Back to Journals » Journal of Inflammation Research » Volume 16
M1-Type Macrophages Secrete TNF-α to Stimulate Vascular Calcification by Upregulating CA1 and CA2 Expression in VSMCs
Authors Song X, Song Y, Ma Q, Fang K, Chang X
Received 20 March 2023
Accepted for publication 8 July 2023
Published 19 July 2023 Volume 2023:16 Pages 3019—3032
DOI https://doi.org/10.2147/JIR.S413358
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Xianqin Song,1 Yu Song,1 Quanping Ma,2 Kehua Fang,3 Xiaotian Chang1
1Medical Research Center of the Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China; 2Clinical Laboratory, The fourth People’s Hospital of Jinan, Jinan, Shandong, People’s Republic of China; 3Clinical Laboratory of the Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China
Correspondence: Xiaotian Chang, Medical Research Center of the Affiliated Hospital of Qingdao University, Qingdao, Shandong, 266000, People’s Republic of China, Tel/Fax +86 0532-82917331, Email [email protected]
Purpose: Vascular calcification is a hallmark of atherosclerosis (AS). We and others confirmed that carbonic anhydrase I (CA1) and CA2 increased expression and catalyzed calcium deposition in atherosclerotic aortas. Macrophages have been demonstrated to be strongly related to AS. This study aimed to clarify how and which macrophage subtypes regulate CA1 and CA2 expression to stimulate aortic calcification.
Methods and Results: THP-1 cells were induced to form M0, M1 and M2 macrophage subtypes. These cells and their culture supernatants were separately incubated with human vascular smooth muscle cells (VSMCs). Calcification was strongly increased in VSMCs treated with β-GP, a chemical inducer of cellular calcification, following incubation with M1 macrophages or their culture supernatants, and was much higher than that in VSMCs treated with β-GP alone. Meanwhile, the expression of CA1 and CA2, as well as calcification marker genes, including Runx2, BMP-2 and ALP, was increased in VSMCs during this process. TNF-α levels were also increased in the culture medium of M1 macrophages. M0 and M2 macrophages or their supernatants did not significantly stimulate calcification in VSMCs. Following transfection with anti-CA1 or CA2 siRNAs, β-GP-induced VSMCs showed decreased calcification, but the calcification level was partially increased when those VSMCs were incubated with the supernatants of M1 macrophages, while CA1 and CA2 expression as well as TNF-α levels were also elevated. When VSMCs were treated with TNF-α without β-GP induction, calcification and the expression of CA1 and CA2 were also significantly increased.
Conclusion: The results of this study suggest that M1 macrophages can increase CA1 and CA2 expression to promote atherosclerotic calcification in VSMCs by secreting TNF-α.
Keywords: atherosclerosis, calcification, carbonic anhydrase I, carbonic anhydrase II, macrophages, TNF-α
Introduction
Atherosclerosis (AS) is characterized by the presence of lesions called atheromatous plaques. A plaque is focal thickening of the intima that is caused by the proliferation of smooth muscle cells and the deposition of cholesterol, lipids, hydroxyapatite and fibrous connective tissue. The essential step in the process is calcium precipitation, which traps cholesterol as well as other plaque precursor matrices to form atherosclerotic lesions.1,2 There is a high correlation between calcification degree and AS severity.3 Thus, calcification is a clinical marker of AS.4,5 Although various factors have been studied and are thought to contribute to vascular calcification in AS, the exact mechanism by which calcification is induced needs to be further elucidated.
Carbonic anhydrases (CAs) are a group of isoenzymes that catalyze the reversible conversion of carbon dioxide into bicarbonate. Bicarbonic acid is unstable and quickly combines with calcium ions to form calcium carbonate deposits. CAs contribute to calcification in many human tissues, such as bone and soft tissue. This group of isoenzymes is also involved in pathological calcifications such as ankylosing spondylitis, dermatomyositis, bile and kidney stone formation and carcinoma-associated calcification.6–8 We previously reported that CA1, which is a CA family member, stimulated AS by promoting calcium precipitation. In an AS model induced by a high-fat diet in apolipoprotein E (ApoE−/−) mice, CA1 expression was significantly increased in the aortic lesions, but its expression was dramatically decreased in mice that were treated with methazolamide (MZ), a CA inhibitor that can effectively treat glaucoma by reducing aqueous humor secretion, and the mouse AS symptoms were simultaneously relieved.9 Calcification is associated with vascular smooth muscle cells (VSMCs) and macrophages in AS.4 CA1 is highly expressed in human AS aortic tissues and in VSMCs with β-glycerophosphate (β-GP)-induced calcification. Inhibiting CA 1 expression with siRNA or MZ reduced calcification in β-GP-induced VSMCs.9 We also found that AS patients were more likely to suffer from glaucoma because CA1 expression is involved in both AS aortic tissues and glaucoma aqueous humor.10 Others also detected autoantigenicity of CA1 in patients with abdominal aortic aneurysm, which often occurs in AS.11 Additionally, a study detected CA2 expression near calcium deposits in atherosclerotic plaques.12 Thirty-five frequently used cardiac drugs showed potent inhibition of CA1 and CA2, especially drugs that were primarily used for AS treatment.13 These results demonstrated that CA1 and CA2 were involved in the regulation of calcification in AS progression. Our previous study did not detect the involvement of other CA members in AS progression.9 It is not clear how CA1 and CA2 expression in VSMCs is activated when they stimulate calcification in AS.
Macrophages have been considered to play an important regulatory role in the occurrence, development and vascular calcification in cardiovascular events.14 Macrophages can undergo two distinct polarization states: the proinflammatory M1 phenotype and the anti-inflammatory M2 phenotype. Both M1 and M2 macrophages are present in human AS plaques, and M1- and M2-type macrophage responses are predictive of adverse outcomes in human AS.15 Macrophage polarization in perivascular fat is associated with coronary AS.16 However, the molecular mechanisms of macrophages during plaque progression, especially the effect of macrophages on CA1 and CA2 expression in VSMCs during calcification, have not been well elucidated.
This study aimed to clarify which subtype and how macrophages regulate CA1 and CA2 expression in VSMCs to contribute to AS plaque calcification. In the present study, a model of macrophage polarization in THP-1 cells was established by routine methods. Three types of macrophages, including M0, M1 or M2 subtype macrophages, and their culture supernatants were collected and separately cultured with VSMCs to observe the induction of calcification in VSMCs. CA1 and CA2 expression, as well as the expression of calcification-related genes, was examined in VSMCs during the induction of calcification. To verify the key role of CA1 and CA2 in vascular calcification, anti-CA1 and anti-CA2 siRNA were used to treat VSMCs during calcification induction. Additionally, we measured proinflammatory cytokine production in the culture medium and investigated the effect of TNF-α on VSMC calcification. The study showed that M1 macrophages but not M0 or M2 macrophages stimulated CA1 and CA2 expression in VSMCs to promote atherosclerotic calcification by increasing TNF-α production.
Materials and Methods
Culture and Polarization of Macrophages
Human monocytic THP-1 cells were commercially obtained from Procell Life Science&Technology Co., Ltd. (Wuhan, China) and cultured in high-glucose Dulbecco’s modified Eagle medium (DMEM, Procell) with 10% fetal bovine serum (FBS, Procell). THP-1 monocytes were differentiated into macrophages (M0) by 24 h of incubation in serum-free DMEM containing 320 nM phorbol 12-myristate 13-acetate (PMA, Sigma, P8139). M1 macrophages were obtained by incubating M0 macrophages in serum-free DMEM containing 20 ng/mL IFN-γ (MCE, HY-P7025) and 10 ng/mL LPS (Sigma, L2280), and M2 macrophages were obtained by incubating M0 macrophages with serum-free DMEM containing 20 ng/mL interleukin 4 (Il-4, MCE, HY-P7042) and 20 ng/mL interleukin 13 (IL-13, MCE, HY-P7033). The three subtypes of macrophages were washed with PBS and cultured in new serum-free DMEM for 24 h. The induction scheme refers to Rahal’s article.17
Culture and Calcification of Human VSMCs
VSMCs were kindly provided by the Institute for Translational Medicine, Qingdao University, and the cells were cultured in DMEM (Procell) with 10% fetal bovine serum (FBS, Procell). VSMCs in the 3rd passage were plated at a density of 1×105 cells. Upon reaching 80% confluence, the cells were cultured in calcification-inducing medium that contained DMEM, 10% FBS and 10 mM β-GP (Sigma, USA) for 14 days, and the medium was changed every 2 days. The experimental design was based on the study by Bin‐Yao Tian.18
Coculturing VSMCs and Macrophages
THP-1 cells (1x106 in each well) were seeded onto a 6-well plate and induced into M0, M1 or M2 cells as described above. After polarization was complete, the macrophages were carefully washed with PBS. VSMCs were seeded into wells (1x105 in each well) that contained each type of macrophage. The macrophage subtypes and VSMCs were cocultured for 14 days in calcification-inducing medium that contained 10 mM β-GP.
Coculturing VSMCs and Macrophage Subtypes in Transwells
THP-1 cells were seeded onto the upper chambers of a Transwell plate (3422, Corning, NY, USA) and then induced into the different macrophage subtypes (M0, M1 or M2) as described above. On the day before polarization was complete, VSMCs were seeded in the lower wells of another Transwell plate and allowed to attach overnight. After polarization was complete, macrophages in the upper chambers were carefully washed with PBS, and the upper chamber was transferred to the plate in which VSMCs had been seeded in the lower chambers. VSMCs and polarized macrophages were cocultured for 14 days in calcification-inducing medium that contained 10 mM β-GP.
Culturing VSMCs with Macrophage Culture Supernatants
Human VSMCs were placed into 6-well plates. After the cells had adhered, the culture medium was replaced with the supernatants of M0, M1 or M2 macrophages plus calcification-inducing medium containing 10 mM β-GP (1:1 proportion).
Flow Cytometric Analysis of Macrophages
FITC-conjugated anti-human CD11b (Biolegend, USA) and PE-conjugated anti-human CD163 (Biolegend, USA) were used to detect phenotypic changes in macrophages. When the different types of macrophages were successfully induced, the cells were collected from the plate and centrifuged. The cells were resuspended in PBS and transferred to a 1.5 mL centrifuge tube. CD11b or CD163 antibodies were added and incubated with the cells at 4°C for 90 min without light. Then, PBS was used to suspend the cells following centrifugation, and the cells were examined using an ACEA flow cytometer (NovoCyte D2040R, USA).
Flow Cytometric Analysis of Cytokines
The culture supernatants of THP-1 cells, M0, M1, and M2 macrophages and VSMCs were collected by centrifugation. The supernatant was examined using LEGENDplex™ Human Inflammation Panel 1 (13-plex) (Biolegend Inc., USA), which is a multiplex bead-based assay that can detect 13 inflammatory cytokines, including IL-1β, IFN-α, IFN-γ, TNF-α, MCP-1 (CCL2), IL-6, IL-8 (CXCL8), IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33. Flow cytometry was performed using an ACEA (NovoCyte D2040R, USA). The raw flow cytometry data files were analyzed using FlowJo (BD Biosciences, USA) and the LEGENDplex chip platform, and the MCF was calculated according to the standard curve.
Alizarin Red S Staining
When induction of VSMC calcification was complete, the cells were washed with phosphate buffer saline (PBS), fixed with 95% ethanol for 60 min, and stained with 0.5% (w/v) alizarin red S (AR-S, Solabio) (pH = 4.2) for 30 min at room temperature. VSMCs were observed under a microscope to examine the formation of calcified nodules.
Quantification of Calcification Using Cetylpyridinium Chloride
Cultured VSMCs were treated with 10% (W/V) cetylpyridine chloride and incubated at 37°C for 1 h. The optical density (OD) was measured at 562 nm using a spectrophotometer (Molecular device Tecan, Austria).
Western Blotting
Total protein samples were extracted from VSMCs, and the proteins were separated by 10% SDS/PAGE (20 μg for each total protein sample). The proteins were transferred to a PVDF membrane (Millipore, Burlington, MA, USA), which was then blocked with 5% nonfat milk for 2 h. The membrane was incubated overnight at 4°C with primary antibodies against CA1, CA2 (13198-2-AP/16961-1-AP, Proteintech) or GAPDH (AF0502, Elabscience). The membranes were then incubated with HRP–conjugated goat anti-rabbit IgG (SY0102, Elabscience) at room temperature for 1 h after being washed three times with 1× TBST. Immunostaining was performed using ECL substrate (Affinity, USA).
Quantitative Real-Time PCR
Total RNA was extracted from VSMCs using TRIzol reagent (TaKaRa, Dalian, China), followed by reverse transcription of the RNA into cDNA. Fluorescence real-time quantitative PCR was performed with a SYBR Green qPCR kit (Vazyme, Nanjing, China) to comparatively quantify the expression of CA1, CA2, Runx2, BMP2 and ALP. GAPDH expression was used to normalize the expression of the target genes. The primer sequences were designed as follows:
CA1: F: 5’-CTGACAGCTACAGGCTCTTTC-3’;
CA1: R: 5’-CTACGTGAAGCTCGGCAGAAT-3’;
CA2: F: 5’- TGTTGACATCGACACTCATACA-3’;
CA2: R: 5’-GTCATCAAACTCCACGTTGAAA-3’;
BMP2: F: 5’-ACTACCAGAAACGAGTGGGAA-3’;
BMP2: R: 5’-GCATCTGTTCTCGGAAAACCT-3’;
Runx2: F: 5’- CGCCTCACAAACAACCACAG-3’;
Runx2: R: 5’- TCACTGTGCTGAAGAGGCTG-3;
ALP: F: 5’-CCGCTATCCTGGCTCCGT-3’;
ALP: R: 5’- AGATTTCCCAGCGTCCTTGG-3’;
GAPDH: F: 5’-CAGAACATCATCCCTGCCTCTAC-3’;
GAPDH: R: 5’-TTGAAGTCAGAGGAGACCACCTG-3’.
SiRNA Transfection
VSMCs (1 × 105) were seeded in a six-well plate and cultured until they reached 80% confluence. VSMCs were transfected with siRNA using GenePharma SiRNA-MateTM (GenePharma, China.) according to the manufacturer’s instructions. The siRNA sequences used were as follows:
Anti-CA1: 5’-GCCACAGCCAAAGAAAUUATTUAAUUUCUUUGGCUGUGGCTT-3’; and
Anti-CA2: 5’-GGCAAAUCAAAGCUUCCUUTTAAGGAAGCUUUGAUUUGCCTT-3’.
Statistical Analysis
SPSS 19.0 (SPSS Inc., Chicago, USA) software was used for statistical analyses. The means ± SDss were calculated for the measurement data. ANOVA were performed to compare the data among different groups, and p < 0.05 was considered statistically significant.
Results
M1 Macrophages Stimulated Calcification in VSMCs
To determine whether macrophages can induce calcification, THP-1 cells were differentiated into M0, M1 and M2 macrophages. Macrophage polarization was confirmed by measuring the expression of surface markers using flow cytometry, and the subtypes were then induced to undergo calcification with β-GP. As shown in Supplementary Figure 1a, the expression of CD11b was markedly upregulated in M1 macrophages, while the expression of CD163 was upregulated in M2 macrophages, indicating successful polarization of macrophages from THP-1 cells. The morphology of THP-1, M0, M1 and M2 cells is shown in Supplementary Figure 1b. In the culture medium, the levels of IL-1β, IL-6, IL-8, TNF-α, IFN-a2, MCP-1, IFN-γ, IL-18, IL-23 and IL-33 were significantly increased when THP-1 cells were induced into M0, M1 or M2 macrophages. The levels of Il-1β and Il-6 were the highest in the M0 subtype, and these levels were decreased in the culture medium of the M1 and M2 subtypes compared with that of the M0 subtype. The level of TNF-α was the highest in the culture medium of the M1 subtype compared with that in the culture medium of M0 and M2 macrophages (Supplementary Figure 1c). M0, M1 and M2 macrophages were then cultured in DMEM supplemented with 10% FBS and 10 mM β-GP for 14 days to induce cellular calcification. Alizarin red staining did not detect obvious calcified nodules in any of the macrophage subtypes, regardless of the presence of β-GP (Supplementary Figure 1d). These results indicated that the three types of macrophages were not calcified regardless of β-GP induction and that M1 macrophages produced high levels of TNF-α.
To confirm calcification of VSMCs and the best experimental conditions for calcification induction, human VSMCs were cultured in calcification-induced medium (DMEM with 10% FBS and 10 mM β-GP). The culture medium was changed every 2 days, and calcification was examined at 3, 5, 7, 10, 12 and 14 days following calcification induction. Calcified nodules were observed in VSMCs beginning on the 5th day following calcification induction, and the highest number of nodules was observed on the 14th day (Supplementary Figure 2a). Calcification quantification by cetylpyridinium chloride assay also detected increased absorbance of calcification at the 5th day following induction, and the calcification level remained high until the 14th day (Supplementary Figure 2b). The above observation indicated that calcification can occur in VSMCs following calcification induction, and calcification peaked on the 14th day following induction, but the level of calcification was generally low.
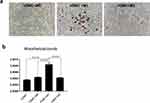
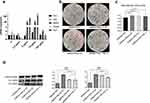
To determine whether macrophages can induce calcification in VSMCs, M0, M1 and M2 macrophages that were induced from THP-1 cells were seeded onto 6-well plates (1x106 cells in each well) and then cocultured with VSMCs (1x105 cells in each well). After 14 days of culture in calcification-inducing medium containing 10 mM β-GP, many calcification nodules were observed in the cells cocultured with M1 macrophages, and few calcification nodules were observed in the cells cocultured with M0 or M2 macrophages (Figure 1a). The cetylpyridinium chloride assay showed an increased ratio of absorbance to cell count in VSMC and M1 macrophage cocultures compared with VSMCs that were cocultured with M0 or M2 macrophages, as well as VSMCs that were cultured alone (Figure 1b). The results indicated that M1 macrophages but not M0 or M2 macrophages could significantly stimulate calcification in VSMCs, although macrophages alone cannot undergo calcification.
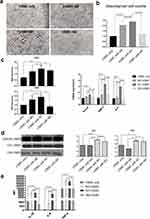
To investigate whether macrophage subtypes affected VSMC calcification through indirect contact, VSMCs were cocultured with the 3 macrophage subtypes in transwells, and the expression of CA1 and CA2 in VSMCs, as well as the levels of proinflammatory cytokines in the culture medium, were measured. Polarized macrophages and VSMCs were separately cultured in the upper chamber and lower chamber of the Transwell without direct contact for 14 days in calcification-inducing medium that contained 10 mM β-GP. Alizarin red staining showed much more calcification in VSMCs that were cocultured with M1 macrophages than in VSMCs that were cocultured with M2 or M0 cells and VSMCs that were not cocultured (Figure 2a). The cetylpyridine chloride assay showed higher calcification levels in the coculture medium of VSMCs and M1 macrophages than in the coculture medium of M0 and M2 macrophages (Figure 2b). Real-time PCR showed an increase in CA1 and CA2 mRNA expression in VSMCs that were cocultured with M1 macrophages compared with VSMCs that were not cocultured or with those that were cultured with M0 or M2 macrophages. Real-time PCR also showed increased levels of Runx2, BMP2 and ALP mRNA in cocultured VSMCs, indicating increased calcification in VSMCs that were cocultured with M1 macrophages (Figure 2c). Western blot analysis showed that CA1 and CA2 protein expression was significantly increased in VSMCs that were cocultured with M1 macrophages compared with that in VSMCs that were not cocultured or with those that were cocultured with M0 or M2 macrophages (Figure 2d). Additionally, the culture medium of VSMCs that were cocultured with M0 and M1 macrophages, especially M1 macrophages, showed considerably increased levels of IL-1β, IL-6 and TNF-α compared with the medium of VSMCs that were not cocultured or those that were cocultured with M2 macrophages (Figure 2e). These results indicated that calcification was induced and significantly increased in VSMCs that were indirectly cocultured with M1 macrophages, and CA1 and CA2, as well as IL-1β, Il-6 and TNF-α, levels were increased in VSMCs that were cultured with M1 macrophages.
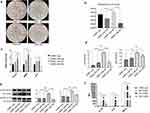
To further investigate how M1 macrophages affect calcification in VSMCs, VSMCs were separately cultured with the supernatants of the different macrophage subtypes. THP-1 cells were induced to differentiate into M0, M1 or M2 macrophages. The culture supernatants were collected and cultured with VSMCs for 14 days in the presence of calcification-inducing medium that contained 10 mM β-GP (1:1 ratio of macrophage culture medium supernatant: calcification-inducing medium). Alizarin red staining showed a significant increase in calcification in VSMCs cultured with the supernatant of M1 macrophages compared with those that were cultured with M0 or M2 macrophage supernatants, which was higher than that in VSMCs that were not cocultured. Very few calcified nodules were observed in VSMCs that were cultured with M0 or M2 macrophage culture supernatants (Figure 3a). The cetylpyridinium chloride assay showed similar results (Figure 3b). Real-time PCR showed a significant increase in the mRNA levels of BMthe P2, ALP and Runx2, three calcification-related genes, in VSMCs that were cultured with supernatant from M1 macrophages compared to those in VSMCs cultured with supernatants from M0 or M2 macrophages or that were not cocultured (Figure 3c), indicating that the culture supernatant of M1 macrophages strongly induced calcification in VSMCs. Real-time PCR also showed increased expression of CA1 and CA2 in VSMCs that were cultured with M1 macrophage supernatant compared with VSMCs that were cocultured with M0 or M2 supernatant (Figure 3d). Western blot analysis showed increased protein expression of CA1 and CA2 in VSMCs that were cultured with M1 macrophage supernatant compared with VSMCs that were cultured with M0 or M2 macrophage supernatants or that were not cocultured (Figure 3e). Additionally, the medium of VSMCs that were cultured with M1 macrophage supernatant showed significantly higher levels of IL-1β, IL-6 and TNF-α than the medium of VSMCs cultured with the supernatants of M0 or M2 macrophages (Figure 3f). TNF-α in the coculture medium of VSMCs and M1 macrophage supernatants had the highest levels among all the groups. These results indicated that the supernatants of M1 but not M0 or M2 macrophages could significantly stimulate calcification in VSMCs and increase the expression levels of CA 1 and CA2 in cells. The supernatants of M1 macrophages also increased the levels of IL-1β, IL-6, and especially TNF-α in the culture medium.
M1 Macrophages Stimulated Calcification in VSMCs by Regulating CA1 and CA2 Expression
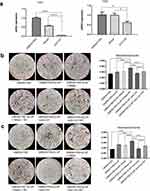
To determine the essential roles of CA1 and CA2 in VSMC calcification, VSMCs were transfected with anti-CA1 siRNA or anti-CA2 siRNA, and Allstar siRNA was used as a control. The cells were cultured in M1 supernatant and calcification-inducing medium containing β-GP (1:1 proportion). Other controls were prepared, including VSMCs cultured in normal culture medium plus 10% FBS and VSMCs cultured in DMEM plus 10% FBS and β-GP. Real-time PCR showed decreased CA1 and CA2 mRNA expression in VSMCs following siRNA transfection, indicating successful siRNA transfection (Figure 4a). After 14 days of culture, AR-S staining showed a few calcification nodules in VSMCs and Allstar siRNA-transfected VSMCs that were cultured in calcification-inducing medium, and quantification of calcification with a cetylpyridine chloride assay verified this observation, indicating successful calcification induction in VSMCs. The staining showed the most calcified nodules in VSMCs that were cultured with M1 supernatant plus calcification-inducing medium containing β-GP, although these VSMCs were transfected with Allstar siRNA. When VSMCs were transfected with anti-CA1 siRNA, the calcified nodules were significantly decreased even though the cells were cultured in calcification-inducing medium. When the anti-CA1 siRNA-transfected VSMCs were cultured in M1 supernatant plus calcification-inducing medium containing β-GP, the number of calcified nodules was increased, but the level was not as high as that in untransfected VSMCs (Figure 4b). VSMCs that were transfected with anti-CA2 siRNA showed similar results (Figure 4c). This observation indicated that CA1 and CA2 expression played essential roles in VSMC calcification, and M1 supernatant can alleviate the suppressive effect of anti-CA1 and anti-CA2 siRNA on VSMC calcification.
Real-time PCR and Western blotting showed the highest level of CA1 mRNA in VSMCs that were transfected with Allstar siRNA and incubated in M1 macrophage supernatant plus β-GP, and the level was lowest in VSMCs that were transfected with anti-CA1 siRNA and cultured in calcification-inducing medium. Moreover, M1 macrophage supernatant partially alleviated the suppressive effect of anti-CA1 siRNA on CA1 expression in VSMCs (Figure 5a and b). Real-time PCR and Western blotting showed similar CA2 expression levels in VSMCs transfected with anti-CA2 siRNA (Figure 5c and d). IL-1β, IL-6 and TNF-α levels were significantly increased in the medium of VSMCs that were cultured in M1 supernatant plus calcification-inducing medium containing β-GP compared with the controls, and these levels were even higher than those in VSMCs cultured in calcification-inducing medium. The levels of IL-1β, IL-6 and TNF-α were decreased in the culture medium of VSMCs transfected with anti-CA1 siRNA, and M1 supernatant partially increased the levels of IL-1β, IL-6 and TNF-α in the culture medium (Figure 5e). The levels of IL-1β, IL-6 and TNF-α were decreased in the culture medium of VSMCs that were transfected with anti-CA2 siRNA. The levels of IL-1β, IL-6 and TNF-α were completely restored to their original levels in the culture of VSMCs that were transfected with Allstar siRNA and cultured in M1 supernatant (Figure 5f). These results indicated that anti-CA1 and anti-CA2 siRNA suppressed CA1 and CA2 expression in VSMCs and reduced IL-6, IL-1β and TNF-α levels in the culture medium. IL-6, IL-1β and TNF-α levels in the culture medium of VSMCs incubated in M1 supernatant and transfected with anti-CA1 or anti-CA2 siRNA were lower than those in the culture medium of VSMCs incubated with an equal volume of M1 supernatant, indicating that VSMCs also produced IL-1β, IL-6 and TNF-α under the control of CA1 and CA2 expression.
TNF-α Increased CA1 and CA2 Expression and Induced Calcification in VSMCs
To determine the stimulatory effect of TNF-α on calcification of VSMCs, VSMCs were treated with TNF-α at different concentrations (0, 2.5, 5, 10, and 100 ng/mL) for 24 h without β-GP induction. Real-time PCR demonstrated that TNF-α treatment increased the mRNA expression of CA1 and CA2, as well as the calcification-associated genes BMP2, ALP and Runx2, in VSMCs, and these levels were the highest when VSMCs were treated with 10 ng/mL TNF-α (Figure 6a). VSMCs were then cultured in DMEM containing FBS and TNF-α (10 ng/mL) for 14 days without β-GP induction. VSMCs cultured in M1 macrophage supernatant or DMEM plus FBS supplemented with β-GP were used as controls. VSMCs treated with TNF-α, M1 supernatant or β-GP showed calcification morphology by AR-S staining, although the levels in VSMCs treated with TNF-α were not as high as those in VSMCs induced with β-GP (Figure 6b). Quantification of calcification using the cetylpyridinium chloride assay showed high calcification levels in VSMCs cultured with calcification-inducing medium, M1 macrophage supernatant or TNF-α (Figure 6c). Western blot analysis showed that TNF-α treatment increased CA1 and CA2 expression in VSMCs compared with VSMCs in normal culture without β-GP induction, although the level was not as high as that in the cells induced with β-GP or M1 macrophage supernatant (Figure 6d). These results indicated that TNF-α and the supernatant of M1 macrophages could induce calcification in VSMCs, as well as CA1 and CA2 expression.
Discussion
We and others found that CA1 and CA2 expression was upregulated in atherosclerotic aortas and stimulated aortic calcification.9,12,19 In this study, we investigated how CA1 and CA2 were regulated by macrophages to promote calcification in atherosclerotic aortas. Three subtypes of macrophages or their supernatants were separately cultured with VSMCs. The results indicated that M1 macrophages but not M0 and M2 macrophages stimulated VSMC calcification and further found that M1 macrophages increased the production of certain proinflammatory cytokines, especially TNF-α, to stimulate calcification in VSMCs by upregulating their CA1 and CA2 expression.
In addition, M0, M1 and M2 macrophages were induced from THP-1 cells. These subtypes were directly cocultured with VSMCs in transwells. The culture supernatants of the 3 macrophage subtypes were also separately added to VSMC culture in calcification-inducing medium. M1 macrophages and their supernatants but not M0 and M2 macrophages or their supernatants enhanced VSMC calcification, and the calcification level was much higher than that in VSMCs that were treated with calcification-inducing medium alone. These results suggest that the secretions of M1 macrophages can promote VSMC calcification in the tissue microenvironment. A pathological accumulation of proinflammatory M1-like macrophages has been observed in AS coronary arteries.19 M1 macrophages are a prominent phenotype in unstable plaques, and plaques contain macrophages that mainly express M1 markers.20 The distribution of M1 and M2 macrophages in different types of atherosclerotic lesions indicates that macrophages exhibit a high degree of phenotypic plasticity in response to various conditions in the microenvironment.21 Reportedly, M1 macrophage deposition in VSMCs induces VSMCs to differentiate into osteoblasts.15 M1 macrophages promote microcalcification and ossification in VSMCs through vesicle-mediated mineralization as a result of macrophage and VSMC apoptosis.15 Our study further confirmed the stimulatory effect of M1 macrophages on calcification in VSMCs in the tissue microenvironment, which corresponded to the findings of others.
We further explored the mechanism by which M1 macrophages enhanced calcification. When VSMCs were cultured with the supernatants of M1 macrophages in the presence of β-GP, CA1 and CA2 expression in VSMCs was increased and was much higher than that in VSMCs induced by β-GP alone. The calcification levels were significantly decreased following transfection of anti-CA1 and CA2 siRNA in VSMCs, while the calcification level was partially restored when the transfected VSMCs were incubated with the supernatants of M1 macrophages, and the CA1 and CA2 expression levels were also partially recovered. This observation indicated that M1 macrophages stimulated VSMC calcification by increasing CA1 and CA2 expression in VSMCs, and CA1 and CA2 expression in VSMCs played an essential role in atherosclerotic aortic calcification. Furthermore, the calcification level and expression of calcification-related genes were significantly increased, and CA1 and CA2 expression was also increased when VSMCs were treated with TNF-α. When M1 macrophages were induced from THP-1 cells, TNF-α levels were also increased. These results indicated that M1 macrophages stimulated VSMC calcification through TNF-α secretion. When VSMCs transfected with anti-CA1 and anti-CA2 siRNA were cultured with the supernatants of M1 macrophages, TNF-α levels were increased in the culture medium. Because VSMCs cultured with M1 supernatants showed lower IL-6, IL-1β and TNF-α levels following siRNA transfection than those cultured with an equal volume of M1 supernatant without siRNA transfection, the results showed that VSMCs also produced IL-1β, IL-6 and TNF-α under the control of CA1 and CA2 expression. We previously used acetazolamide (AZ), a CA inhibitor with a chemical structure similar to that of MTZ, to treat VSMCs. AZ markedly suppressed calcification and reduced CA1, IL-6, IFN-γ, GM-CSF, and TNF-α expression in cultured VSMCs. Anti-CA1 siRNA significantly suppressed calcification, cell proliferation, and migration, promoted apoptosis of VSMCs, and reduced IL-6, IFN-γ, GM-CSF, and TNF-α secretion in cultured VSMCs.9 These results suggest that M1 macrophages secrete proinflammatory cytokines, especially TNF-α, to stimulate CA1 and CA2 expression in VSMCs, which promotes calcification and TNF-α production in VSMCs. An inflammatory cytokine signature exists in AS. Increased levels of TNF-α and IL-6 were reportedly associated with myocardial infarction,22 and ApoE−/− mice fed a high-fat diet exhibited AS symptoms in the aorta and high serum levels of TNF-α and IFN-γ.23 Gonzalez et al found that TNF-α promotes and exacerbates calcification in heart valve myofibroblasts.24 Our study further confirmed that M1 macrophages stimulate VSMC calcification by secreting TNF-α to elevate CA1 and CA2 expression.
Many studies have confirmed that AS is a degenerative inflammatory disease of the vascular wall, and monocytes and macrophages play the most important role by accumulating redundant LDL particles in their oxidized form and producing proinflammatory cytokines.25 M1 macrophages are associated with an increased risk of coronary thrombosis and correlate with histological components of plaque progression and destabilization. M2 macrophages are correlated with plaque size, calcification, necrotic content and vasa vasorum in the adventitia layer.16 Importantly, M1 markers and Th1-associated cytokines are highly expressed in symptomatic plaques, whereas the expression of mannose receptor (MR) and CD163, M2 macrophage markers, and Th2 cytokines is inversely related to disease progression.15 TNF-α antagonists may have beneficial effects on preventing the progression of subclinical AS and arterial stiffness.26 Increased levels of TNF-α and the presence of polymorphisms in the TNF-α gene have been implicated in cardiovascular disease pathogenesis.22,27 These studies support that M1 macrophages promote calcification by increasing TNF-α secretion.
This study has limitations because most data in this study were derived from an in vitro cellular system. The constitution of AS aorta tissues is very complicated, and an in vitro culture system is a good model for the determination of relations and regulations between cells in the tissue.
Conclusion
Our results showed that M1 macrophages had increased TNF-α secretion to stimulate atherosclerotic calcification by increasing CA1 and CA2 expression in VSMCs. This finding may be helpful for understanding the regulatory mechanism of aortic calcification, which is a key step in AS progression.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gamble W. Atherosclerosis: the carbonic anhydrase, carbon dioxide, calcium concerted theory. J Theor Biol. 2006;239(1):16–21. doi:10.1016/j.jtbi.2005.07.008
2. Chinetti-Gbaguidi G, Daoudi M, Rosa M, et al. Human alternative macrophages populate calcified areas of atherosclerotic lesions and display impaired RANKL-induced osteoclastic bone resorption activity. Circ Res. 2017;121(1):19–30. doi:10.1161/CIRCRESAHA.116.310262
3. Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–336. doi:10.1161/01.ATV.0000110786.02097.0c
4. Villa-Bellosta R. New insights into endogenous mechanisms of protection against arterial calcification. Atherosclerosis. 2020;306:68–74. doi:10.1016/j.atherosclerosis.2020.03.007
5. Waring OJ, Skenteris NT, Biessen EAL, Donners M. Two-faced Janus: the dual role of macrophages in atherosclerotic calcification. Cardiovasc Res. 2022;118(13):2768–2777. doi:10.1093/cvr/cvab301
6. Adeva-Andany MM, Fernández-Fernández C, Sánchez-Bello R, Donapetry-García C, Martínez-Rodríguez J. The role of carbonic anhydrase in the pathogenesis of vascular calcification in humans. Atherosclerosis. 2015;241(1):183–191. doi:10.1016/j.atherosclerosis.2015.05.012
7. Zamanova S, Shabana AM, Mondal UK, Ilies MA. Carbonic anhydrases as disease markers. Expert Opin Ther Pat. 2019;29(7):509–533. doi:10.1080/13543776.2019.1629419
8. Rahman S, Bibi S, Javed T, et al. Review: therapeutic potential of carbonic anhydrase inhibitors. Pak J Pharm Sci. 2019;32(2):709–720.
9. Yuan L, Wang M, Liu T, et al. Carbonic anhydrase 1-mediated calcification is associated with atherosclerosis, and methazolamide alleviates its pathogenesis. Front Pharmacol. 2019;10:766. doi:10.3389/fphar.2019.00766
10. Song X, Li P, Li Y, et al. Strong association of glaucoma with atherosclerosis. Sci Rep. 2021;11(1):8792. doi:10.1038/s41598-021-88322-4
11. Ando T, Iizuka N, Sato T, et al. Autoantigenicity of carbonic anhydrase 1 in patients with abdominal aortic aneurysm, revealed by proteomic surveillance. Hum Immunol. 2013;74(7):852–857. doi:10.1016/j.humimm.2013.02.009
12. Oksala N, Levula M, Pelto-Huikko M, et al. Carbonic anhydrases II and XII are up-regulated in osteoclast-like cells in advanced human atherosclerotic plaques-Tampere Vascular Study. Ann Med. 2010;42(5):360–370. doi:10.3109/07853890.2010.486408
13. Argan O, Çıkrıkçı K, Baltacı A, Gencer N. The effects of cardiac drugs on human erythrocyte carbonic anhydrase I and II isozymes. J Enzyme Inhib Med Chem. 2020;35(1):1359–1362. doi:10.1080/14756366.2020.1781844
14. Li Y, Sun Z, Zhang L, et al. Role of macrophages in the progression and regression of vascular calcification. Front Pharmacol. 2020;11:661. doi:10.3389/fphar.2020.00661
15. de Gaetano M, Crean D, Barry M, Belton O. M1- and M2-type macrophage responses are predictive of adverse outcomes in human atherosclerosis. Front Immunol. 2016;7:275. doi:10.3389/fimmu.2016.00275
16. Farias-Itao DS, Pasqualucci CA, de Andrade RA, et al. Macrophage polarization in the perivascular fat was associated with coronary atherosclerosis. J Am Heart Assoc. 2022;11(6):e023274. doi:10.1161/JAHA.121.023274
17. Rahal OM, Wolfe AR, Mandal PK, et al. Blocking Interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100(4):1034–1043. doi:10.1016/j.ijrobp.2017.11.043
18. Tian BY, Yao L, Sheng ZT, et al. Specific knockdown of WNT8b expression protects against phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt-β-catenin signaling pathway. J Cell Physiol. 2019;234(4):3469–3477. doi:10.1002/jcp.26827
19. Eshghjoo S, Kim DM, Jayaraman A, Sun Y, Alaniz RC. Macrophage Polarization in Atherosclerosis. Genes. 2022;13(5):756. doi:10.3390/genes13050756
20. Momtazi-Borojeni AA, Abdollahi E, Nikfar B, Chaichian S, Ekhlasi-Hundrieser M. Curcumin as a potential modulator of M1 and M2 macrophages: new insights in atherosclerosis therapy. Heart Fail Rev. 2019;24(3):399–409. doi:10.1007/s10741-018-09764-z
21. Nikiforov NG, Elizova NV, Bukrinsky M, et al. Use of primary macrophages for searching novel immunocorrectors. Curr Pharm Des. 2017;23(6):915–920. doi:10.2174/1381612823666170125110128
22. DePalma RG, Hayes VW, Cafferata HT, et al. Cytokine signatures in atherosclerotic claudicants. J Surg Res. 2003;111(2):215–221. doi:10.1016/S0022-4804(03)00075-1
23. Cao H, Jia Q, Shen D, et al. Bushen Jiangzhi formula reduces atherosclerosis in apoE-/- mice through autophagy. J Tradit Chin Med. 2020;40(4):593–601. doi:10.19852/j.cnki.jtcm.2020.04.008
24. Gonzalez Rodriguez A, Schroeder ME, Grim JC, et al. Tumor necrosis factor-α promotes and exacerbates calcification in heart valve myofibroblast populations. FASEB J. 2021;35(3):e21382. doi:10.1096/fj.202002013RR
25. Králová A, Králová Lesná I, Poledne R. Immunological aspects of atherosclerosis. Physiol Res. 2014;63(Suppl 3):S335–S342. doi:10.33549/physiolres.932858
26. Tam LS, Kitas GD, González-Gay MA. Can suppression of inflammation by anti-TNF prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology. 2014;53(6):1108–1119. doi:10.1093/rheumatology/ket454
27. Bennet AM, van Maarle MC, Hallqvist J, et al. Association of TNF-alpha serum levels and TNFA promoter polymorphisms with risk of myocardial infarction. Atherosclerosis. 2006;187(2):408–414. doi:10.1016/j.atherosclerosis.2005.09.022
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.