Back to Journals » International Journal of Nanomedicine » Volume 19
Macrophage-Derived Exosomes as Advanced Therapeutics for Inflammation: Current Progress and Future Perspectives
Authors Song Y , Hu J , Ma C, Liu H, Li Z, Yang Y
Received 22 November 2023
Accepted for publication 10 February 2024
Published 19 February 2024 Volume 2024:19 Pages 1597—1627
DOI https://doi.org/10.2147/IJN.S449388
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Yanjuan Song,1 Jing Hu,2 Chunlian Ma,3– 5 Hua Liu,3– 5 Zhanghua Li,6 Yi Yang3– 5
1Graduate School, Wuhan Sports University, Wuhan, Hubei Province, People’s Republic of China; 2Wuhan Children’s Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan, Hubei Province, People’s Republic of China; 3Fitness Monitoring and Chronic Disease Intervention Research Center, Wuhan Sports University, Wuhan, Hubei Province, People’s Republic of China; 4College of Sports Medicine, Wuhan Sports University, Wuhan, Hubei Province, People’s Republic of China; 5Hubei Key Laboratory of Exercise Training and Monitoring, Wuhan Sports University, Wuhan, Hubei Province, People’s Republic of China; 6Department of Orthopedics, Wuhan Third Hospital, Tongren Hospital of Wuhan University, Wuhan, Hubei Province, People’s Republic of China
Correspondence: Yi Yang, Fitness Monitoring and Chronic Disease Intervention research center, Wuhan Sports University, Wuhan, Hubei Province, 430079, People’s Republic of China, Tel +86 139 7129 3990, Email [email protected] Zhanghua Li, Department of Orthopedics, Wuhan Third Hospital, Tongren Hospital of Wuhan University, Wuhan, Hubei Province, 430074, People’s Republic of China, Tel +86 189 7161 0121, Email [email protected]
Abstract: The development of numerous diseases is significantly influenced by inflammation. Macrophage-derived exosomes (M-Exos) play a role in controlling inflammatory reactions in various conditions, including chronic inflammatory pain, hypertension, and diabetes. However, the specific targets and roles of M-Exos in regulating inflammation in diseases remain largely unknown. This review summarizes current knowledge on M-Exos biogenesis and provides updated information on M-Exos’ biological function in inflammation modulation. Furthermore, this review highlights the functionalization and engineering strategies of M-Exos, while providing an overview of cutting-edge approaches to engineering M-Exos and advancements in their application as therapeutics for inflammation modulation. Finally, multiple engineering strategies and mechanisms are presented in this review along with their perspectives and challenges, and the potential contribution that M-Exos may have in diseases through the modulation of inflammation is discussed.
Keywords: inflammation, macrophage-derived exosomes, biological functions, inflammation modulation, engineering strategies
Introduction
Inflammation is a natural biological response dominated by defense in local tissues to stimulation by pathogens, injured cells, chemicals, or physical trauma, the cardinal symptoms of which are typically characterized by redness, swelling, heat, pain, and dysfunction.1 Inflammation can maintain homeostasis by repairing damaged tissues and organ function, increasing the number of leukocytes, and enhancing metabolism.2,3 If inflammation is not promptly addressed, it will lead to a series of chronic inflammatory diseases (eg, diabetes, rheumatoid arthritis, cancer, inflammatory bowel diseases (IBD), cardiovascular disease, and neurologic system disorders).4–6 Conventional drugs approaches to inflammation include steroids, glucocorticoids, nonsteroidal anti-inflammatory drugs or inhibitors targeting specific pro-inflammatory cytokines.4,7,8 However, the aforementioned medicines have limited effect on inflammation regulation and cannot completely prevent disease progression. In addition, long-term administration of these medications can cause gastrointestinal adverse effects (eg nausea, vomiting) and osteoporosis, hyperglycemia.9–11 Therefore, promising treatment approaches with durable efficacy and few side effects are urgently needed.
Exosomes, which are nanosized vesicles derived from endocytosis, contain bioactive molecules such as proteins, DNA, and RNA.12 These vesicles play a crucial role in facilitating material exchange between cells and participate in numerous physiological and pathological processes within the body.13,14 With the deepening of research on exosomes, a high volume of evidence indicated that exosomes are involved in the regulation of inflammation and different types of inflammatory diseases as mediators and potential therapeutic targets.15 This makes exosomes a promising candidate for inflammation.
Macrophages are generally thought to be terminally differentiated immune cells that develop from monocytes, which originate from hematopoietic stem cells in the bone marrow.16 After undergoing differentiation steps, monocytes enter the peripheral blood and become circulating monocytes, including inflammatory and resident monocytes.17 After migrating to tissues, they differentiate into tissue-specific macrophages, including the skeletal system (osteoclasts), central nervous system (microglia), lungs (alveolar macrophages), liver (Kupffer cells), and connective tissue (histiocytes), as well as the spleen, gastrointestinal tract, and peritoneum.18–20 Within tissues, macrophages are multifunctional cell types that can react to factors in the microenvironment (eg, damaged cells, microbial products).21,22 Macrophages can display different phenotypes and functions under the influence of changes in surrounding microenvironment factors, which are a heterogenous population with the function of engulfing microbial infections and dead cells to resist pathogen invasion.23 Depending on their activation status, macrophages can be polarized into different functional phenotypes: classically activated macrophages (M1) and alternatively activated macrophages (M2).24 Moreover, M2 macrophages can be further subdivided into subsets called M2a, M2b, M2c, and M2d. M1 macrophages are induced by pro-inflammatory mediators like lipopolysaccharide (LPS) and interferon-γ (IFN-γ) to secrete different pro-inflammatory cytokines and chemokines, such as tumor necrosis factor alfa (TNF-α), interleukin 6 (IL-6), interleukin 1alfa (IL-1α), interleukin 1beta (IL-1β), C-X-C motif chemokine ligand 9 (CXCL9).25 In contrast, M2 macrophages are activated by anti-inflammatory mediators like interleukin 4 (IL-4) and interleukin 13 (IL-13) to release different anti-inflammatory factors, such as transforming growth factor beta (TGF-β), interleukin 10 (IL-10), CC motif chemokine ligand 1 (CCL1), CC motif chemokine ligand 17 (CCL17), C-C motif chemokine ligand 18 (CCL18), and are involved in anti-inflammatory response, tissue repair and remodeling, immunomodulation.26,27 Among them, M2 macrophages can be further subdivided into four subtypes: M2a, M2b, M2c, and M2d. M2a is induced by IL-4, IL-13, or fungal and parasitic infections; M2b is induced by immune complexes and LPS; M2c is induced by IL-10, TGF-β, glucocorticoids, M2d can be induced by IL-6 and adenosine.28 Exosomes are key mediators of intercellular communication, which carry biological information on macrophages. They are involved in various inflammatory diseases and consistently regulate the inflammatory microenvironment.29,30 Also, evidence suggests that the regulation of inflammation by M-Exos is pivotal.15,31
As a result, this review aims to provide inspiration for future investigations by summarizing the functions of M-Exos, exploring the approaches for engineering inflammation-related disease applications, and discussing their potential as drug delivery platforms for anti-inflammatory purposes.
Biogenesis and Composition of Exosomes
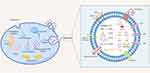
Exosomes are a type of nano-sized extracellular vesicle generated from multivesicular bodies (MVBs), generally range in size from 30 to 150 nm in diameter and are enriched in selected proteins, lipids, nucleic acids, and glycoconjugates.32 Current studies have shown that the invagination of the cytoplasmic membrane forms early endosomes (ESEs), early endosomes (ESEs) are formed by invagination of the cytoplasmic membrane, early endosomes form late endosomes (LSEs) by exchange of material with organelles or interfusion between endosomes and late endosomes. Late endosomes and endosomes produce many ILVs, which then subsequently evolve into multivesicular bodies (MVBs) that are released and eventually form exosomes.33,34 Exosomes can be released by various cell types such as dendritic cells (DCs), mast cells, and macrophages.35 This etiologic heterogeneity led to find exosomes in various kinds of biological fluids, including plasma, serum, saliva, cerebrospinal fluid, amniotic fluid, urine, pleural effusion or ascites, and breast milk.36,37 Exosomes have been confirmed to be vital carriers of unique cargo of protein, lipid, DNA, mRNA, and microRNA (miRNA), which can mediate intercellular communication via these cargo molecules.38 As vectors for intercellular communication, exosomes from donor cells are internalized by micropinocytosis to fuse with the membranes to release its contents of lipids, proteins and RNAs, inducing subsequent physiological changes in recipient cells.39,40 As mentioned above, due to these characteristics that exosomes have, exosomes seem to be capable of acting as vehicles for drug delivery to convey their RNA and protein contents.38,41 In recent years, researchers have discovered that exosomes can also be involved in processes such as the inflammatory response, antigen presentation, pathogen transmission, the immune response, programmed cell death, angiogenesis, and coagulation.42–44 Exosomes have been reported to play various roles in the inflammatory response and pathogenesis of inflammatory diseases, such as in inflammatory bowel diseases, exosomes can induce intestinal barrier repair through TSG-6 overexpression.45 Another study also showed that Schwann cell-derived exosomes containing MFG-E8 can play an anti-inflammatory role by modifying macrophage/microglial polarization, and attenuate inflammation via the SOCS3/STAT3 pathway after spinal cord injury.46
Different models show the involvement of different proteins, which are usually highly abundant in exosomes and play crucial roles in cargo sorting and wrapping of exosomes. These proteins usually involve tetraspanins (CD9, CD63, CD37, CD81 or CD82), as well as heat shock proteins (HSP70), Rab family proteins Tsg101 and Alix, which can be used to confirm the presence of exosomes.47,48 There is also a class of specific proteins, which are only derived from cells and tissues, such as tetraubiquitin, MHC class II, transferrin receptor, etc.49,50 Moreover, the biogenesis of exosome is regulated by multiple factors (eg, cell type, contact inhibition)51 and changes in pathological states (eg, diabetes and neuronal degeneration)52 can affect the yield and content of exosomes, which increases the feasibility of exosome content as a biomarker for disease diagnosis and prognosis. Based on the characteristics and prospects of exosomes as new potential diagnostic and therapeutic tools, current isolation methods for exosomes are also constantly evolving, including ultracentrifugation, affinity-based capture technology, filtration, chromatography, precipitation, and microfluidics.37,53 However, these technologies have some limitations. For example, the ultracentrifugation method is time-consuming and at high cost of special equipment,54 other molecules co-precipitated with exosomes in the precipitation method may be contaminated, causing the purity of exosomes to be questioned.55 Overall, in the future, it is necessary to develop and investigate exosome isolation strategies that are widely recognized or can be used as the gold standard to promote the realization of their applications.
Taken together, exosomes have been implicated in a broad range of biological processes and play essential roles in many facets of human health and disease, including development, immune regulation, inflammation, cancer progression and metastasis, and neuronal communication. Therefore, based on their potential to manipulate drug delivery, the specific targeting and homing nature,56,57 exosomes can be considered as “professional transporters and messengers” to systemic level. Since exosomes protect their cargo from being degraded by enzymes in the circulation, all of the above offer a potential approach for disease diagnosis and assessing therapeutic efficacy of specific drugs. Biogenesis and composition of exosomes are presented in (Figure 1).
The Biogenesis, Biological Functions, and Applications of M-Exos
The formation of M-Exos is mainly derived from three stages: exosome biogenesis; sorting of cargo into exosomes; exosome release.58 Different phenotypes of M-Exos have different biological information, resulting in different executive functions. And their formation is precisely regulated by a process that involves multiple proteins.21 Until now, current research has dug deeper into three types of M-Exos, including unpolarised M0 macrophage-derived exosomes (M0-Exos), polarised M1-Exos and M2-Exos.59 So far, there is still no clear biomarker for distinguishing M1-Exos from M2-Exos. Furthermore, in macrophages, the amphisomes (amphisomes are hybrid organelles produced through the fusion of endosomes with autophagosomes within cells) would not fuse with the lysosome, which causes exosomes to be released in greater quantities.60 The amount of secretion with M-Exos has different effects on the outcome of different diseases, which also reflects the influence of distinct cell types and diverse cellular environments in governing exosome secretion (Figure 2).
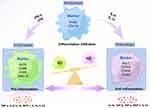 |
Figure 2 Different typologies of macrophages and their cell expression markers. By Figdraw. |
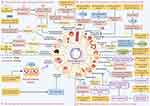
Studies have confirmed that M-Exos play critical functions in the treatment of diseases including cancer, atherosclerosis, diabetes, heart disease, and inflammation.61–65 It has been reported that all types of M2-Exos can reduce the severity of IBD, among which M2b-Exos (M2b macrophage-derived exosomes) have the best effect.66 Recent studies have confirmed that M-Exos can be used as drug delivery tools, gene or protein delivery vehicles. Consequently, they have a significant impact on the diagnosis, prevention, and treatment of future diseases (Figures 3 and 4).21
 |
Figure 3 Related diseases involving exosomes derived from different macrophages. By Figdraw. |
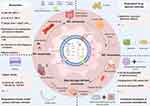 |
Figure 4 The biogenesis, biological functions, and applications of macrophage-derived exosomes. By Figdraw. Notes: This schematic demonstrates that exosomes can serve as biomarkers (eg miR-155,67 miR-17,68 IL-1β,69 TNF-α70), nucleic acid and protein delivery vehicles (eg BDNF,71 PPAR-γ,72 miR-24-3p,73 miR-37074), drug delivery vehicles (eg berberine,75 curcumin,76 melatonin,77 silibinin78) and the combined strategy of exosome biomaterials (eg The M2 Exo/pDNA/BSP co-delivery system,79 engineering of BP-loaded HNV80) play a role in diagnosis and treatment of diseases. |
M-Exos Diagnostic Value and Biomarkers to Monitor Inflammation Progression
Recent research has demonstrated the involvement of M-Exos in the pathological and physiological processes of inflammation and related diseases and have been studied as a potential diagnostic and prognostic markers of inflammation and associated diseases.65 M-Exos cargoes from different pathological conditions (bacterial infection, viral infection and disease states) may show distinct genomic and proteomic profiles for distinguishing and identifying the diagnosis and development status assessment of different inflammatory stages and different inflammatory diseases, so as to serve as a reference for exploring the pathogenesis of inflammation and related diseases. For instance, mycobacterium avium glycopeptidolipids (GPLs) were found in exosomes released by infected macrophages, resulting in GPLs transfer from infected to uninfected macrophages.81 Notably, the exosomes released by Toxoplasma gondii-infected macrophages stimulated proinflammatory responses in Toll-like receptors and MyD88 in uninfected macrophages.70 Proteomic result of M-Exos after infection with live or heat-killed Escherichia coli showed that OmpA, GroL1, DegP, CirA, and FepA are potential biomarkers for exosome-mediated inflammation.82 Another study demonstrated that the Shiga toxins (Stxs)-containing M-Exos can cause cytotoxicity and inflammation, which leads to cell death in toxin-sensitive cells. Compared with purified Stx2a, exosomes released from Stx2-treated human macrophages contained pro-inflammatory cytokine mRNAs and proteins, resulting in more severe inflammation. The above studies imply that human macrophages may act as a significant role in the exacerbation of renal inflammation.69 Interestingly, in a study of atmospheric particulate matter (PM), researchers found that the exosomes derived from PM2.5-treated macrophages could stimulate the release of inflammatory cytokine and alter the inflammatory phenotype of recipients via activating the TLR-4-NF-κB signaling pathway, thereby inducing inflammatory responses in mouse lung.83
The regulation of inflammation by M-Exos has different features in different homeostasis and disease states. In cell experiments simulating diabetic nephropathy, high glucose-treated M-Exos contained a large amount of IL-1β and iNOS, which could regulate NF-κB p65 signaling pathway in vitro, induce the activation of M1 and the increased release of inflammatory cytokines, leading to the kidney damage.84 Another similar study reported that high glucose-stimulated M-Exos accumulated more inflammation factors and accelerated kidney injury by promoting NLRP3 inflammasome to be activated and impairing mesangial cell autophagy in diabetic nephropathy. The above studies have highlighted that the pathological process of inflammation will be further aggravated in high glucose state.85 Furthermore, exosomes can also be used as messengers to monitor the body’s inflammatory response to reflect its development process and pathogenesis so that the body can make corresponding adjustments. There are data indicating that exosomes could be the means by which the placenta responds to these non-contact-dependent messages from activated macrophages, which subsequently signaled to the placental unit to facilitate the response to maternal inflammation and infection. In this way, a protective placental immune response might be mediated during pregnancy through the exosomal pathway.86 Taken together, M-Exos provide a novel mode for maternal-placental messaging.
Noncoding RNA such as miRNA, lncRNA, and circRNA is an important biologically active molecule in exosomes. Additionally, they also contribute significantly to the regulation of the occurrence of inflammatory responses and development of inflammatory diseases. Recent research has illustrated that activated macrophages secreted miR-155-enriched exosomes, which could downregulate suppressor of cytokine signaling 1 expression to induce fibroblast inflammation in the case of cardiac injury.67 Similarly, another study found that proinflammatory M1-Exos released a large number of high expression of pro-inflammatory exosomes miR-155 after myocardial infarction. miR-155 was transported to endothelial cells, resulting in the downregulation of its novel target genes, including Rac family small GTPase 1 (RAC1), p21 (RAC1)-activated kinase 2 (PAK2), Sirtuin 1 (Sirt1), and protein kinase AMP-activated catalytic Subunit alpha 2 (AMPKα2). While M1-Exos inhibited Sirt1/AMPKα2-endothelial nitric oxide synthase and RAC1-PAK2 signaling pathways by targeting the five molecular genes to reduce the angiogenic capacity of endothelial cells and aggravate myocardial injury.61 According to this, miR-155 in M-Exos is associated with the inflammation development of various diseases and is an effective diagnostic biomarker. Interestingly, one study has found that exosomal miRNAs such as miR-155-5p, miR-132-3p, miR-1246, miR-210-3p, and miR-330-5p secreted by inflamed macrophages in peripheral tissue might cross the blood–brain barrier and activate microglia and astrocytes to produce proinflammatory cytokines, thereby inducing neuroinflammation and contributing to the progression of Parkinson’s disease (PD).87 In conclusion, M-Exos exert significant functions in immune surveillance, signal mediation and promotion of disease progression in the pathogenesis and pathological process of inflammation and related diseases. We have tabulated M-Exos-related biomarkers from recent studies as useful references for the detection of inflammation and its associated diseases (Table 1 and Figure 5).
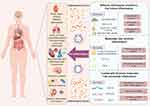 |
Figure 5 Macrophage-derived exosomes-related biomarkers from recent studies as useful references for the detection of inflammation and its associated diseases. By Figdraw. |
 |
Table 1 Biomarkers in M-Exos with Inflammation and Related Diseases |
Advanced Therapeutic Strategies for M-Exos to Improve Inflammation in Diseases
Immunomodulatory and Anti-Inflammatory Effects of M-Exos in Inflammation
The infiltration of inflammatory cells has important functions in various diseases, the modulation of immune function may help delay inflammation progression.93 Conventional approaches adopt inhibiting inflammatory cytokines and driving the conversion of dominant phenotype from M1 to M2 to ameliorate inflammation.94–96 A study has shown that M-Exos could possess anti-inflammatory properties by suppressing the release of pro-inflammatory enzymes and cytokines. Additionally, M-Exos were shown to promote diabetic wound healing by speeding up the process of angiogenesis and re-epithelialization.97 Noteworthy, besides the mentioned inhibition of inflammation methods, the present study found that peripheral M-Exos (PM-Exos) triggered microglial autophagy by suppressing PI3K/AKT/mTOR signaling pathway, promoted the polarization of anti-inflammatory type microglia and stimulated the anti-inflammatory properties. This also suggested that PM-Exos possess immense potential in promoting repair after spinal cord injury.98 Nevertheless, clinical applications of unmodified M-Exos are limited due to insufficient targeting capabilities (Supplementary Table 1).
Engineered M-Exos as Therapeutic Agents for Inflammation
Although M-Exos have shown certain anti-inflammatory effects, previous studies indicated that the therapeutic efficiency of exosomes might be restricted by some intrinsic limitations, including short half-life, low specific organ retention, low targeting efficiency, and low production yield.99 Hence, M-Exos must be functionalized using advanced engineering techniques and carry out the targeted modification of exosomes to address these issues and increase their potency and efficiency as inflammation therapeutics. In the following sections, we give a brief overview of the present modification methods and efficacy of engineered M-Exos in different inflammation and related diseases models (Supplementary Table 2 and Figure 6).
Preconditioned M-Exos for Inflammation Therapy
Preconditioned M-Exos can be utilized for the treatment of inflammation. In general, macrophage cells can be preconditioned with pathological stimuli to generate M-Exos with desirable profiles that are beneficial for relieving inflammation (Supplementary Table 2). A previous study demonstrated that the stimulation of RAW 264.7 cells with LPS increased the levels of inflammatory chemokines and RNAs in exosomes. The NF-κB signaling cascade was activated, causing a shift towards anti-inflammatory gene transcription later in inflammation progression.100 Lipopolysaccharides (LPS)-stimulated M-Exos attenuated paw swelling and thermal hyperalgesia associated with complete Freund’s adjuvant (CFA)-induced inflammatory pain.101 Similarly, Zheng et al found that LPS-stimulated M-Exos prevented cerebral ischemia via shifting microglial functional polarity from M1 toward an anti-inflammatory M2 phenotype, which revealed the anti-inflammatory and neuroprotection effects of LPS-Ex.102 Additionally, a recent study revealed that Talaromyces marneffei infected M-exos would trigger the innate immune responses to control inflammation, which were mainly manifested in promoting the activation of ERK1/2 and autophagy, the release of IL‐10 and TNF‐α in human macrophages.103
In addition to the mentioned M-Exos precondition methods, the anti-inflammatory capacity of M-Exos can also be increased by preconditioning with anti-inflammatory cytokines. For instance, IL-4 polarized human M-Exos (THP1-IL4-exo) promoted macrophages towards the anti-inflammatory phenotype and reprogramed energy metabolism via upregulating miR-21/99a/146b/378a while suppressing miR-33. Besides this, the above intervention also might reprogramme the inflammatory signaling of circulating Ly6Chi monocytes.104 Bouchareychas et al indicated that exosomes secreted from naive bone marrow-derived macrophages (BMDM) contained anti-inflammatory miR-99a/146b/378a, which was upregulated in the case of BMDM polarized with IL-4. These exosomal microRNAs inhibited inflammation via targeting NF-kB and TNF-α signaling and promoted M2 polarization in recipient macrophages. Moreover, this study has suggested that exosomes secreted from IL-4-induced M2-like macrophages could be used to improve inflammatory diseases such as atherosclerosis in mice.105 As a result, rather than adopting sophisticated engineering methods, the application of exosomes derived from these preconditioned macrophages may offer a simple route for the treatment of inflammation.
M-Exos as Delivery Vehicles for the Treatment of Inflammation
Exosomes are highly complex vesicles, there are many physiological and pathological functions involved in the molecular information of each exosome. As their bilayer structure and the capacity of cargo transfer, it is possible for exosomes to serve as natural carriers of therapeutic agents and prevent them from degradation in the body.106–108 Currently, techniques for loading cargoes into exosomes can be divided into endogenous loading including genetic modification of exosome donor cells, and exogenous loading including coincubation, electroporation, sonication of drugs with isolated exosomes.109–113 M-Exos can be loaded with a wide variety of therapeutics, including nucleic acids, proteins, and drugs. Engineered M-Exos with promoted therapeutic properties and organ targeting efficacy can be gained from gene-modified donor cells, such as the overexpression or knockdown of gene or protein. For example, after septic mice were injected miR-24-3p-modified M2-Exos or siRNA of Tnfsf10, the levels of miR-24-3p decreased and the levels of Tnfsf10 increased in the myocardium. In short, M2-Exos-derived miR-24-3p could improve cardiac function and reduce serum inflammation in the myocardial tissue through reducing Tnfsf10 levels.73 Li et al have reported a strategy that M2-Exos could relieve lung injury and inflammation, and delay asthma progression through carrying miR-370 and inhibiting the FGF1/MAPK/STAT1 axis.74 And the latest research proved that lean adipose tissue M-Exos induced M2 polarization in vivo and in vitro, upregulated miR-222-3p level and decreased the expression of Bim to inhibit the levels of inflammatory mediators and accelerate diabetic wound healing. This also implied that the exosomes had a positive regulatory role in macrophage polarization.114 A previous study demonstrated that M2-Exos improved inflammatory cell infiltration and contributed to the recovery of damaged pubococcygeus muscle in stress urinary incontinence models, in which M2-Exos miR-501 might have the potential as a therapeutic drug target for treating diseases caused by muscle injury.115 Similarly, recent studies also have shown that a possible mechanism by which M2-Exos promote osteoprotection may be through the transport of IL-10 mRNA to BMSCs and BMDM, thereby upregulating the expression of the IL-10 cytokine. Subsequently, activation of the IL-10/IL-10R pathway modulated cell differentiation and prevented bone loss in murine periodontitis. The findings provided a novel therapeutic strategy for periodontitis.116 In another study, in the early stage of inflammation, M1-Exos may promote osteogenesis in BMSCs via miR-21a-5p. This study contributes to the understanding of the regulatory role of M1-Exos miRNAs on BMSCs and has tremendous potential in designing effective strategies to improve regenerative outcomes and optimize fracture treatment.117 Moreover, genetic modifications can enhance the targeting capability of M-Exos. A recent study showed that M2-Exos miR-590-3p attenuated DSS-induced mucosal injury and reduced inflammation via regulating LATS1 and the transcription of YAP/β-catenin. This discovery would potentially open up new avenues for treating ulcerative colitis in clinical practice.118 LncRNA is a major component transported by exosomes in many inflammatory diseases. A research result on the pathogenesis of silicosis illustrated that M-Exos lncRNA MSTRG.91634.7 inhibited fibroblast activation by targeting PINK1, and overexpression of PINK1 suppressed silica-induced lung inflammation and fibrosis in mice. This also revealed that the modification of M-Exos targeted genes played a major role in the regulation of inflammation.119 In summary, these studies suggest that genetic engineering is an effective approach for improving the targeting function and therapeutic effects of M-Exos.
Proteins, as important regulatory molecules, act as a pivotal function in the treatment of inflammation. Growing evidence has demonstrated that genetically engineered M-Exos containing therapeutic proteins were used for treating inflammation. For instance, Yuan et al further proved that following intravenous administration, naïve macrophage (Mf) exosomes could across the blood-brain barrier and deliver the cargo protein of brain-derived neurotrophic factor (BDNF) to the inflamed brain. To summarize, these findings have important implications for utilizing Mf-derived exosomes as natural nanocarriers for delivering therapeutic proteins to the brain to treat diseases of the central nervous system.71 Noteworthy, Meng et al demonstrated that exosomal PPARγ derived from macrophages could serve as a mediator of intercellular communication to inhibit LPS‑induced peritonitis by negatively modulating the CD14/TLR4 axis.72
In addition, M-Exos can also be used for delivering drugs to improve the therapeutic index. Wang et al used M1-Exos as Paclitaxel (PTX) carriers to the preparation of a nano-formulation (PTX-M1-Exos), and subsequently used the sonication method to formulate a drug delivery system. They found that M1-Exos could boost the antitumor activity of paclitaxel through triggering macrophage-mediated inflammation.120 Recent studies have demonstrated that M2-Exos were encapsulated with an FDA-approved hexyl 5-aminolevulinate hydrochloride (HAL) by using electroporation technology. After systemic administration, the engineered M2-Exos exerted inflammatory tropism and anti-inflammatory effects through surface-bound chemokine receptors as well as released anti-inflammatory cytokines from anti-inflammatory M2 macrophages. Additionally, the encapsulated HAL could promote the production of anti-inflammatory carbon monoxide and bilirubin to improve the anti-inflammatory ability and relieve atherosclerosis, which would open an expanding new avenue for atherosclerosis therapeutics.121 Researchers designed M2-type primary peritoneal macrophages exosomes as a drug carrier for berberine (Exos-Ber), which could deliver the drug to the injured spinal cord across the BBB. Exos-Ber could suppress iNOS and increase CD206 to diminish inflammation such as TNF-α, IL-1β via inducing the polarization of macrophages/ microglia from M1 phenotype to M2 phenotype. Overall, Exos-Ber is a promising anti-inflammatory strategy for spinal cord injury.75 Similarly, Li et al have reported a strategy that curcumin-loaded M-Exos (Exos-cur) could not only reduce ROS in vitro, regulate mitochondrial membrane potential, and alleviate inflammation, but also suppress the inflammatory response via activating the Nrf2/ARE pathway, promote angiogenesis, and accelerate wound healing process of diabetic rats in vivo.76 Also, engineered M2- exosomes loading with melatonin (Mel@M2-exos) could target inflammatory regions and lead to a macrophage reprogramming from M1 to M2 type, which relieved chronic inflammation and improved periodontitis. This confirms that Mel@M2-exos is a promising treatment for periodontitis.77 Innovatively, Huo et al designed biomimetic silibinin-loaded M-Exos (Exos-Slb), which could highly selectively bind to Aβ1-42 and inhibit Aβ1-42 aggregation to diminish astrocyte activation and inhibit secretion of inflammatory cytokines. This also inspired researchers to use Exos-Slb as an innovative strategy for multifunctional drug delivery systems for AD treatment in the future.78
Taken together, the limitations of exosomes such as low targeting efficiency, and low concentration of functional molecules will be solved through the purposeful design of engineered exosomes, which will make exosomes an ideal functional carrier for delivering genetic materials and drugs to disease lesions (Tables 2, 3 and Figure 6).99
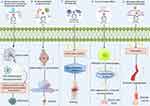 |
Figure 7 Combined strategies of macrophage-derived exosomes-biomaterials for treatment of inflammation. By Figdraw. |
 |
Table 2 M-Exos as Engineered Nucleic Acids/Protein Delivery Vehicles for the Treatment of Inflammation |
 |
Table 3 M-Exos as Drug Delivery Vehicles for the Treatment of Inflammation |
Different Loading Capacities of M-Exos in Nucleic Acids, Proteins and Drugs
Currently, there are many methods for loading nucleic acids, proteins, and drugs into exosomes, including endogenous loading and exogenous loading.122 Among them, miRNA is generally loaded into exosomes by chemical transfection, fusion expression, and electroporation. mRNA is generally loaded into exosomes by cell overexpression, electroporation, fusion expression, and nanoporation, and proteins are mostly loaded into exosomes by electroporation, chemical transfection, ultrasound, fusion expression, and other methods for loading exosomes, and for drugs, exosome loading methods include electroporation, co-incubation, ultrasound and other methods. Various substances have different loading strategies depending on the purpose of delivery.123,124 This review summarizes the loading methods of M-Exos in nucleic acids, proteins, and drugs. The loading of nucleic acids and proteins mainly relies on transfection methods, while the loading methods of drugs are mainly ultrasound, electroporation, and co-incubation. Among them, the loading capacity of M-Exos in nucleic acids is mostly not clearly defined. It only describes the injection volume of exosomes containing relevant nucleic acids, while the loading capacity of M-Exos in proteins and drugs is clearly described. Therefore, the specific loading of miRNA, mRNA, etc. in exosomes requires further exploration of quantifiable objective indicators as a loading reference. Since drugs have pharmacokinetics, drug uptake time experiments, and a large number of basic and clinical studies on various packaging materials, their loading capacity and loading efficiency already have a mature research basis for reference. Overall, in the future, more in-depth and refined experimental quantitative studies should be carried out for evaluation, and efficiency comparisons between different loading methods should be carried out to provide data reference for the specific loading amounts and loading efficiencies of miRNA and mRNA (Tables 4 and 5).
 |
Table 4 M-Exos Loading Capacity for Nucleic Acids/Proteins |
 |
Table 5 M-Exos Loading Capacity for Drugs |
Combined Strategies of M-Exos-Biomaterials for Treatment of Inflammation
In addition to the mentioned engineered M-Exos modification methods, current evidence indicates that functional biomaterials have been applied in innovative strategies of M-Exos to improve inflammation and related diseases. Combination of such combined strategies may be a prospective strategy for advanced anti-inflammatory therapy. For example, the study illustrated that M1-Exos might be served as vaccine adjuvants and enhance cancer vaccine via creating a pro-inflammatory microenvironment in the lymph nodes.125 In another study, the researchers encapsulated a plasmid DNA encoding the anti-inflammatory cytokine interleukin 10 (IL-10 pDNA) and the chemotherapeutic drug betamethasone sodium phosphate (BSP) into the biomimetic vector M2-Exos. The co-delivery system of M2-Exos/pDNA/BSP nanoparticles not only showed better targeting and anti-inflammatory properties, but also alleviated inflammation in joint lesions with the help of the synergistic effect of pDNA and BSP in rheumatoid arthritis (RA). Moreover, this delivery system showed negligible toxicity.79 Similarly, Zhao et al developed novel hybrid exosome-mimic nanovesicles (HNV) with broad-spectrum anti-inflammatory function for RA treatment by fusing M1 macrophage membranes with M2-Exos-mimicking nanovesicles. The experimental results also demonstrated that the HNV loaded with black phosphorus targeted and accumulated at the inflamed knee joints, which exerted a multimodal rheumatoid arthritis treatment by comprehensively inhibiting inflammation combined with NIR irradiation.80 Otherwise, Zeng et al designed a novel nanoagent (MEXI) for spinal cord injury (SCI) therapy through conjugating bioactive IKVAV peptides to the surface of M2-Exos by click chemistry method. The engineered exosomes could target the injured site of the spinal cord, and MEXI promoted motor function recovery in SCI mice via reducing macrophage infiltration, decreasing pro-inflammatory factors, and speeding regeneration of damaged nerve tissue.126 Besides the mentioned exosome encapsulation methods, the M2-Exos-loaded photosensitive hydrogel microneedles (PMN)-based wound dressing system (M2-Exos@PMN) was designed to treat diabetic wounds. The experimental results showed that M2-Exos@PMN suppressed the inflammation and drove angiogenesis to promote diabetic wound healing with the combined action of M2-Exos and the photothermal effects produced by PMN, which was a promising cell-free approach.127
Collectively, these results suggest that biomaterial-based M-Exos engineering is an efficient approach for enhanced anti-inflammatory therapy. The combination of various engineering strategies can further enhance the anti-inflammatory potency of M-Exos-biomaterials therapies, which can improve bioavailability, targeting capability and potentiate therapeutic effect (Table 6 and Figure 7).
 |
Table 6 Combined Strategies of M-Exos-Biomaterials for Treatment of Inflammation |
M-Exos for Inflammation Therapy in Clinical Trials
To date, even though the number of related in vitro and animal studies is on the rise, and growing evidence has demonstrated that M-Exos are an effective anti-inflammatory strategy, few clinical trials have been conducted associated with direct inflammation. The majority of them are still in the early stages, clinical evaluations of M-Exos remain limited, and the current state is still far from clinical applications. At present, there are extremely few previous studies regarding inflammation-related clinical trials. In a clinical study, twenty silicosis patients and twenty-nine health workers were recruited to collect peripheral blood to isolate serum exosomes. According to high-throughput sequencing, researchers found that the expression of M-Exos lncRNA MSTRG.91634.7 was reduced in silicosis patients. Subsequent animal experiments showed that the target protein PINK1 of lncRNA MSTRG.91634.7 was involved in inhibiting silica-induced lung inflammation in mice.119 However, the study did not mention the blinding method of the clinical trial and the rigor of the experimental design, and the relevant clinical design still needs to be further improved. Overall, current evidence indicates that there are still some practical problems that needed to be taken into account and addressed before further translation of M-Exos into clinical applications: (1) The lack of standardized isolation methods, safety and dosage for clinical use. (2) Safe production and quality control criteria of M-Exos. (3) Feasible scheme for mass production of M-Exos. (4) The gold standard for the characterization and quantitation of M-Exos. (5) The Advanced technology need to be further optimized for the co-delivery of drugs and genes in clinics. (6) Implementation of standardized, large-scale, multicenter clinical trials. (7) Monitoring and evaluation of adverse reactions in patients.
Conclusion and Future Perspectives
Inflammation is an alternating process, so it is important to consider the role of M-Exos from a dynamic point of view.21 Recent studies provide evidences that the advantage of specific biomarkers of exosomes endow a method for the identification of specific diseases, and the heterogeneity of exosomes sizes and contents can reflect the state and types of source.128 Hence, it can be seen that exosomes can provide a useful bank of biomarkers for diagnostics of a variety of diseases.129 Accumulating evidence indicates that M-Exos exhibit distinct genomic and proteomic features in inflammatory responses in different pathologic conditions such as bacterial infection, virus infection, and disease states. The nature of miRNAs, lncRNAs and circRNAs in exosomes determines the function of exosomes in inflammation and their role as biomarkers, which is helpful for evaluating and diagnosing the development of inflammation and inflammatory diseases, and exploring their specific pathogenesis.130 In terms of functional therapeutic, M-Exos can be engineered for immunomodulation and generate content that contributes to inflammatory resolution. Moreover, due to the good biocompatibility, accurate targeting, low toxicity and immunogenicity of M-Exos, they can be used as delivery vehicles by virtue of advanced engineering techniques.106,131 M-Exos are capable of selectively delivering proteins, nucleic acids and drugs to the site of inflammation through the interaction between their surface-antibody or modified ligand with cell surface receptors. This will increase the targeting effect and decrease the dose used, which will facilitate the translation of exosomes towards clinical application.132 Although research on exosomes as carriers is in the early stages, the powerful targeted delivery ability of M-Exos has been well documented. All in all, engineered M-Exos are an emerging player in the field of inflammation and have great potential for diagnosis, therapy, and clinical translation.
Nevertheless, M-Exos still faces a number of challenges before it can be widely used in clinical practice. For future studies in this field, several directions may be helpful: (1) There is currently no standardized method for isolating exosomes to get high-purity and high-yield exosomes. And existing research progress shows that the methods for exosome separation, quantification and characterization vary greatly. Consequently, a unified protocol for exosome isolation, purification and storage needs to be further explored.133,134 (2) Exosomes are unstable, tend to aggregate, and have a short half-life. Their long-term effects still need to be studied with the help of the continued evolving advanced technologies. (3) Controversy still exists in the dosage, measurement standards, and administration routes of exosomes. Limited pharmacokinetic research has been conducted on the utilization of M-Exos as vehicles for drug delivery. Thus, follow-up studies still need to clarify the specific distribution, survival time and metabolism of M-Exos in vivo through pharmacokinetic studies and toxicological studies. From the above, determining the optimal dose, time and route of injection about M-Exos is of great significance for enhancing clinical efficacy and minimizing the side effects.135 (4) The difficulty in isolating subtypes of M-Exos, low drug loading efficiency, complicated preparation steps, and the low yield of specific exosome subtypes limits their application as targeted drug delivery systems. Strategies for isolating specific subtypes of M-Exos and methods for analyzing exosomal contents with high sensitivity are needed to identify the origin of specific exosome subtypes, thereby improving exosome production efficiency. (5) Most previous studies about inflammation have focused on the therapeutic effects of M-Exos on miRNAs, but proteins, lncRNAs, circRNAs and some other bioactive molecules may also participate in the function of exosomes. Therefore, more attention should be paid to other bioactive molecules present in M-Exos.136 (6) Current research on the anti-inflammatory effects of M-Exos primarily relies on small animal models, such as mice or rats, with limited studies conducted on large animal models. Given the immense variation in genetics and physiology between humans and small animals, these models are unlikely to fully mimic the pathology of human patients.137 As a result, future research requires standard and large animal models that better mimic human inflammation and related diseases in this field. (7) With regard to clinical application, drug delivery vehicles based on M-Exos can deliver targeted drugs to designated locations with low cost, minimal or no immune response or toxicity. However, in order to achieve the best clinical effect and safety, there are still some technical issues such as route of administration, injection rate, and frequency of transplantation that need to be resolved. Considering the above factors and concerns, regulators and stakeholders should collaborate to develop quality control (QC) standards for M-Exos and safety specifications for evaluating clinical trials and approving exosome-related products.138 This is helpful for exosomes to shift from bench top science to clinical area, and advance the clinical diagnosis and treatment.132,139,140 Besides this, more large-scale, multi-sample, multi-center, and long-term follow-up studies are needed to validate the effectiveness and safety of M-Exos in the treatment of clinical inflammation and related diseases. In the future, it can be foreseen that with the development of biotechnology and the significant improvement of emerging methodologies, M-Exos may achieve large-scale production with good stability. Consequently, this development will pave the way for novel prospects in clinical applications, specifically in the diagnosis and therapy of inflammation and related diseases (Supplementary Figure 1).
Abbreviations
M-Exos, macrophage-derived exosomes; IBD, inflammatory bowel disease; M1, classically activated macrophages; M2, alternatively activated macrophages; MVBs, multivesicular bodies; ESEs, early endosomes; LSEs, late endosomes; DCs, dendritic cells; MSCs, mesenchymal stromal cells; miRNA, microRNA; LPS, lipopolysaccharide; IFN-γ, interferon-γ; IL-4, interleukin 4; IL-13, interleukin 13; M0-Exos, unpolarised M0 macrophage-derived exosomes; M1-Exos, polarised M1 macrophage-derived exosomes; M2-Exos, M2 macrophage-derived exosomes; Stxs, Shiga toxins; PM, particulate matter; RAC1, Rac family small GTPase 1; PAK2, p21-activated kinase 2; Sirt1, Sirtuin 1; AMPKα2, protein kinase AMP-activated catalytic Subunit alpha 2; PD, Parkinson’s disease; COM, calcium oxalate monohydrate; PM-Exos, peripheral macrophage-derived exosomes; HS, hemorrhagic shock; ROS, reactive oxygen species; PMNs, polymorphonuclear neutrophils; BALF, bronchoalveolar lavage fluid; ALI, acute lung injury; CFA, complete Freund’s adjuvant; BMDM, bone marrow-derived macrophages; Mf, naïve macrophage; PTX, Paclitaxel; HAL, hexyl 5-aminolevulinate hydrochloride; Exos-cur, curcumin-loaded M-Exos; Mel@M2-exos, M2-exosomes loading with melatonin; Exos-Slb, silibinin-loaded M-Exos; BDNF, brain-derived neurotrophic factor; LCP, lipid calcium phosphate; BSP, betamethasone sodium phosphate; RA, rheumatoid arthritis; HNV, hybrid exosome-mimic nanovesicles; SCI, spinal cord injury; PMN, photosensitive hydrogel microneedles.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81970261; 82100440), the 14th Five-Year-Plan Advantageous and Characteristic Disciplines (Groups) of Colleges and Universities in Hubei Province for Exercise and Brain Science from Hubei Provincial Department of Education in China, Research and innovation team project of Wuhan Sports University (No. 21KT04). Thanks to Figdraw for giving the drawing support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tian Y, Cheng C, Wei Y, Yang F, Li G. The role of exosomes in inflammatory diseases and tumor-related inflammation. Cells. 2022;11(6):1005. doi:10.3390/cells11061005
2. Gupta S, Sarangi PP. Inflammation driven metabolic regulation and adaptation in macrophages. Clin Immunol. 2023;246:109216. doi:10.1016/j.clim.2022.109216
3. Zhong J, Shi G. Editorial: regulation of inflammation in chronic disease. Front Immunol. 2019;10:737. doi:10.3389/fimmu.2019.00737
4. Panigrahy D, Gilligan MM, Serhan CN, Kashfi K. Resolution of inflammation: an organizing principle in biology and medicine. Pharmacol Ther. 2021;227:107879. doi:10.1016/j.pharmthera.2021.107879
5. Feehan KT, Gilroy DW. Is resolution the end of inflammation? Trends Mol Med. 2019;25(3):198–214. doi:10.1016/j.molmed.2019.01.006
6. Miao L, Liu C, Cheong MS, et al. Exploration of natural flavones’ bioactivity and bioavailability in chronic inflammation induced-type-2 diabetes mellitus. Crit Rev Food Sci Nutr. 2023;63(33):11640–11667. doi:10.1080/10408398.2022.2095349
7. Custodero C, Mankowski RT, Lee SA, et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: a systematic review and meta-analysis. Ageing Res Rev. 2018;46:42–59. doi:10.1016/j.arr.2018.05.004
8. Wang S, Lei B, Zhang E, et al. Targeted therapy for inflammatory diseases with mesenchymal stem cells and their derived exosomes: from basic to clinics. Int j Nanomed. 2022;17:1757–1781. doi:10.2147/ijn.S355366
9. Jordan F, Quinn TJ, McGuinness B, et al. Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst Rev. 2020;4(4):Cd011459. doi:10.1002/14651858.CD011459.pub2
10. Fragoulis GE, Nikiphorou E, Dey M, et al. 2022 EULAR recommendations for screening and prophylaxis of chronic and opportunistic infections in adults with autoimmune inflammatory rheumatic diseases. Ann Rheumatic Dis. 2023;82(6):742–753. doi:10.1136/ard-2022-223335
11. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi:10.1016/j.mce.2010.04.005
12. Chen S, Zhang M, Li J, et al. β-catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J Extracell Vesicles. 2022;11(3):e12203. doi:10.1002/jev2.12203
13. Arya SB, Collie SP, Parent CA. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2023. doi:10.1016/j.tcb.2023.06.006
14. Ozansoy M, Mikati H, Velioglu HA, Yulug B. Exosomes: a missing link between chronic systemic inflammation and Alzheimer’s disease? Biomed Pharmacother. 2023;159:114161. doi:10.1016/j.biopha.2022.114161
15. Chen Z, Larregina AT, Morelli AE. Impact of extracellular vesicles on innate immunity. Curr Opinion Organ Transpl. 2019;24(6):670–678. doi:10.1097/mot.0000000000000701
16. Yahara Y, Barrientos T, Tang YJ, et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat Cell Biol. 2020;22(1):49–59. doi:10.1038/s41556-019-0437-8
17. Bian Z, Gong Y, Huang T, et al. Deciphering human macrophage development at single-cell resolution. Nature. 2020;582(7813):571–576. doi:10.1038/s41586-020-2316-7
18. Li F, Okreglicka KM, Piattini F, Pohlmeier LM, Schneider C, Kopf M. Gene therapy of Csf2ra deficiency in mouse fetal monocyte precursors restores alveolar macrophage development and function. JCI Insight. 2022;7(7):e152271.
19. Lee JW, Lee IH, Iimura T, Kong SW. Two macrophages, osteoclasts and microglia: from development to pleiotropy. Bone Res. 2021;9(1):11. doi:10.1038/s41413-020-00134-w
20. Chauhan A, Sheriff L, Hussain MT, et al. The platelet receptor CLEC-2 blocks neutrophil mediated hepatic recovery in Acetaminophen induced acute liver failure. Nat Commun. 2020;11(1):1939. doi:10.1038/s41467-020-15584-3
21. Shan X, Zhang C, Mai C, et al. The biogenesis, biological functions, and applications of macrophage-derived exosomes. Front Mol Biosci. 2021;8:715461. doi:10.3389/fmolb.2021.715461
22. Li J, Xue H, Li T, et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(-/-) mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510(4):565–572. doi:10.1016/j.bbrc.2019.02.005
23. Baig MS, Roy A, Rajpoot S, et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflammation Res. 2020;69(5):435–451. doi:10.1007/s00011-020-01318-0
24. Dini L, Tacconi S, Carata E, Tata AM, Vergallo C, Panzarini E. Microvesicles and exosomes in metabolic diseases and inflammation. Cytokine Growth Factor Rev. 2020;51:27–39. doi:10.1016/j.cytogfr.2019.12.008
25. Fu SP, Chen SY, Pang QM, et al. Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury. Front Immunol. 2022;13:1014013. doi:10.3389/fimmu.2022.1014013
26. Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 2019;10:792. doi:10.3389/fimmu.2019.00792
27. Kim W, Lee EJ, Bae IH, et al. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J Extracell Vesicles. 2020;9(1):1793514. doi:10.1080/20013078.2020.1793514
28. Sedighzadeh SS, Khoshbin AP, Razi S, Keshavarz-Fathi M, Rezaei N. A narrative review of tumor-associated macrophages in lung cancer: regulation of macrophage polarization and therapeutic implications. Transl Lung Cancer Res. 2021;10(4):1889–1916. doi:10.21037/tlcr-20-1241
29. Wu Y, Li J, Zeng Y, et al. Exosomes rewire the cartilage microenvironment in osteoarthritis: from intercellular communication to therapeutic strategies. Int J Oral Sci. 2022;14(1):40. doi:10.1038/s41368-022-00187-z
30. Ma X, Liu B, Fan L, et al. Native and engineered exosomes for inflammatory disease. Nano Res. 2023;16(5):6991–7006. doi:10.1007/s12274-022-5275-5
31. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
32. Gharavi AT, Hanjani NA, Movahed E, Doroudian M. The role of macrophage subtypes and exosomes in immunomodulation. Cell Mol Biol Lett. 2022;27(1):83. doi:10.1186/s11658-022-00384-y
33. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi:10.1007/s00018-017-2595-9
34. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signaling. 2021;19(1):47. doi:10.1186/s12964-021-00730-1
35. Nail HM, Chiu CC, Leung CH, Ahmed MMM, Wang HD. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J Biomed Sci. 2023;30(1):69. doi:10.1186/s12929-023-00964-w
36. de Rivero Vaccari JP, Brand F, Adamczak S, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochemistry. 2016;136(1):39–48. doi:10.1111/jnc.13036
37. Pang H, Luo S, Xiao Y, et al. Emerging roles of exosomes in T1DM. Front Immunol. 2020;11:593348. doi:10.3389/fimmu.2020.593348
38. Xiong YY, Gong ZT, Tang RJ, Yang YJ. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics. 2021;11(3):1046–1058. doi:10.7150/thno.53326
39. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi:10.7150/thno.52570
40. Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–1762. doi:10.1016/j.cmet.2021.08.006
41. Gao J, Dong X, Wang Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods. 2020;177:114–125. doi:10.1016/j.ymeth.2019.11.012
42. Li M, Rai AJ, DeCastro GJ, et al. An optimized procedure for exosome isolation and analysis using serum samples: application to cancer biomarker discovery. Methods. 2015;87:26–30. doi:10.1016/j.ymeth.2015.03.009
43. Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinform. 2015;13(1):17–24. doi:10.1016/j.gpb.2015.02.001
44. Li KL, Huang HY, Ren H, Yang XL. Role of exosomes in the pathogenesis of inflammation in Parkinson’s disease. Neural regenerat res. 2022;17(9):1898–1906. doi:10.4103/1673-5374.335143
45. Yang S, Liang X, Song J, et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12(1):315. doi:10.1186/s13287-021-02404-8
46. Ren J, Zhu B, Gu G, et al. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023;14(1):70. doi:10.1038/s41419-023-05607-4
47. Zhang B, Yang Y, Xiang L, Zhao Z, Ye R. Adipose-derived exosomes: a novel adipokine in obesity-associated diabetes. J Cell Physiol. 2019;234(10):16692–16702. doi:10.1002/jcp.28354
48. Mathieu M, Névo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389. doi:10.1038/s41467-021-24384-2
49. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi:10.1186/s12943-019-0991-5
50. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428–445.e18. doi:10.1016/j.cell.2019.02.029
51. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4). doi:10.3390/cells8040307
52. Chang W, Wang J. Exosomes and their noncoding RNA Cargo are emerging as new modulators for diabetes mellitus. Cells. 2019;8(8). doi:10.3390/cells8080853
53. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int j Nanomed. 2020;15:6917–6934. doi:10.2147/ijn.S264498
54. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
55. Wu R, Gao W, Yao K, Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol. 2019;10:648. doi:10.3389/fimmu.2019.00648
56. Rehman FU, Liu Y, Zheng M, Shi B. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949. doi:10.1016/j.biomaterials.2022.121949
57. Zhang M, Hu S, Liu L, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transd Target Ther. 2023;8(1):124. doi:10.1038/s41392-023-01382-y
58. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
59. Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–195. doi:10.1111/imm.12910
60. Liu J, Wu F, Zhou H. Macrophage-derived exosomes in cancers: biogenesis, functions and therapeutic applications. Immunol Lett. 2020;227:102–108. doi:10.1016/j.imlet.2020.08.003
61. Liu S, Chen J, Shi J, et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. 2020;115(2):22. doi:10.1007/s00395-020-0781-7
62. Nguyen MA, Karunakaran D, Geoffrion M, et al. Extracellular vesicles secreted by atherogenic macrophages transfer MicroRNA to inhibit cell migration. Arteriosclerosis Thrombosis Vasc Biol. 2018;38(1):49–63. doi:10.1161/atvbaha.117.309795
63. Tian F, Tang P, Sun Z, et al. miR-210 in exosomes derived from macrophages under high glucose promotes mouse diabetic obesity pathogenesis by suppressing NDUFA4 expression. J Diabetes Res. 2020;2020:6894684. doi:10.1155/2020/6894684
64. Wang B, Wang ZM, Ji JL, et al. Macrophage-derived exosomal Mir-155 regulating cardiomyocyte pyroptosis and hypertrophy in uremic cardiomyopathy. JACC. 2020;5(2):148–166. doi:10.1016/j.jacbts.2019.10.011
65. Ye C, Li H, Bao M, Zhuo R, Jiang G, Wang W. Alveolar macrophage - derived exosomes modulate severity and outcome of acute lung injury. Aging. 2020;12(7):6120–6128. doi:10.18632/aging.103010
66. Yang R, Liao Y, Wang L, et al. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi:10.3389/fimmu.2019.02346
67. Wang C, Zhang C, Liu L, et al. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther. 2017;25(1):192–204. doi:10.1016/j.ymthe.2016.09.001
68. Osada-Oka M, Shiota M, Izumi Y, et al. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res. 2017;40(4):353–360. doi:10.1038/hr.2016.163
69. Lee KS, Lee J, Lee P, et al. Exosomes released from Shiga toxin 2a-treated human macrophages modulate inflammatory responses and induce cell death in toxin receptor expressing human cells. Cell Microbiol. 2020;22(11):e13249. doi:10.1111/cmi.13249
70. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110(9):3234–3244. doi:10.1182/blood-2007-03-079152
71. Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi:10.1016/j.biomaterials.2017.07.011
72. Meng M, Lu M, Feng J, et al. Exosomal PPARγ derived from macrophages suppresses LPS-induced peritonitis by negative regulation of CD14/TLR4 axis. Inflammation Res. 2023;72(8):1567–1581. doi:10.1007/s00011-023-01765-5
73. Sun X, Liu Y, Wang J, Zhang M, Wang M. Cardioprotection of M2 macrophages-derived exosomal microRNA-24-3p/Tnfsf10 axis against myocardial injury after sepsis. Mol Immunol. 2022;141:309–317. doi:10.1016/j.molimm.2021.11.003
74. Li C, Deng C, Zhou T, et al. MicroRNA-370 carried by M2 macrophage-derived exosomes alleviates asthma progression through inhibiting the FGF1/MAPK/STAT1 axis. Int J Bio Sci. 2021;17(7):1795–1807. doi:10.7150/ijbs.59715
75. Gao ZS, Zhang CJ, Xia N, et al. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–223. doi:10.1016/j.actbio.2021.03.018
76. Li D, Zhang C, Gao Z, et al. Curcumin-loaded macrophage-derived exosomes effectively improve wound healing. Mol Pharmaceut. 2023;20(9):4453–4467. doi:10.1021/acs.molpharmaceut.3c00062
77. Cui Y, Hong S, Xia Y, et al. Melatonin engineering M2 macrophage-derived exosomes mediate endoplasmic reticulum stress and immune reprogramming for periodontitis therapy. Adv Sci. 2023;10(27):e2302029. doi:10.1002/advs.202302029
78. Huo Q, Shi Y, Qi Y, Huang L, Sui H, Zhao L. Biomimetic silibinin-loaded macrophage-derived exosomes induce dual inhibition of Aβ aggregation and astrocyte activation to alleviate cognitive impairment in a model of Alzheimer’s disease. Mater Sci Eng C Mater Biol Appl. 2021;129:112365. doi:10.1016/j.msec.2021.112365
79. Li H, Feng Y, Zheng X, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30. doi:10.1016/j.jconrel.2021.11.019
80. Zhao C, Song W, Ma J, Wang N. Macrophage-derived hybrid exosome-mimic nanovesicles loaded with black phosphorus for multimodal rheumatoid arthritis therapy. Biomater Sci. 2022;10(23):6731–6739. doi:10.1039/d2bm01274j
81. Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282(35):25779–25789. doi:10.1074/jbc.M702277200
82. Imamiya R, Shinohara A, Yakura D, et al. Escherichia coli-Derived Outer Membrane Vesicles Relay Inflammatory Responses to Macrophage-Derived Exosomes. mBio. 2023;14(1):e0305122. doi:10.1128/mbio.03051-22
83. Cui X, Lai W, Zhao Y, Chen C. The exosome-mediated cascade reactions for the transfer and inflammatory responses of fine atmospheric particulate matter in macrophages. Environ Sci Technol. 2023;57(21):7891–7901. doi:10.1021/acs.est.3c01436
84. Zhu M, Sun X, Qi X, Xia L, Wu Y. Exosomes from high glucose-treated macrophages activate macrophages andinduce inflammatory responses via NF-κB signaling pathway in vitro and in vivo. Int Immunopharmacol. 2020;84:106551. doi:10.1016/j.intimp.2020.106551
85. Liu Y, Li X, Zhao M, et al. Macrophage-derived exosomes promote activation of NLRP3 inflammasome and autophagy deficiency of mesangial cells in diabetic nephropathy. Life Sci. 2023;330:121991. doi:10.1016/j.lfs.2023.121991
86. Holder B, Jones T, Sancho Shimizu V, et al. Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic. 2016;17(2):168–178. doi:10.1111/tra.12352
87. Jin Y, Wu R, Li L, Shen L, Gu Y, Sun C. Exosomes from Inflamed macrophages promote the progression of parkinson’s disease by inducing neuroinflammation. Mol Neurobiol. 2023;60(4):1914–1928. doi:10.1007/s12035-022-03179-6
88. Chen F, Li J, She J, Chen T, Yuan Z. Exosomal microRNA-16-5p from macrophage exacerbates atherosclerosis via modulating mothers against decapentaplegic homolog 7. Microvascular Res. 2022;142:104368. doi:10.1016/j.mvr.2022.104368
89. Singhto N, Kanlaya R, Nilnumkhum A, Thongboonkerd V. Roles of Macrophage Exosomes in Immune Response to Calcium Oxalate Monohydrate Crystals. Front Immunol. 2018;9:316. doi:10.3389/fimmu.2018.00316
90. Singhto N, Thongboonkerd V. Exosomes derived from calcium oxalate-exposed macrophages enhance IL-8 production from renal cells, neutrophil migration and crystal invasion through extracellular matrix. J Proteom. 2018;185:64–76. doi:10.1016/j.jprot.2018.06.015
91. Jiao Y, Li Z, Loughran PA, et al. Frontline science: macrophage-derived exosomes promote neutrophil necroptosis following hemorrhagic shock. J Leukocyte Biol. 2018;103(2):175–183. doi:10.1189/jlb.3HI0517-173R
92. Esser J, Gehrmann U, D’Alexandri FL, et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010;126(5):1032–40, 1040.e1–e4. doi:10.1016/j.jaci.2010.06.039
93. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Ann Rev Physiol. 2010;72:219–246. doi:10.1146/annurev-physiol-021909-135846
94. Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Bio Sci. 2014;10(5):520–529. doi:10.7150/ijbs.8879
95. Bachwich PR, Chensue SW, Larrick JW, Kunkel SL. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986;136(1):94–101. doi:10.1016/0006-291x(86)90881-8
96. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi:10.1016/j.cell.2006.01.007
97. Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Biotechnol. 2019;47(1):3793–3803. doi:10.1080/21691401.2019.1669617
98. Zhang B, Lin F, Dong J, Liu J, Ding Z, Xu J. Peripheral macrophage-derived exosomes promote repair after spinal cord injury by inducing local anti-inflammatory type microglial polarization via increasing autophagy. Int J Bio Sci. 2021;17(5):1339–1352. doi:10.7150/ijbs.54302
99. Xu M, Feng T, Liu B, et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11(18):8926–8944. doi:10.7150/thno.62330
100. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspect Biol. 2009;1(6):a001651. doi:10.1101/cshperspect.a001651
101. McDonald MK, Tian Y, Qureshi RA, et al. Functional significance of macrophage-derived exosomes in inflammation and pain: erratum. Pain. 2022;163(2):e383–e384. doi:10.1097/j.pain.0000000000002533
102. Zheng Y, He R, Wang P, Shi Y, Zhao L, Liang J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7(5):2037–2049. doi:10.1039/c8bm01449c
103. Ji G, Feng S, Ren H, Chen W, Chen R. Exosomes released from macrophages infected with Talaromyces marneffei activate the innate immune responses and decrease the replication. Immun inflam dis. 2023;11(6):e881. doi:10.1002/iid3.881
104. Phu TA, Ng M, Vu NK, Bouchareychas L, Raffai RL. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol Ther. 2022;30(6):2274–2297. doi:10.1016/j.ymthe.2022.03.008
105. Bouchareychas L, Duong P, Covarrubias S, et al. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via MicroRNA Cargo. Cell Rep. 2020;32(2):107881. doi:10.1016/j.celrep.2020.107881
106. Hwang HS, Kim H, Han G, et al. Extracellular vesicles as potential therapeutics for inflammatory diseases. Int J Mol Sci. 2021;22(11):5487.
107. Sil S, Dagur RS, Liao K, et al. Strategies for the use of extracellular vesicles for the delivery of therapeutics. J Neuroimmune Pharmacol. 2020;15(3):422–442. doi:10.1007/s11481-019-09873-y
108. Tang TT, Wang B, Lv LL, Liu BC. Extracellular vesicle-based nanotherapeutics: emerging frontiers in anti-inflammatory therapy. Theranostics. 2020;10(18):8111–8129. doi:10.7150/thno.47865
109. Gámbaro F, Li Calzi M, Fagúndez P, et al. Stable tRNA halves can be sorted into extracellular vesicles and delivered to recipient cells in a concentration-dependent manner. RNA Biol. 2020;17(8):1168–1182. doi:10.1080/15476286.2019.1708548
110. Mehryab F, Rabbani S, Shahhosseini S, et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. doi:10.1016/j.actbio.2020.06.036
111. Xi XM, Xia SJ, Lu R. Drug loading techniques for exosome-based drug delivery systems. Die Pharmazie. 2021;76(2):61–67. doi:10.1691/ph.2021.0128
112. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Delivery Rev. 2016;106(Pt A):148–156. doi:10.1016/j.addr.2016.02.006
113. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
114. Xia W, Liu Y, Jiang X, et al. Lean adipose tissue macrophage derived exosome confers immunoregulation to improve wound healing in diabetes. J Nanobiotechnol. 2023;21(1):128. doi:10.1186/s12951-023-01869-4
115. Zhou M, Li B, Liu C, et al. M2 Macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int Immunopharmacol. 2021;101(Pt B):108223. doi:10.1016/j.intimp.2021.108223
116. Chen X, Wan Z, Yang L, et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnol. 2022;20(1):110. doi:10.1186/s12951-022-01314-y
117. Liu K, Luo X, Lv ZY, et al. Macrophage-derived exosomes promote bone mesenchymal stem cells towards osteoblastic fate through microRNA-21a-5p. Front Bioeng Biotechnol. 2021;9:801432. doi:10.3389/fbioe.2021.801432
118. Deng F, Yan J, Lu J, et al. M2 macrophage-derived exosomal miR-590-3p attenuates DSS-induced mucosal damage and promotes epithelial repair via the LATS1/YAP/ β-Catenin signalling axis. J Crohn’s Colitis. 2021;15(4):665–677. doi:10.1093/ecco-jcc/jjaa214
119. Ban J, Liu F, Zhang Q, Chang S, Zeng X, Chen J. Macrophage-derived exosomal lncRNA MSTRG.91634.7 inhibits fibroblasts activation by targeting PINK1 in silica-induced lung fibrosis. Toxicol Lett. 2023;372:36–44. doi:10.1016/j.toxlet.2022.10.004
120. Wang P, Wang H, Huang Q, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–1727. doi:10.7150/thno.30716
121. Wu G, Zhang J, Zhao Q, et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem. 2020;59(10):4068–4074. doi:10.1002/anie.201913700
122. Piffoux M, Volatron J, Cherukula K, et al. Engineering and loading therapeutic extracellular vesicles for clinical translation: a data reporting frame for comparability. Adv Drug Delivery Rev. 2021;178:113972. doi:10.1016/j.addr.2021.113972
123. Silva AM, Lázaro-Ibáñez E, Gunnarsson A, et al. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J Extracell Vesicles. 2021;10(10):e12130. doi:10.1002/jev2.12130
124. Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Delivery Rev. 2020;159:332–343. doi:10.1016/j.addr.2020.04.004
125. Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther. 2017;25(7):1665–1675. doi:10.1016/j.ymthe.2017.02.007
126. Zeng J, Gu C, Sun Y, Chen X. Engineering of M2 macrophages-derived exosomes via click chemistry for spinal cord injury repair. Adv Healthcare Mater. 2023;12(11):e2203391. doi:10.1002/adhm.202203391
127. Zeng J, Sun Z, Zeng F, Gu C, Chen X. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater Today Bio. 2023;20:100649. doi:10.1016/j.mtbio.2023.100649
128. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi:10.7150/thno.21945
129. Deb A, Gupta S, Mazumder PB. Exosomes: a new horizon in modern medicine. Life Sci. 2021;264:118623. doi:10.1016/j.lfs.2020.118623
130. Ashrafizadeh M, Kumar AP, Aref AR, Zarrabi A, Mostafavi E. Exosomes as promising nanostructures in diabetes mellitus: from insulin sensitivity to ameliorating diabetic complications. Int j Nanomed. 2022;17:1229–1253. doi:10.2147/ijn.S350250
131. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi:10.1186/s13578-019-0282-2
132. Wang C, Xu M, Fan Q, Li C, Zhou X. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian J Pharm Sci. 2023;18(1):100772. doi:10.1016/j.ajps.2022.100772
133. Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi:10.1016/j.ymeth.2015.02.019
134. Lai JJ, Chau ZL, Chen SY, et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv Sci. 2022;9(15):e2103222. doi:10.1002/advs.202103222
135. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
136. Wang C, Li Z, Liu Y, Yuan L. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11(8):3996–4010. doi:10.7150/thno.56035
137. Lv K, Wang Y, Lou P, et al. Extracellular vesicles as advanced therapeutics for the resolution of organ fibrosis: current progress and future perspectives. Front Immunol. 2022;13:1042983. doi:10.3389/fimmu.2022.1042983
138. DiStefano TJ, Vaso K, Danias G, Chionuma HN, Weiser JR, Iatridis JC. Extracellular vesicles as an emerging treatment option for intervertebral disc degeneration: therapeutic potential, translational pathways, and regulatory considerations. Adv Healthcare Mater. 2022;11(5):e2100596. doi:10.1002/adhm.202100596
139. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2. doi:10.3402/jev.v2i0.20360
140. Mateescu B, Kowal EJ, van Balkom BW, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles. 2017;6(1):1286095. doi:10.1080/20013078.2017.1286095
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.