation of autophagy has been associated with various cardiovascular diseases.95 Cardiac autophagy is crucial for maintaining cellular homeostasis and responding to stress conditions, including IR injury and nutrient deprivation, to prevent cell death. In response to stimulating conditions, such as energy deprivation, oxidative stress, and calcium changes induced by myocardial IR, autophagy is induced as a protective mechanism.96 In the initial phases of myocardial IR, the accumulation of waste products and apoptotic cells disrupts cardiac homeostasis and worsens myocardial injury. Upregulation of autophagy plays a vital role in clearing apoptotic cells and damaged organelles, contributing to the restoration of cardiac homeostasis and mitigating the detrimental effects of IR injury.96,97 Indeed, enhanced autophagy plays a critical role in maintaining ATP levels and ensuring an adequate nutrient supply during stressful conditions. Upregulation of autophagy under stress is beneficial and protective for cells. Therefore, pharmacological induction of autophagy could be a promising and innovative therapeutic strategy to alleviate IR injury and promote cardiac tissue recovery. The natural flavonoid Galangin has been found to promote autophagy in the process of myocardial IR by activating the PI3K/AKT/mTOR pathway. This activation facilitates the removal and clearance of damaged organelles and misfolded proteins, contributing to cellular homeostasis and protection against IR-induced injury. In addition to promoting autophagy, Galangin has been shown to reduce the infiltration of immune cells (CD45+ cells, neutrophils, and macrophages) and the levels of inflammatory factors (IL-1β and NLRP3). This protective effect helps safeguard impaired cardiac function and limits infarct expansion following myocardial IR. By modulating immune cell infiltration and inflammation, Galangin exhibits potential as a therapeutic agent for mitigating the adverse effects of myocardial IR injury.98
Regulation of Macrophage Polarization in Ischemia-Reperfusion Injury by Interleukins
Effective suppression of excessive inflammatory response following myocardial infarction can alleviate myocardial ischemic injury and improve cardiac function. Considering the crucial role of interleukins in IR injury, interventions targeting them hold promising therapeutic prospects. Indeed, TNF-α and IL-6 are two classic pro-inflammatory cytokines released by macrophages. Their activation leads to a cascade of events, including the activation of macrophages and MAPK/NF-κB signaling pathways. This, in turn, stimulates the production of additional inflammatory factors and promotes the polarization of macrophages from an anti-inflammatory M2 phenotype to a pro-inflammatory M1 phenotype. This shift in macrophage polarization contributes to the intensification of the inflammatory response and exacerbates tissue damage in various inflammatory conditions.99–101 After IR injury, miR-21 can silence KBTBD7 upstream of TNF-α and IL-6, reducing the inflammatory response of cardiac macrophages and thus decreasing the myocardial infarct size. IL-1β is a central cytokine in MI/RI that recruits a large number of inflammatory cells and enhances the synthesis of inflammatory cytokines, exacerbating myocardial injury. Recombinant IL-1 receptor antagonist can reduce cell apoptosis and improve cardiac function after myocardial infarction.102 IL-18, a member of the IL-1 superfamily, exerts cardioprotective effects when neutralized after MI/RI.103 IL-7 enhances the cytotoxic activity of macrophages,104,105 induces the secretion of various pro-inflammatory cytokines by monocyte-derived macrophages (such as MCP-1, MIP, IL-1β),106 modulates the interaction between different components in the inflammatory process,107 and increases the expression of chemokine receptors (CCRs) on monocytes, such as CCR1, CCR2, and CCR5.108 Anti-IL-7 antibodies significantly reduce myocardial cell apoptosis and macrophage infiltration, influence the production of cytokines by Th1 and Th2 cells, and promote macrophage polarization towards the M2 phenotype.
IL-38 has been shown to attenuate IR injury by inhibiting macrophage inflammation. This inhibitory effect is partly achieved by suppressing the activation of the NOD-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome, resulting in reduced expression of inflammatory cytokines and decreased cardiomyocyte apoptosis.109 IL-38 has demonstrated multiple beneficial effects in alleviating myocardial IR injury. It promotes the differentiation of M1 macrophages into an M2 phenotype, inhibits the activation of the NLRP3 inflammasome, and enhances the secretion of anti-inflammatory cytokines such as IL-10 and transforming growth factor-beta. These actions collectively contribute to the attenuation of IR injury in the myocardium. Recombinant IL-38, upon full activation, can bind to interleukin 1 receptor accessory protein-like 1 (IL-1RAPL1) and activate the c-jun N-terminal kinase/activator protein 1 (JNK/AP1) pathway, leading to increased production of IL-6. Additionally, IL-38 plays a role in regulating cardiac regulatory T cells induced by dendritic cells, thereby modulating macrophage polarization and ultimately improving ventricular remodeling after myocardial infarction. The diverse mechanisms of IL-38 action highlight its potential as a promising therapeutic target for the treatment of myocardial IR injury and related cardiovascular conditions.110
Emerging Therapeutic Approaches Targeting Macrophages
Biomimetic Targeted Delivery Systems Based on Monocyte/Macrophage
After myocardial infarction, platelets are activated early on and interact with monocytes within the vasculature, leading to their subsequent entry into the ischemic myocardium.111 Monocyte-platelet aggregates are formed through the interaction of P-selectin with P-selectin glycoprotein ligand-1 (PSGL-1) and are associated with the severity of inflammation in acute myocardial infarction.112,113 Therefore, simulating the interaction between platelets and monocytes may facilitate efficient targeted delivery of therapeutic molecules by preferentially binding to circulating monocytes. Tan et al114 engineered platelet-like fusogenic liposomes (PLPs) that carry mesoporous silica nanospheres loaded with miR-21. Under the coating of PLPs, these nanospheres were specifically delivered to circulating monocytes in mice with IR injury, where they directly entered the cytoplasm of monocytes through membrane fusion, leading to their reprogramming into a reparative phenotype. Zhou et al115 achieved reversibly camouflaged nanocomplexes (NCs) using a hybrid membrane derived from platelets and macrophages. By exploiting the inflammation homing mediated by macrophage membrane and the microthrombus-targeting effect of platelet membrane, the NCs, when systemically administered after myocardial IR injury, actively aggregated in the damaged myocardium and internalized into cardiomyocytes. In rats and pigs, the NCs effectively delivered siSav1, resulting in significant downregulation of Sav1 in the injured myocardium, thereby inhibiting the Hippo pathway, promoting myocardial regeneration, and suppressing apoptosis. Resolvin D1 (RvD1) is known for its role in mediating the active resolution of acute inflammation. However, its systemic administration as a treatment for IR injury is severely limited due to its biological instability and lack of targeting ability. These challenges have hindered its clinical application as a therapeutic agent for IR injury. Weng et al116 developed a platelet-inspired, ROS-responsive RvD1 delivery platform by combining ROS-responsive liposomes loaded with RvD1 and platelet membranes. This delivery platform inherited the ability of platelets to interact with monocytes, allowing platelets to reach the site of heart injury through monocyte chemotaxis after intravenous injection. At the site of injury, abundant ROS disrupts the delivery platform, enabling rapid release of RvD1. In mammals, the NOX protein family plays a critical role in ROS generation. Considering the trans-activation of NOX genes in MOF-programmed macrophages, Wang et al117 developed macrophage-targeted NCs to efficiently co-deliver siRNA against MOF (siMOF) and microRNA-21 (miR21) into cardiac macrophages. The NCs were effectively internalized by cardiac macrophages after systemic administration, where branched poly(β-amino ester) (BPAE-SS) was degraded by intracellular glutathione into small fragments, promoting the release of siMOF/miR21 and ultimately inducing effective gene silencing.
Treatment of Myocardial Ischemia-Reperfusion Injury with MSCs
MSCs are a unique type of stromal cells that have been shown to effectively reduce infarct size and promote angiogenesis in ischemic heart disease.118 Studies have demonstrated that MSCs can also reduce the production of pro-inflammatory cytokines by macrophages and induce their polarization towards an anti-inflammatory M2 phenotype, thereby alleviating the inflammatory cascade response.119
The interaction between MSCs and the inflammatory niche relies heavily on intercellular communication mediated by the secretion of immunomodulatory secretomes.120,121 Extracellular vesicles derived from MSCs (MSC-Exo) have shown promising effects in improving overall cardiac function and attenuating ventricular remodeling by inhibiting stress-induced cell apoptosis, reducing oxidative stress, and promoting angiogenesis in the setting of myocardial ischemic injury.122,123 Furthermore, MSC-Exo have demonstrated therapeutic potential in animal models of tissue injury and inflammatory diseases.124,125
Certain microRNAs present in MSC-derived extracellular vesicles have been identified as important regulators of immune modulation. For example, miR-182 has been shown to participate in the M1 to M2 polarization of macrophages mediated by MSC-Exo through the targeting of TLR4/NF-κB/PI3K/Akt signaling cascade.119 miR-98-5p can suppress inflammation levels and macrophage infiltration by reducing TLR4 expression and activating the PI3K/Akt signaling pathway.126 miR-125a-5p, enriched in MSC-Exos, enhances M2 macrophage polarization and angiogenesis while attenuating fibroblast proliferation and activation, thereby improving myocardial cell apoptosis and inflammation.127 Moreover, several other microRNAs, such as miR-21, miR-223, miR-146a, and miR-181b, have demonstrated therapeutic potential in improving cardiac IR, regulating macrophage polarization, and modulating the inflammatory cascade.119 These findings highlight the broad application prospects of microRNAs as therapeutic molecules in the context of cardiac IR, macrophage polarization, and inflammatory response modulation.
However, the administration route for therapeutic MSC-Exo remains a pressing issue. In animal experiments, intramyocardial injection of MSC-Exo has been widely used as an efficacy assessment method but has limited clinical translational value. Although EVs have low immunogenicity and high stability, their systemic administration via intravenous route faces the risk of being engulfed by the mononuclear phagocyte system. Unmodified EVs have a short half-life in serum, lasting only 6 hours in nude mice and 1–3 hours in normal mice,128,129 which severely hampers their therapeutic efficacy. Wei et al130 developed a two-step EV delivery approach involving genetic modification followed by therapeutic cargo electroporation. They constructed CD47-EVs loaded with mir-21 through electroporation in MSCs overexpressing CD47, enabling successful transportation of exogenous miR-21 to the left ventricle myocardium after cardiac IR injury via peripheral vein injection and significantly prolonging EV retention in vivo. Based on the aforementioned biomimetic molecular delivery system, Li et al131 introduced platelet membrane-engineered MSC-derived EVs (named P-EVs) for targeted immunomodulatory therapy in cardiac repair. P-EVs bind to Ly6Chigh monocytes in peripheral blood, and monocytes carry P-EVs into the ischemic myocardium. After transportation, monocytes preferentially differentiate into M1 macrophages,132 leading to in situ engulfment of surface-anchored P-EVs by M1 macrophages. In a mouse model of myocardial IR injury, P-EVs achieved endosomal escape upon engulfment by macrophages, releasing therapeutic miRNAs into the cytoplasm and promoting polarization of M1 macrophages toward an M2 phenotype, thereby mediating cardiac repair.131
Conclusion
Coronary reperfusion is necessary after acute myocardial infarction, but it can also trigger myocardial IR injury, in which macrophages play a crucial role. Different subtypes of macrophages participate in the inflammatory response and myocardial anti-inflammatory repair after myocardial infarction, generating complex effects during IR injury. Although macrophages play a positive role in clearing cellular debris and initiating tissue repair, excessive infiltration and activation can exacerbate myocardial damage and remodeling. M1 macrophages release numerous pro-inflammatory cytokines, intensifying the inflammatory response and increasing the generation of reactive oxygen species, leading to cell membrane damage and mitochondrial dysfunction. In contrast, M2 macrophages possess anti-inflammatory and reparative functions. They secrete anti-inflammatory factors, helping to alleviate the inflammatory response and promote cardiac cell repair and regeneration. Additionally, M2 macrophages are involved in cardiac tissue remodeling and angiogenesis, facilitating damaged tissue repair. Therefore, regulating the polarization state of macrophages towards M2 may be a key strategy to alleviate myocardial IR injury. By utilizing emerging biotechnologies, such as biomimetic molecular targeting delivery systems and MSC-derived extracellular vesicles, more precise interventions may be achieved by targeting specific signaling pathways and molecular targets. This approach holds promise for modulating the immune-inflammatory response, alleviating myocardial IR injury, and promoting cardiac repair and regeneration. These potential therapeutic strategies could pave the way for novel avenues in the treatment of cardiovascular diseases, yielding improved clinical outcomes.
Despite significant progress in the field of macrophages and myocardial IR injury research, there are still challenges to overcome. Future investigations should delve into the specific functions of macrophage subtypes and their regulatory mechanisms, as well as their interactions with other immune and myocardial cells. Furthermore, conducting clinical studies is essential to validate the safety and efficacy of potential therapeutic strategies in the human body. In conclusion, gaining a deeper understanding of the mechanisms by which macrophages contribute to myocardial IR injury is of great significance for developing new anti-inflammatory intervention strategies and improving the prognosis of patients with acute myocardial infarction. By continuously exploring the regulation and intervention of macrophages, we may bring new hope for the treatment of cardiovascular diseases and make greater contributions to preserving myocardial health.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by The Natural Science Foundation of Guangdong Province [2020A1515010216], Science and technology project of Shenzhen [JCYJ20190808100807422] and Public health project of Shenzhen Futian [FTWS2020010] to Huanji Zhang. It was also supported by The National Natural Science Foundation of China [81600351] to Kun Zhang.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Algoet M, Janssens S, Himmelreich U, et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 2022;33:357–366. doi:10.1016/j.tcm.2022.02.005
2. Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17(12):773–789. doi:10.1038/s41569-020-0403-y
3. Davidson SM, Ferdinandy P, Andreadou I, et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol. 2019;73(1):89–99. doi:10.1016/j.jacc.2018.09.086
4. Shen Y, Liu X, Shi J, Wu X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol. 2019;125:496–502. doi:10.1016/j.ijbiomac.2018.11.190
5. Wang J, Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta pharmaceutica Sinica B. 2020;10(10):1866–1879. doi:10.1016/j.apsb.2020.03.004
6. Heusch G. Myocardial ischemia: lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res. 2016;119(2):194–196. doi:10.1161/CIRCRESAHA.116.308925
7. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. doi:10.1016/j.jacc.2018.08.1038
8. Peet C, Ivetic A, Bromage DI, Shah AM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116(6):1101–1112. doi:10.1093/cvr/cvz336
9. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136(12):1155–1166. doi:10.1161/CIRCULATIONAHA.117.029870
10. Frangogiannis NG. Pathophysiology of myocardial infarction. Compreh Physiol. 2015;5(4):1841–1875.
11. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Investig. 2013;123(1):92–100. doi:10.1172/JCI62874
12. Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94(2):276–283. doi:10.1093/cvr/cvs018
13. Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9(7):465–479. doi:10.1038/nri2569
14. Veltman D, Wu M, Pokreisz P, et al. Clec4e-receptor signaling in myocardial repair after ischemia-reperfusion injury. J Am Coll Cardiol Basic Trans Science. 2021;6(8):631–646. doi:10.1016/j.jacbts.2021.07.001
15. Sánchez-Hernández CD, Torres-Alarcón LA, González-Cortés A, Peón AN. Ischemia/reperfusion injury: pathophysiology, current clinical management, and potential preventive approaches. Mediators Inflammation. 2020;2020:8405370. doi:10.1155/2020/8405370
16. Hahn JY, Song YB, Kim EK, et al. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128(17):1889–1896. doi:10.1161/CIRCULATIONAHA.113.001690
17. Engstrøm T, Kelbæk H, Helqvist S, et al. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol. 2017;2(5):490–497. doi:10.1001/jamacardio.2017.0022
18. Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373(11):1021–1031. doi:10.1056/NEJMoa1505489
19. Selker HP, Beshansky JR, Sheehan PR, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307(18):1925–1933. doi:10.1001/jama.2012.426
20. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi:10.1038/nature12034
21. Hulsmans M, Clauss S, Xiao L, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;169(3):510–522.e520. doi:10.1016/j.cell.2017.03.050
22. Honold L, Nahrendorf M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res. 2018;122(1):113–127. doi:10.1161/CIRCRESAHA.117.311071
23. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18(12):733–744. doi:10.1038/s41577-018-0065-8
24. Pinto AR, Paolicelli R, Salimova E, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7(5):e36814. doi:10.1371/journal.pone.0036814
25. Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi:10.1016/j.immuni.2013.11.019
26. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi:10.1016/j.immuni.2014.06.013
27. Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res. 2016;118(10):1498–1511. doi:10.1161/CIRCRESAHA.115.308270
28. Bajpai G, Schneider C, Wong N, et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24(8):1234–1245. doi:10.1038/s41591-018-0059-x
29. Bajpai G, Bredemeyer A, Li W, et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124(2):263–278. doi:10.1161/CIRCRESAHA.118.314028
30. Jian Y, Zhou X, Shan W, et al. Crosstalk between macrophages and cardiac cells after myocardial infarction. Cell Commun Signal. 2023;21(1):109. doi:10.1186/s12964-023-01105-4
31. Dutta P, Sager HB, Stengel KR, et al. Myocardial infarction activates CCR2(+) hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;16(5):477–487. doi:10.1016/j.stem.2015.04.008
32. Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014;102(2):240–248. doi:10.1093/cvr/cvu025
33. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15(2):117–129. doi:10.1038/nri3800
34. Majmudar MD, Keliher EJ, Heidt T, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–2046. doi:10.1161/CIRCULATIONAHA.112.000116
35. Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Translat Res. 2018;191:15–28. doi:10.1016/j.trsl.2017.10.001
36. Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol. 2014;63(3):185–195. doi:10.1097/FJC.0000000000000003
37. Jung K, Kim P, Leuschner F, et al. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res. 2013;112(6):891–899. doi:10.1161/CIRCRESAHA.111.300484
38. Kubota A, Frangogiannis NG. Macrophages in myocardial infarction. Am J Physiol Cell Physiol. 2022;323(4):C1304–c1324. doi:10.1152/ajpcell.00230.2022
39. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119(1):91–112. doi:10.1161/CIRCRESAHA.116.303577
40. Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118(3):400–409. doi:10.1161/CIRCRESAHA.115.307778
41. Wan E, Yeap XY, Dehn S, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113(8):1004–1012. doi:10.1161/CIRCRESAHA.113.301198
42. Jia D, Chen S, Bai P, et al. Cardiac resident macrophage-derived legumain improves cardiac repair by promoting clearance and degradation of apoptotic cardiomyocytes after myocardial infarction. Circulation. 2022;145(20):1542–1556. doi:10.1161/CIRCULATIONAHA.121.057549
43. Zagórska A, Través PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol. 2014;15(10):920–928. doi:10.1038/ni.2986
44. Cai B, Thorp EB, Doran AC, et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Investig. 2017;127(2):564–568. doi:10.1172/JCI90520
45. Cai B, Thorp EB, Doran AC, et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci USA. 2016;113(23):6526–6531. doi:10.1073/pnas.1524292113
46. Zhang S, Yeap XY, DeBerge M, et al. Acute CD47 blockade during ischemic myocardial reperfusion enhances phagocytosis-associated cardiac repair. J Am Coll Cardiol Basic Trans Science. 2017;2(4):386–397. doi:10.1016/j.jacbts.2017.03.013
47. Tang X, Liu Z, Li X, Wang J, Li L. Cannabinoid receptors in myocardial injury: a brother born to rival. Int J Mol Sci. 2021;22(13). doi:10.3390/ijms22136886
48. Defer N, Wan J, Souktani R, et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 2009;23(7):2120–2130. doi:10.1096/fj.09-129478
49. van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7(1):30–37. doi:10.1038/nrcardio.2009.199
50. Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10(1):15–26. doi:10.1038/nrcardio.2012.158
51. Kologrivova I, Shtatolkina M, Suslova T, Ryabov V. Cells of the Immune System in Cardiac Remodeling: main Players in Resolution of Inflammation and Repair After Myocardial Infarction. Front Immunol. 2021;12:664457. doi:10.3389/fimmu.2021.664457
52. Ferraro B, Leoni G, Hinkel R, et al. Pro-angiogenic macrophage phenotype to promote myocardial repair. J Am Coll Cardiol. 2019;73(23):2990–3002. doi:10.1016/j.jacc.2019.03.503
53. Kubota A, Suto A, Suzuki K, Kobayashi Y, Nakajima H. Matrix metalloproteinase-12 produced by Ly6C(low) macrophages prolongs the survival after myocardial infarction by preventing neutrophil influx. J Mol Cell Cardiol. 2019;131:41–52. doi:10.1016/j.yjmcc.2019.04.007
54. Ramos G, Hofmann U, Frantz S. Myocardial fibrosis seen through the lenses of T-cell biology. J Mol Cell Cardiol. 2016;92:41–45. doi:10.1016/j.yjmcc.2016.01.018
55. Toor IS, Rückerl D, Mair I, et al. Eosinophil deficiency promotes aberrant repair and adverse remodeling following acute myocardial infarction. J Am Coll Cardiol Basic Trans Science. 2020;5(7):665–681. doi:10.1016/j.jacbts.2020.05.005
56. Xu JY, Xiong YY, Tang RJ, et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovasc Res. 2022;118(9):2165–2178. doi:10.1093/cvr/cvab237
57. Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209(1):123–137. doi:10.1084/jem.20111009
58. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi:10.1016/j.cell.2011.04.005
59. Patel B, Bansal SS, Ismahil MA, et al. CCR2(+) monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. J Am Coll Cardiol Basic Trans Science. 2018;3(2):230–244. doi:10.1016/j.jacbts.2017.12.006
60. Yan X, Anzai A, Katsumata Y, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi:10.1016/j.yjmcc.2013.04.023
61. Zhang F, Dang Y, Li Y, Hao Q, Li R, Qi X. Cardiac contractility modulation attenuate myocardial fibrosis by inhibiting TGF-β1/Smad3 signaling pathway in a rabbit model of chronic heart failure. Cell Physiol Biochem. 2016;39(1):294–302. doi:10.1159/000445624
62. Ismail N, Wang Y, Dakhlallah D, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121(6):984–995. doi:10.1182/blood-2011-08-374793
63. Ge X, Meng Q, Wei L, et al. Myocardial ischemia-reperfusion induced cardiac extracellular vesicles harbour proinflammatory features and aggravate heart injury. J Extracell Vesicles. 2021;10(4):e12072. doi:10.1002/jev2.12072
64. Chen H, Hou Y, Zhai Y, et al. Peli1 deletion in macrophages attenuates myocardial ischemia/reperfusion injury by suppressing M1 polarization. J Leukoc Bio. 2023;113(2):95–108. doi:10.1093/jleuko/qiac012
65. DeBerge M, Glinton K, Subramanian M, et al. Macrophage AXL receptor tyrosine kinase inflames the heart after reperfused myocardial infarction. J Clin Investig. 2021;131(6). doi:10.1172/JCI139576
66. Fan Q, Tao R, Zhang H, et al. Dectin-1 contributes to myocardial ischemia/reperfusion injury by regulating macrophage polarization and neutrophil infiltration. Circulation. 2019;139(5):663–678. doi:10.1161/CIRCULATIONAHA.118.036044
67. Cadenas S, Aragonés J, Landázuri MO. Mitochondrial reprogramming through cardiac oxygen sensors in ischaemic heart disease. Cardiovasc Res. 2010;88(2):219–228. doi:10.1093/cvr/cvq256
68. Wallert M, Ziegler M, Wang X, et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019;26:101292. doi:10.1016/j.redox.2019.101292
69. Yao M, Lu Y, Shi L, et al. A ROS-responsive, self-immolative and self-reporting hydrogen sulfide donor with multiple biological activities for the treatment of myocardial infarction. Bioact Mater. 2022;9:168–182. doi:10.1016/j.bioactmat.2021.07.011
70. Fromen CA, Kelley WJ, Fish MB, et al. Neutrophil-particle interactions in blood circulation drive particle clearance and alter neutrophil responses in acute inflammation. ACS nano. 2017;11(11):10797–10807. doi:10.1021/acsnano.7b03190
71. Zhao T, Wu W, Sui L, et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47–72. doi:10.1016/j.bioactmat.2021.06.006
72. Wang Y, Li L, Zhao W, et al. Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. acs nano. 2018;12(9):8943–8960. doi:10.1021/acsnano.8b02037
73. Kim SY, Jeong JM, Kim SJ, et al. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat Commun. 2017;8(1):2247. doi:10.1038/s41467-017-02325-2
74. Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–922. doi:10.1161/01.RES.0000261924.76669.36
75. Yu L, Yang G, Zhang X, et al. Megakaryocytic leukemia 1 bridges epigenetic activation of NADPH oxidase in macrophages to cardiac ischemia-reperfusion injury. Circulation. 2018;138(24):2820–2836. doi:10.1161/CIRCULATIONAHA.118.035377
76. Li N, Guo X, Li R, Zhou J, Yu F, Yan X. p-Coumaric acid regulates macrophage polarization in myocardial ischemia/reperfusion by promoting the expression of indoleamine 2, 3-dioxygenase. Bioengineered. 2021;12(2):10971–10981. doi:10.1080/21655979.2021.2001924
77. Xu L, Zeng Z, Niu C, et al. Normothermic ex vivo heart perfusion with NLRP3 inflammasome inhibitor Mcc950 treatment improves cardiac function of circulatory death hearts after transplantation. Front Cardiovasc Med. 2023;10:1126391. doi:10.3389/fcvm.2023.1126391
78. Sun L, Lu WX, Li H, Feng DY, Nie JX. Total saponins of Aralia elata (Miq.) Seem. alleviate myocardial ischemia-reperfusion injury by promoting NLRP3-inflammasome inactivation via PI3K/Akt signaling. Kaohsiung J Med Sci. 2023;39(3):290–301. doi:10.1002/kjm2.12627
79. Cheng P, Yang G, Zhao X, et al. Precisely and efficiently enzyme response microspheres with immune removal escape loaded with MCC950 ameliorate cardiac dysfunction in acute myocardial infarction. J Biomed Nanotech. 2020;16(2):153–165. doi:10.1166/jbn.2020.2885
80. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi:10.1152/physrev.00044.2005
81. Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid. Redox Signaling. 2018;29(17):1727–1745. doi:10.1089/ars.2017.7342
82. Rothzerg E, Ingley E, Mullin B, Xue W, Wood D, Xu J. The hippo in the room: targeting the hippo signalling pathway for osteosarcoma therapies. J Cell Physiol. 2021;236(3):1606–1615. doi:10.1002/jcp.29967
83. Xu B, Zhang J, Strom J, Lee S, Chen QM. Myocardial ischemic reperfusion induces de novo Nrf2 protein translation. BBA. 2014;1842(9):1638–1647. doi:10.1016/j.bbadis.2014.06.002
84. Fei Q, Liu J, Qiao L, et al. Mst1 attenuates myocardial ischemia/reperfusion injury following heterotopic heart transplantation in mice through regulating Keap1/Nrf2 axis. Biochem Biophys Res Commun. 2023;644:140–148. doi:10.1016/j.bbrc.2022.12.087
85. Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi:10.1038/nature18629
86. Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192.
87. Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi:10.1038/nature18590
88. Wang Y, Chen Q, Jiao F, et al. Histone deacetylase 2 regulates ULK1 mediated pyroptosis during acute liver failure by the K68 acetylation site. Cell Death Dis. 2021;12(1):55. doi:10.1038/s41419-020-03317-9
89. Shi H, Gao Y, Dong Z, et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R Injury. Circ Res. 2021;129(3):383–396. doi:10.1161/CIRCRESAHA.120.318629
90. Ye B, Chen X, Dai S, et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. 2019;13:975–990. doi:10.2147/DDDT.S195412
91. Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13(2):397–406. doi:10.1074/mcp.M113.035600
92. Rieckmann JC, Geiger R, Hornburg D, et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat Immunol. 2017;18(5):583–593. doi:10.1038/ni.3693
93. Ye X, Zhang P, Zhang Y, et al. GSDMD contributes to myocardial reperfusion injury by regulating pyroptosis. Front Immunol. 2022;13:893914. doi:10.3389/fimmu.2022.893914
94. Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812–1824. doi:10.1161/CIRCRESAHA.117.311082
95. Sciarretta S, Maejima Y, Zablocki D, Sadoshima J. The role of autophagy in the heart. Annu Rev Physiol. 2018;80:1–26. doi:10.1146/annurev-physiol-021317-121427
96. Ma LL, Ma X, Kong FJ, et al. Mammalian target of rapamycin inhibition attenuates myocardial ischaemia-reperfusion injury in hypertrophic heart. J Cell & Mol Med. 2018;22(3):1708–1719. doi:10.1111/jcmm.13451
97. Li Y, Liang P, Jiang B, et al. CARD9 promotes autophagy in cardiomyocytes in myocardial ischemia/reperfusion injury via interacting with Rubicon directly. Bas Res Cardiol. 2020;115(3):29. doi:10.1007/s00395-020-0790-6
98. Zhang J, Hu S, Gao Y, et al. Galangin alleviated myocardial ischemia-reperfusion injury by enhancing autophagic flux and inhibiting inflammation. Eur J Pharmacol. 2023;945:175621. doi:10.1016/j.ejphar.2023.175621
99. Gombozhapova A, Rogovskaya Y, Shurupov V, et al. Macrophage activation and polarization in post-infarction cardiac remodeling. J Biom Sci. 2017;24(1):13. doi:10.1186/s12929-017-0322-3
100. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242–253. doi:10.1016/j.imbio.2018.11.010
101. Lu Y, Li C, Chen Q, et al. Microthrombus-targeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Advan Mater. 2019;31(21):e1808361. doi:10.1002/adma.201808361
102. Abbate A, Salloum FN, Vecile E, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117(20):2670–2683. doi:10.1161/CIRCULATIONAHA.107.740233
103. Woldbaek PR, Tønnessen T, Henriksen UL, et al. Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse; a potential role in cardiac dysfunction. Cardiovasc Res. 2003;59(1):122–131. doi:10.1016/S0008-6363(03)00339-0
104. Kim SJ, Chang HJ, Volin MV, et al. Macrophages are the primary effector cells in IL-7-induced arthritis. Cell Mol Immunol. 2020;17(7):728–740. doi:10.1038/s41423-019-0235-z
105. Zhu J, Zhang W, Zhang L, et al. IL-7 suppresses macrophage autophagy and promotes liver pathology in Schistosoma japonicum-infected mice. J Cell & Mol Med. 2018;22(7):3353–3363. doi:10.1111/jcmm.13610
106. Bao C, Wang B, Yang F, Chen L. Blockade of interleukin-7 receptor shapes macrophage alternative activation and promotes functional recovery after spinal cord injury. Neuroscience. 2018;371:518–527. doi:10.1016/j.neuroscience.2017.10.022
107. Shive CL, Clagett B, McCausland MR, et al. Inflammation perturbs the IL-7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquir Immune Defic Syndr. 2016;71(5):483–492. doi:10.1097/QAI.0000000000000913
108. Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–184. doi:10.1038/ni.1689
109. Wei Y, Xing J, Su X, et al. IL-38 attenuates myocardial ischemia-reperfusion injury by inhibiting macrophage inflammation. Immun Inflamm Dis. 2023;11(6):e898. doi:10.1002/iid3.898
110. Li Z, Ding Y, Peng Y, et al. Effects of IL-38 on macrophages and myocardial ischemic injury. Front Immunol. 2022;13:894002. doi:10.3389/fimmu.2022.894002
111. Liu Y, Gao XM, Fang L, et al. Novel role of platelets in mediating inflammatory responses and ventricular rupture or remodeling following myocardial infarction. Arterioscler Thromb Vasc Biol. 2011;31(4):834–841. doi:10.1161/ATVBAHA.110.220467
112. Ziegler M, Wang X, Peter K. Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target. Cardiovasc Res. 2019;115(7):1178–1188. doi:10.1093/cvr/cvz070
113. An G, Wang H, Tang R, et al. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117(25):3227–3237. doi:10.1161/CIRCULATIONAHA.108.771048
114. Tan H, Song Y, Chen J, et al. Platelet-like fusogenic liposome-mediated targeting delivery of mir-21 improves myocardial remodeling by reprogramming macrophages post myocardial ischemia-reperfusion injury. Advan Sci. 2021;8:15.
115. Zhou Y, Liang Q, Wu X, et al. siRNA delivery against myocardial ischemia reperfusion injury mediated by reversibly camouflaged biomimetic nanocomplexes. Advan Mater. 2023;35(23):e2210691. doi:10.1002/adma.202210691
116. Weng X, Tan H, Huang Z, et al. Targeted delivery and ROS-responsive release of Resolvin D1 by platelet chimeric liposome ameliorates myocardial ischemia-reperfusion injury. J Nanobiotechnol. 2022;20(1):454. doi:10.1186/s12951-022-01652-x
117. Wang Y, Hou M, Duan S, et al. Macrophage-targeting gene silencing orchestrates myocardial microenvironment remodeling toward the anti-inflammatory treatment of ischemia-reperfusion (IR) injury. Bioact Mater. 2022;17:320–333. doi:10.1016/j.bioactmat.2022.01.026
118. Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev. 2016;96(3):1127–1168. doi:10.1152/physrev.00019.2015
119. Zhao J, Li X, Hu J, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115(7):1205–1216. doi:10.1093/cvr/cvz040
120. Xian P, Hei Y, Wang R, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956–5975. doi:10.7150/thno.33872
121. Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10(3):244–258. doi:10.1016/j.stem.2012.02.005
122. Xiao C, Wang K, Xu Y, et al. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ Res. 2018;123(5):564–578. doi:10.1161/CIRCRESAHA.118.312758
123. Xiao J, Pan Y, Li XH, et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7(6):e2277. doi:10.1038/cddis.2016.181
124. Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110(3):319–330. doi:10.1093/cvr/cvw054
125. Song Y, Dou H, Li X, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35(5):1208–1221. doi:10.1002/stem.2564
126. Zhang L, Wei Q, Liu X, et al. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int Immunopharmacol. 2021;101(Pt B):107592. doi:10.1016/j.intimp.2021.107592
127. Gao L, Qiu F, Cao H, et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. 2023;13(2):685–703. doi:10.7150/thno.73568
128. Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38(3):201–211. doi:10.1093/eurheartj/ehw240
129. Liu B, Lee BW, Nakanishi K, et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018;2(5):293–303. doi:10.1038/s41551-018-0229-7
130. Wei Z, Chen Z, Zhao Y, et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials. 2021;275:121000. doi:10.1016/j.biomaterials.2021.121000
131. Li Q, Huang Z, Wang Q, et al. Targeted immunomodulation therapy for cardiac repair by platelet membrane engineering extracellular vesicles via hitching peripheral monocytes. Biomaterials. 2022;284:121529. doi:10.1016/j.biomaterials.2022.121529
132. van der Laan AM, Ter Horst EN, Delewi R, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35(6):376–385. doi:10.1093/eurheartj/eht331
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.