Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Lower ATG7 Levels are Associated with a Higher Risk of Gestational Diabetes Mellitus: A Cross-Sectional Study
Authors Lu L, Ma Y, Deng J, Xie J, Huang C
Received 2 June 2022
Accepted for publication 30 July 2022
Published 4 August 2022 Volume 2022:15 Pages 2335—2343
DOI https://doi.org/10.2147/DMSO.S377041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Ling Lu,1 Yan Ma,2 Jie Deng,1 Jiaqiong Xie,1 Chaolin Huang1
1Department of Gynaecology, The First Affiliated Hospital of Chengdu Medical College, Chengdu, People’s Republic of China; 2Department of Obstetrics, The First Affiliated Hospital of Chengdu Medical College, Chengdu, People’s Republic of China
Correspondence: Chaolin Huang; Jiaqiong Xie, Department of Gynaecology, The First Affiliated Hospital of Chengdu Medical College, Chengdu, People’s Republic of China, Email [email protected]; [email protected]
Objective: This study aimed to investigate ATG7 levels in pregnant women with and without gestational diabetes mellitus (GDM) and explore the potential associations between ATG7 levels and GDM.
Methods: This was a cross-sectional study conducted in a large tertiary hospital in Chengdu, China. The ATG7 levels in pregnant women at between 24 and 28 weeks of gestation with (n=84) and without GDM (n=649) were measured by using an ELISA kit. Glucose, HbA1c, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were measured by an automatic biochemistry analyser. The homeostasis model assessment of insulin resistance (HOMA-IR) and insulin secretion (HOMA-β) were calculated according to published formulas. The associations of ATG7 levels with laboratory parameters, GDM, and insulin resistance were evaluated using correlation analysis or a regression model.
Results: The ATG7 levels were significantly lower in pregnant women with GDM than in those without GDM. The correlation analyses found that ATG7 levels correlated positively with HOMA-β but correlated negatively with HOMA-IR, oral glucose tolerance test (OGTT) glucose levels, TGs, and LDL-C. There were no significant correlations between ATG7 levels and HbA1c, HDL-C, or TC. After adjusting for potential confounders, lower ATG7 levels were shown to be associated with a higher risk of GDM. Furthermore, multiple linear regression analyses showed that ATG7 levels were negatively associated with insulin resistance.
Conclusion: ATG7 levels are significantly lower in pregnant women with GDM than in those without GDM, and lower ATG7 levels are associated with a higher risk of GDM. ATG7 levels were negatively associated with insulin resistance. Autophagy deficiency, which is caused by lower ATG7 levels, may be the underlying mechanism that mediates insulin resistance in the development of GDM.
Keywords: ATG7, autophagy, cross-sectional study, gestational diabetes mellitus, insulin resistance
Introduction
Gestational diabetes mellitus (GDM) is one of the most common complications among pregnant women, affecting 6–18% of all pregnancies.1,2 GDM is associated with a higher risk of adverse pregnancy outcomes, such as premature birth, stillbirth, polyhydramnios, macrosomia, intrauterine growth retardation, and neonatal respiratory distress.3–6 It has also been reported that women with a history of GDM have a significantly higher risk of developing type 2 diabetes mellitus and cardiovascular diseases later in life.7,8 In recent years, GDM has attracted much attention from the scientific community because of its growing incidence and adverse effects on mothers and offspring. Although great progress has been made in understanding GDM, the exact pathogenesis of GDM is not yet fully understood.
ATG7 is a core autophagy-related protein that induces autophagy and removes damaged organelles or degraded macromolecules. Previous studies have reported that ATG7 regulates immunity, cell death, protein secretion, and adipogenesis.9–11 Impaired ATG7 activity is associated with human pathologies, including neurodegeneration, neurodevelopmental disorders, cancer, and infection.10,12,13 In addition, several studies reported that ATG7 was associated with β-cell mass homeostasis and pancreatic secretory function.14–16 The studies found that mice with β-cell-specific deletion of ATG7 showed a reduction in β-cell mass as well as impaired pancreatic insulin secretory function. As a result, β-cell-specific ATG7-null mice showed significant hyperglycaemia, glucose intolerance, and hypoinsulinaemia.14–16 Moreover, another study reported that ATG7-null mice showed increased insulin resistance, lipid metabolism disorder, and inflammatory changes, which are characteristic of GDM.17 Therefore, the above findings indicate that there may be an association between ATG7 and the risk of GDM. However, the relationship between serum ATG7 levels and GDM has not yet been reported.
In the current study, we evaluated ATG7 levels in pregnant women with GDM and those without GDM. We further explored the potential association between ATG7 levels and the risk of GDM. Since lipid metabolism disorder is a feature of GDM, we also investigated the relationship between ATG7 levels and lipid profiles in women with GDM. Our results could provide useful information for the diagnosis and treatment of GDM.
Materials and Methods
Study Design and Data Collection
This study was conducted at The First Affiliated Hospital of Chengdu Medical College, Department of Obstetrics and Gynaecology. The First Affiliated Hospital of Chengdu Medical College is a large tertiary hospital in Chengdu, China, with 1800 inpatient beds, and more than 800,000 patients visit the hospital every year. The study complied with the Declaration of Helsinki and good clinical practice and was approved by the Ethics Committee of The First Affiliated Hospital of Chengdu Medical College (No. CYFY17032022). Informed consent was obtained from all participants.
From September 2021 to February 2022, pregnant women who visited the outpatient clinic at The First Affiliated Hospital of Chengdu Medical College for their prenatal examinations and met the following inclusion criteria were recruited: (1) plan to have antenatal care and delivery at the study hospital; (2) age between 18 and 40 years old; (3) gestational age between 24 and 28 weeks; and (4) agreement to participate in the study. The exclusion criteria were as follows: pregestational diabetes mellitus; hypertensive disorders; multiple pregnancies; disorders known to affect glucose metabolism, including polycystic ovary syndrome (PCOS) and chronic renal and liver diseases; and a history of foetal malformation, chronic inflammatory, or infectious diseases. Ultimately, a total of 732 eligible pregnant women were recruited for this study, including 83 patients with GDM and 649 patients without GDM. The sample size was calculated using the formula described in previous studies: n= Z2×P×(1-P)/e2, n= required sample size; Z= 1.96 at 95% confidence interval (CI); P= prevalence of GDM (14.8%); e= margin of error (5%).18–20 The total sample size was calculated to be 194. However, 732 eligible participants were recruited for this study. The flowchart of the study design is shown in Figure 1.
 |
Figure 1 Flow chart of the study design. |
The trained medical staff administered standard questionnaires to collect patient information, including maternal characteristics, demographic information, prepregnancy weight, pregnancy history, smoking status, alcohol intake, past medical history, and family history of diabetes.
Sample Collection and Laboratory Data Measurement
Maternal venous blood was collected with a 3 mL automatic blood collection tube in the morning (8:00–10:00 am) at a gestational age between 24 and 28 weeks when OGTT screening was carried out after an overnight fast of at least 8 h. The serum samples were obtained by centrifugation at 2000 rpm for 10 min and were then stored at −80 °C until the day of analysis. Serum ATG7 levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Abcam, Shanghai, China). The levels of fasting glucose, glycated haemoglobin (HbA1c), total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured by an automatic biochemistry analyser (Olympus AU640, Japan). The homeostasis models assessment of insulin resistance (HOMA-IR) and insulin secretion (HOMA-β) were calculated according to published formulas.21
Diagnosis of GDM
GDM was diagnosed according to the current guidelines of the American Diabetes Association (ADA).22 Briefly, the diagnosis of GDM is made when a 75-g OGTT screening is completed at a gestational age between 24 and 28 weeks and any of the following plasma glucose values are met or exceeded: fasting: 5.1 mmol/L; 1 h: 10.0 mmol/L; and 2 h: 8.5 mmol/L.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD) for normally distributed continuous variables or frequency for categorical variables. The independent samples t-test was used to compare the differences between two groups for normally distributed continuous variables. The chi-square test was performed for the categorical variables. Correlations between maternal plasma ATG7 levels and lipid profiles and glycaemic indices were assessed using Pearson correlation analysis. To assess the relationship between maternal plasma ATG7 levels and GDM, we calculated the adjusted odds ratio (OR) and 95% confidence interval (CI) with a multivariable logistic regression model after controlling for potential confounding factors. To evaluate the relationship between ATG7 levels and insulin resistance, multiple linear regression analyses were performed. All analyses were performed using the Statistical Package for the Social Sciences software version 20.0 (SPSS Inc. Chicago, IL, USA). A two-sided p value <0.05 was considered statistically significant.
Results
Clinical and Laboratory Characteristics of the GDM Group and Non-GDM Group
The clinical and laboratory characteristics were compared between the GDM group and the non-GDM group. As shown in Table 1, there were no significant differences in maternal age, gestational age, or parity between the GDM group and the non-GDM group. However, the prepregnancy BMI was significantly higher in the GDM group than in the non-GDM group. The glucose parameters, including OGTT glucose levels, HbA1c, and HOMA-IR, were significantly higher in the GDM group than in the non-GDM group, while HOMA-β was significantly lower in the GDM group than in the non-GDM group. Regarding the lipid profiles, the levels of TC, TGs, and LDL-C were significantly higher, whereas the HDL-C level was significantly lower in the GDM group than in the non-GDM group. The results also showed that ATG7 levels were significantly lower in the GDM group than in the non-GDM group.
 |
Table 1 Clinical and Laboratory Characteristics Between the GDM Group and Non-GDM Group |
Correlations of ATG7 Levels with Lipid Profiles and Glycaemic Indices in the GDM Group
As mentioned above, the ATG7 levels, lipid profiles, and glycaemic indices were significantly different in the GDM group compared with those in the non-GDM group. We further investigated the correlations of ATG7 levels with lipid profiles and glycaemic indices in the GDM group. Pearson correlation analyses demonstrated that ATG7 levels correlated positively with HOMA-β but correlated negatively with HOMA-IR, OGTT glucose levels, TGs, and LDL-C. However, no significant correlations were observed between ATG7 levels and HbA1c, HDL-C, or TC (Table 2).
 |
Table 2 Correlations of ATG7 Levels with Lipid Profiles and Glycemic Indices in the GDM Group |
Associations Between ATG7 Levels and Risk of GDM Assessed by Logistic Regression Models
ATG7 levels were divided into tertiles according to the distribution cut-off points, and the GDM prevalences in the three tertiles were 15.38% (T1), 13.32% (T2), and 5.32% (T3) (Figure 2). In the unadjusted logistic regression model, lower ATG7 levels were significantly associated with a higher risk of GDM, and the ORs (95% CIs) for GDM across the three ATG7 tertiles were 5.63 (3.25–9.05), 4.73 (2.27–8.74), and 1.00 (reference), respectively (Table 3, crude model, p = 0.001). After adjusting for potential confounders, including gravidity, parity, gestational age, family history of diabetes, prepregnancy BMI, and the levels of TC, TGs, HbA1c, HDL-C, and LDL-C, the adjusted ORs (95% CIs) for GDM across the three ATG7 tertiles were 5.43 (3.04–8.61), 4.42 (2.21–8.25), and 1.00 (reference), respectively (Table 3, Model 1, p = 0.003). This finding suggested that the association between lower ATG7 levels and a higher risk of GDM remained significant when these confounders were controlled.
 |
Table 3 Associations Between ATG7 Levels and Risk of GDM Assessed by Logistic Regression Models |
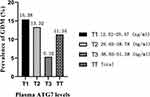 |
Figure 2 Prevalence of GDM at each tertile of plasma ATG7 levels. |
To assess whether the association was mediated by insulin secretion measured by HOMA-β, we added HOMA-β to the adjusted logistic regression model. The results showed that the adjusted ORs (95% CIs) for GDM across the three ATG7 tertiles were 5.13 (2.93–8.32), 4.21 (2.03–7.26), and 1.00 (reference), respectively (Table 3, Model 2, p = 0.013). This finding suggested that the association between lower ATG7 levels and a higher risk of GDM was independent of insulin secretion. However, the association was significantly attenuated after further adjustment for insulin resistance measured by HOMA-IR, and the ORs (95% CIs) for GDM across the three ATG7 tertiles were 2.12 (1.02–6.14), 1.62 (0.56–6.37), and 1.00 (reference), respectively (Table 3, Model 3, p = 0.027).
Association Between ATG7 Levels and Insulin Resistance
Since insulin resistance is a feature of GDM, we investigated the association between ATG7 levels and insulin resistance to understand the potential pathophysiologic role of ATG7 in the development of GDM. Multiple linear regression analyses showed that ATG7 levels were negatively associated with insulin resistance measured by HOMA-IR (Table 4, crude model, β = −0.53±0.13, p<0.001). The association remained significant even after adjusting the model for confounding variables (Table 4, Model 1, β= −0.45±0.07, p = 0.003; Model 2, β= −0.41±0.04, p = 0.021). This finding suggested that the association between ATG7 levels and insulin resistance was independent of these confounding variables.
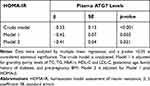 |
Table 4 Association Between ATG7 Levels and Insulin Resistance |
Discussion
In this study, we demonstrated that ATG7 levels were significantly lower in pregnant women with GDM than in those without GDM, and lower ATG7 levels were associated with a higher risk of GDM. Moreover, ATG7 levels correlated positively with HOMA-β but correlated negatively with HOMA-IR, OGTT glucose levels, TGs, and LDL-C. Our results could provide useful information for the diagnosis and treatment of GDM.
Lipid metabolism disorder is a feature of GDM.23,24 Previous studies reported that patients with GDM had lower lipoprotein lipase activity than patients without GDM. This decreased lipoprotein lipase activity reduced TG degradation and increased TG levels and LDL-C levels in the blood.25,26 However, there was higher fatty acid β-oxidation activity in patients with GDM than in patients without GDM. This increased fatty acid β-oxidation produced a large amount of acetyl-CoA, thus leading to a significant increase in TC synthesis and a decrease in HDL-C synthesis.25,26 These findings were consistent with what we have found regarding the changes in lipid profiles in patients with GDM (Table 1). We further investigated the relationship between ATG7 levels and TG levels, LDL-C levels, TC levels, and HDL-C levels in patients with GDM. The results showed that ATG7 levels correlated negatively with TG levels and LDL-C levels. However, there were no significant correlations between ATG7 levels and HDL-C levels or TC levels (Table 2). These findings suggest that there may be an association between ATG7 levels and the risk of GDM.
GDM is characterized by the inability of pancreatic β-cells to respond adequately to the increased insulin resistance of pregnancy, resulting in varying degrees of hyperglycaemia.1,27,28 Previous studies have demonstrated that insulin resistance, chronic inflammation, obesity, genetic factors, oxidative stress, and defective insulin secretion contribute to the development of GDM.1,29 However, the exact mechanisms underlying the pathogenesis of GDM are still largely unknown. In our study, we found that ATG7 levels were significantly lower in pregnant women with GDM than in those without GDM. This finding indicates that ATG7 deficiency might be involved in the pathogenesis of GDM. However, the aetiology of the deficiency of ATG7 in pregnant women with GDM remains to be elucidated.
We further investigated the association between ATG7 levels and the risk of GDM by using logistic regression analysis. Our results showed that lower ATG7 levels were associated with a higher risk of GDM even after adjustment for potential confounders. However, the association was significantly attenuated after further model adjustment for insulin resistance measured by HOMA-IR. We also found that ATG7 levels were negatively associated with insulin resistance. In addition, previous studies found that insulin resistance is a major contributor to the development of GDM.30–32 These findings suggest that ATG7 may mediate insulin resistance in the pathogenesis of GDM.
ATG7 is a core autophagy-related protein that enhances the autophagy process to regulate lysosomal degradation and recycling processes in eukaryotic cells and is responsible for maintaining cellular function and homeostasis between cell survival and cell death.10,33 Enhanced autophagy acts as a protective mechanism against oxidative stress in cells. However, deficiency of autophagy has been implicated in the development of insulin resistance as a result of the induction of oxidative stress.34,35 Our study demonstrated that lower ATG7 levels were associated with a higher risk of GDM. Therefore, the deficiency of autophagy which is caused by the lower ATG7 levels may be the underlying mechanism that mediates insulin resistance in the development of GDM. This idea was corroborated by another study which showed that blocking autophagy pathways with siRNA-mediated knockdown of ATG7 increased insulin resistance by attenuating the abundance of insulin receptor substrate-1, Akt phosphorylation, GLUT4 translocation, and glucose uptake in granulosa cells in polycystic ovary syndrome.36 Further studies should focus on the aetiology of the decrease in ATG7 levels in pregnant women with GDM.
Our study has many strengths. First, this is the first study to investigate the relationship between ATG7 levels and GDM, and it could expand our knowledge regarding the development of GDM. Second, all laboratory and clinical measurements were carried out according to standardized procedures with high reliability. Third, we collected blood samples at between 24 and 28 weeks of gestation when the universal OGTT screenings were performed, which did not add a mental or economic burden to the participants. There are also several limitations that should be acknowledged. First, the study was conducted according to a cross-sectional design, with blood samples collected at only one time point during gestation; therefore, the data could not reveal the changes in ATG7 levels throughout pregnancy. Second, our study had a relatively small sample size and included only pregnant Chinese women. Thus, our findings may not be generalizable to other populations. Third, although the logistic regression analysis was adjusted for a variety of potential confounding factors, we cannot rule out residual confounding factors such as stress, dietary habits, and physical activity.
Conclusions
The present study demonstrates that ATG7 levels are significantly lower in pregnant women with GDM than in those without GDM, and lower ATG7 levels are associated with a higher risk of GDM. ATG7 levels were negatively associated with insulin resistance. These findings indicate that autophagy deficiency, which is caused by lower ATG7 levels, may be the underlying mechanism that mediates insulin resistance in the development of GDM.
Ethics Approval
The study was approved by the Ethics Committee of The First Affiliated Hospital of Chengdu Medical College (No. CYFY17032022). Informed consent was obtained from all participants.
Acknowledgments
This work was supported by grants from The First Affiliated Hospital of Chengdu Medical College (No. CYFY-GQ24, CYZYB20-17), Sichuan Medical Committee (No. Q20075), and Chengdu Science and Technology Bureau (No. 2021-YF05-00269-SN).
Disclosure
The authors declare that they have no competing interests.
References
1. Johns EC, Denison FC, Norman JE, et al. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–754. doi:10.1016/j.tem.2018.09.004
2. Waters TP, Kim SY, Sharma AJ, et al. Longitudinal changes in glucose metabolism in women with gestational diabetes, from late pregnancy to the postpartum period. Diabetologia. 2020;63(2):385–394. doi:10.1007/s00125-019-05051-0
3. Benhalima K, Van Crombrugge P, Moyson C, et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia. 2019;62(11):2118–2128. doi:10.1007/s00125-019-4961-7
4. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199–203. doi:10.3109/14767050903550659
5. Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(Suppl 2):14–20. doi:10.1159/000371628
6. Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636–644. doi:10.1007/s00125-017-4206-6
7. Sun J, Kim GR, Lee SJ, et al. Gestational diabetes mellitus and the role of intercurrent type 2 diabetes on long-term risk of cardiovascular events. Sci Rep. 2021;11(1):21140. doi:10.1038/s41598-021-99993-4
8. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–914. doi:10.1007/s00125-019-4840-2
9. Zhang P, Ling L, Zheng Z, et al. ATG7-dependent and independent autophagy determine the type of treatment in lung cancer. Pharmacol Res. 2021;163:105324. doi:10.1016/j.phrs.2020.105324
10. Collier JJ, Suomi F, Olahova M, et al. Emerging roles of ATG7 in human health and disease. EMBO Mol Med. 2021;13(12):e14824. doi:10.15252/emmm.202114824
11. Wang X, Wu R, Liu Y, et al. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy. 2020;16(7):1221–1235. doi:10.1080/15548627.2019.1659617
12. Collier JJ, Olahova M, McWilliams TG, et al. ATG7 safeguards human neural integrity. Autophagy. 2021;17(9):2651–2653. doi:10.1080/15548627.2021.1953267
13. Collier JJ, Guissart C, Olahova M, et al. Developmental consequences of defective ATG7-mediated autophagy in humans. N Engl J Med. 2021;384(25):2406–2417. doi:10.1056/NEJMoa1915722
14. Jung HS, Chung KW, Won Kim J, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8(4):318–324. doi:10.1016/j.cmet.2008.08.013
15. Quan W, Lim YM, Lee MS. Role of autophagy in diabetes and endoplasmic reticulum stress of pancreatic beta-cells. Exp Mol Med. 2012;44(2):81–88. doi:10.3858/emm.2012.44.2.030
16. Ebato C, Uchida T, Arakawa M, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8(4):325–332. doi:10.1016/j.cmet.2008.08.009
17. Lim YM, Lim H, Hur KY, et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat Commun. 2014;26(5):4934. doi:10.1038/ncomms5934
18. Pandit BR, Vyas A. Clinical symptoms, pathogen spectrum, risk factors and antibiogram of suspected neonatal sepsis cases in tertiary care hospital of southern part of Nepal: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. 2020;58(232):976–982. doi:10.31729/jnma.5094
19. Gao C, Sun X, Lu L, et al. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. 2019;10(1):154–162. doi:10.1111/jdi.12854
20. Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14–17.
21. Monzillo LU, Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr Rev. 2003;61(12):397–412. doi:10.1301/nr.2003.dec.397-412
22. Committee American Diabetes Association Professional Practice. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi:10.2337/dc22-S002
23. Liu Y, Kuang A, Bain JR, et al. Maternal metabolites associated with gestational diabetes mellitus and a postpartum disorder of glucose metabolism. J Clin Endocrinol Metab. 2021;106(11):3283–3294. doi:10.1210/clinem/dgab513
24. Chen Q, Francis E, Hu G, et al. Metabolomic profiling of women with gestational diabetes mellitus and their offspring: review of metabolomics studies. J Diabetes Complications. 2018;32(5):512–523. doi:10.1016/j.jdiacomp.2018.01.007
25. Ryckman KK, Spracklen CN, Smith CJ, et al. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. 2015;122(5):643–651. doi:10.1111/1471-0528.13261
26. Wang GH, Jin J, Liu YQ, et al. The changes of Lp-PLA2 in patients with gestational diabetes and its clinical significance. Medicine. 2021;100(30):e26786. doi:10.1097/MD.0000000000026786
27. Sun YY, Juan J, Xu QQ, et al. Increasing insulin resistance predicts adverse pregnancy outcomes in women with gestational diabetes mellitus. J Diabetes. 2020;12(6):438–446. doi:10.1111/1753-0407.13013
28. Deischinger C, Leitner K, Baumgartner-Parzer S, et al. CTRP-1 levels are related to insulin resistance in pregnancy and gestational diabetes mellitus. Sci Rep. 2020;10(1):17345. doi:10.1038/s41598-020-74413-1
29. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):11. doi:10.3390/ijms19113342
30. Al-Ofi E, Alrafiah A, Maidi S, et al. Altered expression of angiogenic biomarkers in pregnancy associated with gestational diabetes. Int J Gen Med. 2021;14:3367–3375. doi:10.2147/IJGM.S316670
31. Jiang YK, Xin KY, Ge HW, et al. Upregulation of renal GLUT2 and SGLT2 is involved in high-fat diet-induced gestational diabetes in mice. Diabetes Metab Syndr Obes. 2019;12:2095–2105. doi:10.2147/DMSO.S221396
32. He Y, Wu N. Research progress on gestational diabetes mellitus and endothelial dysfunction markers. Diabetes Metab Syndr Obes. 2021;14:983–990. doi:10.2147/DMSO.S295737
33. Xiong J. Atg7 in development and disease: panacea or Pandora’s Box? Protein Cell. 2015;6(10):722–734. doi:10.1007/s13238-015-0195-8
34. Zhang N, Cao MM, Liu H, et al. Autophagy regulates insulin resistance following endoplasmic reticulum stress in diabetes. J Physiol Biochem. 2015;71(2):319–327. doi:10.1007/s13105-015-0384-1
35. Barlow AD, Thomas DC. Autophagy in diabetes: beta-cell dysfunction, insulin resistance, and complications. DNA Cell Biol. 2015;34(4):252–260. doi:10.1089/dna.2014.2755
36. Zhang C, Hu J, Wang W, et al. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB J. 2020;34(7):9563–9574. doi:10.1096/fj.202000605RR
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
