Back to Journals » Journal of Pain Research » Volume 17
Looking for a Beam of Light to Heal Chronic Pain
Authors Xu J , Zhang H, Chen D, Xu K, Li Z, Wu H, Geng X, Wei X, Wu J, Cui W , Wei S
Received 18 December 2023
Accepted for publication 5 March 2024
Published 16 March 2024 Volume 2024:17 Pages 1091—1105
DOI https://doi.org/10.2147/JPR.S455549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Jialing Xu,1,2 Hao Zhang,1,2 Dan Chen,2 Kaiyong Xu,1,2 Zifa Li,1,2 Hongyun Wu,3 Xiwen Geng,1,2 Xia Wei,4 Jibiao Wu,1,2 Wenqiang Cui,3 Sheng Wei1,2
1The Key Laboratory of Traditional Chinese Medicine Classic Theory of Ministry of Education, Shandong University of Traditional Chinese Medicine, Ji’nan, Shandong, People’s Republic of China; 2Chinese Medicine and Brain Science Interdisciplinary Research Institute, Shandong University of Traditional Chinese Medicine, Ji’nan, Shandong, People’s Republic of China; 3Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Ji’nan, Shandong, People’s Republic of China; 4NMPA Key Laboratory for Research and Evaluation of Generic Drugs, Shandong Institute for Food and Drug Control, Ji’nan, Shandong, People’s Republic of China
Correspondence: Sheng Wei, Email [email protected]; Wenqiang Cui, Email [email protected]
Abstract: Chronic pain (CP) is a leading cause of disability and a potential factor that affects biological processes, family relationships, and self-esteem of patients. However, the need for treatment of CP is presently unmet. Current methods of pain management involve the use of drugs, but there are different degrees of concerning side effects. At present, the potential mechanisms underlying CP are not completely clear. As research progresses and novel therapeutic approaches are developed, the shortcomings of current pain treatment methods may be overcome. In this review, we discuss the retinal photoreceptors and brain regions associated with photoanalgesia, as well as the targets involved in photoanalgesia, shedding light on its potential underlying mechanisms. Our aim is to provide a foundation to understand the mechanisms underlying CP and develop light as a novel analgesic treatment has its biological regulation principle for CP. This approach may provide an opportunity to drive the field towards future translational, clinical studies and support pain drug development.
Keywords: chronic pain, light therapy, photoanalgesia, brain regions, molecular targets
Introduction
The eyes are important visual organs. The eyes can perceive light and convert stimuli to electrical signals. Besides their function in imaging, they also have many nonimaging functions, participating in processes such as the circadian rhythm, sleep quality, mood, and learning.1–3 Recent studies also found that optical signals play an analgesic role.4–7 Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.8 It can be categorized as nociceptive (from tissue injury), neuropathic (from nerve injury), or nociplastic (from a sensitive nervous system).9 Chronic pain (CP) is defined by the International Association for the Study of Pain as “persistent or recurrent pain lasting longer than 3 months”.10 Chronic pain is a very common disorder with an estimated global prevalence of about 20%.11 The Global Burden of Disease Study 2019 revealed that CP was the greatest cause of years lived with disability worldwide.12,13
At present, treatment of CP is not efficient, causes side effects, and may lead to drug tolerance,14 which results in poor treatment outcomes and a heavy burden on patients.9,15 Although various nonpharmacological treatment methods are available, including surgery, regional anesthesia, neuromodulation, and rehabilitation programs, pain is not reduced to a sufficient degree in many CP patients. Generally speaking, pain is rarely completely eliminated, and highly effective treatment methods are still lacking.16 There is an urgent need to improve the pain treatment approaches without inducing adverse side effects.
Light therapy has several advantages; it is noninvasive, convenient, and cost-effective. Exposure to light of specific wavelengths and intensities has shown to be effective in the management of various health conditions, such as cancers,17 skin diseases,18 sleep disorders,19 and affective disorder.20 Recently, light therapy has been used for various pain conditions, including chronic nonspecific low back pain,21 fibromyalgia pain,22 migraine headaches,6 and neuropathic pain.23 Based on this technique, optogenetic techniques have been developed that enable the control of cellular activity through ectopic expression of light-activated optogenetic proteins in genetically modified target cells.24,25
In this review, we discuss how light can be used to induce analgesia, including the retinal photoreceptors involved in photoanalgesia and the brain regions associated with these photoreceptors. We also discuss the mechanisms underlying the analgesic effects of light in CP, as well as the principle and application of some derivative technologies in which light plays a therapeutic role. We strive to adequately expound how light serves an analgesic function through the visual pathway and the biological mechanisms through which light acts on pain, as well as the current applications of phototherapy to relieve pain. We hope this review helps to generate new scientific hypotheses, probe physiological mechanisms, develop therapeutic strategies, reveal the value of photoanalgesia techniques, and promote the fundamental research, clinical treatment, and drug development in the area of CP.
Characteristics of Light Exerting Analgesic Effects in CP
Alteration of the intensity of light in the environment impacts several physiological functions. The light environment plays a crucial role in the circadian rhythm, sleep quality, mood, and learning, which are essential for our health and quality of life.1–3 Furthermore, an increasing body of evidence suggests that bright light has an antinociceptive effect in various pain conditions. In the clinic, increased sunlight exposure decreases pain in patients after spinal surgery,26 and bright light appears to improve the symptoms of patients with headache,27 fibromyalgia,28 and chronic low back pain.29,30 A recent study evaluated the effects of exposure to light of various intensities (0, 200, 1000, 3000, and 5000 lux) in wild-type animals through nocifensive behavior tests; it was found that an intensity greater than 3000 lux was required to significantly upregulate the pain threshold.31 Another study found that migraine patients have different pain indices in different seasons; the pain index is reduced in seasons of higher light intensity.32 Similar findings were observed in mice whose cold pain and mechanical pain thresholds changed when they were placed in a dim light environment.33 In addition, two proof of concept studies, one in women with fibromyalgia28 and the other in military veterans with chronic low back pain,29 showed that sitting in broad-spectrum bright white light (>3000 lux) for 1 h per day upon waking in the morning improved pain sensitivity and behavior. All the abovementioned bright light treatments lasted for several hours before analgesic effects were observed. On the contrary, various studies have confirmed that short-term intense light therapy has no analgesic effects, but aggravates pain.34,35 In summary, these results indicate that exposure to high-intensity light for several hours can relieve the sensation of pain.
Beside of intensity, various experiments focused on the therapeutic effect of light wavelength on pain (Table 1). The wavelength of light determines the visual color presentation of our eyes, although the range of the visible spectrum to the human is limited. Recently, short wavelength laser/light therapy has been used for the management of various pain conditions. It may contribute to the photopigment found in retinal ganglion cell is melanopsin and has a peak spectral sensitivity of 480nm, which falls in the blue/cyan range of visible light. It’s understandable that a study reported that red light administered through visual pathways caused thermal hyperalgesia and mechanical allodynia in rats.36 Consistent with the characteristics of melanopsin, most studies have reported the beneficial effects of green/blue light.37,38 For instance, exposure to green light via the visual system resulted in lesser pain in an acute migraine episode compared to the exposure of other wavelengths such as white, blue, amber, and red.39 And Tang et al5 unilateral intra-articular injection of complete Freund’s adjuvant in mice to produced mouse model of arthritic pain, then exposed the models to green light. After six consecutive days with 8 hours daily exposure, hyperalgesia of models had significant relief. Consistently, there is a study have reported that exposing in green light produced long-lasting antinociceptive effects in rats, in the same condition opaque contact lenses prevented antinociception.40 And rats fitted with green contact lenses exposed to room light exhibited antinociception arguing for a crucial role of the visual system. In addition, clinical studies have shown that green light is effective for headache relief.6
 |
Table 1 Summary of Studies on the Effects of Different Light Wavelengths on Pain Through Visual Pathways |
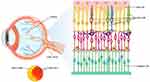 |
Figure 1 Illustration of retinal photoreceptors. |
Retinal Photoreceptors That are Involved in Photoanalgesia
The mammalian eye contains three classes of photoreceptors with distinct peak excitation wavelengths and photo-response characteristics: rods and cones (“canonical” photoreceptors) in the outer retina44 and melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs; “noncanonical” photoreceptors) in the inner retina45 (Figure 1). Cones and rods, which work together to capture visual information and respond to light, are considered as the origin of the visual pathway in the retina.44,46 They show different light sensitivities; in bright environments, our vision is almost completely mediated by the cones, which are not very light-sensitive and provide vision in bright light, yet the proportion of cones is barely 5% (4.6 million out of 92 million) of our retinal photoreceptors. In contrast to rods, cones can perceive different colors.44 There are three types of cones, namely blue-, green-, and red-sensitive cones, while there is only one type of rod, which is green-sensitive.47 Rods, which account for 95% of our retinal photoreceptors, are very light-sensitive; they can detect single photons and contribute to human vision only under low light conditions, for example during twilight.44,47 As the “noncanonical” photoreceptors, ipRGCs capture light by using G protein–coupled receptors (GPCRs) known as melanopsin. The main physiological functions of ipRGC are nonimaging functions, including photic regulation of the circadian rhythm and the pupillary light response.46
Rods/Cones
Photophobia, also termed photo-oculodynia, is defined as an abnormal sensitivity to light. People with photophobia usually consider innocuous visual stimuli as noxious or uncomfortable Photophobia may be caused by a variety of ocular diseases.46 Photophobia is a typical symptom of migraine and has been identified as the most disabling symptom of migraine.27,48 Based on the physical functions of rods and cones, photophobic migraine causes serious difficulties. Given that migraine has also been associated with abnormal color vision and color discrimination,49 maladaptive dysfunction of cones and/or rods could play an additional role in migraine photophobia.
A psychophysical study was performed in migraineurs with normal eyesight to test the effects of colors on headache intensity and other headache features,42 it was found that green light significantly attenuated the headache intensity. Through color selective electroretinography and analysis of visual evoked potentials in migraineurs, it was shown that the perception of headache intensity is selectively modulated by spectral light through photoactivation of cone-driven retinal pathways.50 Similarly, a study focusing on green light analgesia in arthritic mice showed that cone-dominated retinal inputs mediated green light analgesia.5 One study evaluated the sensitivity of the visual system in migraine patients and control subjects, and revealed that retinal cones or the visual cortex did not induce inherent hypersensitivity to light.51
Intrinsically Photosensitive Retinal Ganglion Cells
The neural retina has a layered structure. Rods and cones, the first-order neurons in the visual pathway, initiate the transduction process of visual signals, which are subsequently transmitted to RGCs.52 Intrinsically photosensitive retinal ganglion cells are not only extrinsically activated by rods and cones,53,54 but also intrinsically by virtue of their unique photopigment known as melanopsin. Intrinsically photosensitive retinal ganglion cells have been reported to mediate light signals involved in bright light–induced antinociception.31,55 Hu et al4 found that bright light regulates nocifensive behaviors in mice through a visual circuit. They demonstrated that ON-type RGCs directly innervate the downstream pathway, mediating the antinociceptive effects. Surprisingly, a study showed that exacerbation of migraine headache by light was preserved in blind patients who could sense light in the face of severe degeneration of rod and cone photoreceptors.56 Photomodulatory effects are exerted by novel axonal projections of RGCs that converge to the posterior region of the thalamus consisting of axons of ipRGCs.
These studies elucidate the exact photoreceptor class responsible for light-induced analgesia.
Brain Regions and Circuits Associated with Photoanalgesia
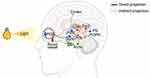
Retinal projections of rods and cones to the brain constitute imaging and nonimaging pathways. Images are formed primarily by activation of corn RGCs, and the functions of the nonimaging pathway are all mediated by a specialized pathway originating from ipRGCs, whose axons project via the optic nerve to the ventral suprachiasmatic nucleus (SCN), intergeniculate leaflet (IGL)/lateral geniculate nucleus (LGN), and olivary pretectal nucleus (OPN).57–59 These brain regions, which may be involved in photoanalgesia, are introduced below (Figure 2).
Suprachiasmatic Nucleus
The suprachiasmatic nucleus is a compact, bilaterally paired cell group in the anterior–ventral hypothalamus. It was first identified as a hypothalamic retinal target in rodents, and by implication it was considered as a potential circadian control point following autoradiographic visualization of its dedicated route of retinal innervation—the retina hypothalamic tract.60 In mammals, it is the site of an endogenous pacemaker that regulates circadian rhythmicity and plays an indispensable role in behavioral, physiological, and hormonal rhythms in response to the environmental light–dark cycle.3,61 Suprachiasmatic nucleus also appears to function as a seasonal clock underlying the measurement of daylength.62 In summary, the SCN is the clock and calendar of vertebrates.
Suprachiasmatic nucleus participates in photoanalgesia probably by projecting to the pineal gland (PG)62,63 and the paraventricular nucleus (PVN).64,65
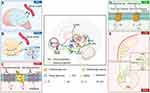
The pineal gland secretes the hormone melatonin.66 Melatonin synthesis follows a daily rhythm, with high levels during night time and low levels during the day time, to regulate the daily rhythms. Melatonin is essential for the integration of seasonal photoperiodic changes in physiology.67 A large body of evidence suggests that melatonin has antinociceptive effects in CP conditions, including migraine headache,67 fibromyalgia,68 irritable bowel syndrome,69 and temporomandibular joint osteoarthritis-induced CP.70 Similarly, Yang et al71 found that intrathecal administration of melatonin changed inflammatory cytokine levels to attenuate bone cancer pain, so they proposed that melatonin may be a promising drug for the clinical treatment of bone cancer pain (Figure 3A).
The paraventricular nucleus is another nucleus which the axons of the SCN project to.64,72 Its magnocellular neurons project mainly to the posterior lobe of the pituitary gland, where they secrete oxytocin (OXT) into the blood and the hypothalamic-neuron hypophysial system,73 as the parvocellular neurons project to the cortex74 (Figure 3B). Repeated OXT administration has been reported to play an analgesic role in preventing central sensitization by regulating synaptic plasticity in a chronic migraine mouse model.72 There is circumstantial evidence that OXT might be involved in migraine headache. The level of circulating OXT increases over the course of pregnancy,75 and the frequency of migraine headaches decreases inversely.76 Consistent with this observation, compared to migraineurs who bottle feed, migraineurs who breastfeed have higher circulating levels of OXT77 and demonstrate a lower rate of postpartum migraine recurrence.76 An experiment on the nocifensive behavior of zebrafish showed that OXT neuron activation is sufficient to generate this defensive behavior, whereas ablation of OXT neurons attenuates this behavior, indicating that OXT neurons are crucial for the generation of appropriate analgesic responses to noxious input. The results of a similar study also showed that evoked OXT release from these OXT neurons suppresses nociception and promotes analgesia in an animal model of inflammatory pain.78,79 These data highlight that OXT neurons of the PVN regulated by ipRGCs may play a potential role in photoanalgesia regulation.
Intergeniculate Leaflet/Lateral Geniculate Nucleus
The rodent ventral LGN (vLGN) and IGL in the visual thalamus are homologous to the primate pregeniculate nucleus.31 The IGL/LGN of the thalamus is the core retinorecipient structure implicated in orchestrating circadian rhythmicity. The IGL/LGN network is highly GABAergic and consists mainly of neuropeptide Y-synthesizing and enkephalinergic neurons.5,31 Enkephalinergic neurons have been reported to provide network inhibition.80 The GABAergic neurons of the IGL/LGN project to the periaqueductal gray area (PAG),81 which is an evolutionarily conserved neurosubstrate providing a key link to the regulation of nocifensive behaviors.82,83 These studies demonstrated that bright light treatment activates the retina–IGL/LGN–PAG pathway, producing antinociceptive effects. Similar to the IGL/LGN, it has been found that green light exposure for several consecutive days can significantly relief complete Freund’s adjuvant-induced thermal hyperalgesia and mechanical allodynia in mice.5 To verify the role of this pathway in their study, they inhibited the retina–IGL/vLGN pathway and completely abolished the analgesic effects, which showed that the retina–IGL/vLGN pathway is responsible for the green light analgesia, and they showed that the IGL/vLGN–dorsal raphe nucleus (DRN) is the crucial region for photoanalgesia as well. Besides, there is another study indicating that morphine plays an analgesic role through stimulating the retina–geniculate–cortex pathway and the thalamus–cortex circuit by regulating the opioid receptors.84
Olivary Pretectal Nucleus
The olivary pretectal nucleus has classically been recognized as a relay in the pupillary light reflex,85,86 which is also an important target of ipRGCs. A study focusing on photosensitivity explored the brain regions that are activated by light, and it was found that pain-modulating neurons in the rostral ventromedial medulla (RVM) unexpectedly respond to light; approximately half of the pain-facilitating “ON-cells” and pain-inhibiting “OFF-cells” sampled exhibited a change in firing with light exposure, shifting the system to a pronociceptive state. The change in neuronal firing was blocked by inactivation of the OPN.35 This means that the OPN is a crucial brain region for photosensitivity. But this finding also implies that light-evoked responses in the RVM do not represent a noxious ocular event, which would presumably be mediated by the trigeminal (TG) system. The role of the TG system in facial and dural sensitivity has been recognized for a long time. Moreover, the TG system has also been considered a prominent actor in brain nociceptive innervation. It is the anatomical substrate of several frequent conditions, such as primary or secondary headaches,87 TG neuralgia,88,89 and other orofacial pains,90 as well as ocular pain.91
Targets Associated with Photoanalgesia
Considering the importance of the characteristics of light-sensitive cells, such as ipRGCs, a novel cross-integration discipline has been developed, optogenetics, whose basic concept is the manipulation of the activity of live cells which generate light-sensitive proteins (opsins) by transducing electrical currents.92,93 This technology has revolutionized the study of neuroscience with single-cell and millisecond precision control of neurons.94 It is possible to manipulate neuronal excitability and network activity to exert analgesia effects in vertebrates. Several potential targets are related to photoanalgesia (Figure 3).
Ion Channel Targets Related to Photoanalgesia
Ion channels such as sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) channels are transmembrane proteins that control the movement of ions across the cell membrane. They affect cellular activity and their permeability is closely related to many life activities, such as the occurrence of receptor potentials, nerve excitation, and regulation of the central nervous system. Nociceptor nerve terminals express ligand-gated and voltage-gated ion channels, which are key molecular transducers of these noxious stimuli.95 For this reason, the generation of photosensitivity proteins in neurons can regulate their activity via ion channels. Researchers conducted a series of studies and found that light changes the state of ion channels through photosensitive proteins to regulate the excitability of cells and exert analgesic effects through downstream molecules. We highlight some known receptors that can change the state of ion channels below.
μ-Opioid receptors
Opioids have a long cultural and medicinal history, and their best-known member, morphine, has been employed since antiquity to alleviate pain and induce euphoria.96 µ-Opioid is an opioid subtype and µ-Opioid receptors (MORs) are widely distributed in the DRN.5 Opioid receptors belong to family A of the GPCRs, which includes many important drug targets.97 GPCRs of this type are closely related to the opsin photoreceptors, which enable vision and shape the circadian rhythm in humans.98 The MOR is the major target of morphine. K+ plays a crucial role in triggering termination of action potentials (APs) and makes a major contribution to maintaining the resting potential. Alberio et al99 investigated light-gated K+ channels and then engineered the light-gated K+ channel BLINK, whose activity is controlled by blue light (455 nm) (Figure 3C). A study from the same group later showed that BLINK has favorable properties for optogenetics. BLINK is an inhibitory tool in long-lasting optogenetic experiments, and activation by light reduced pain in a rat model and inhibited the touch-evoked escape response in zebrafish.100 Subsequently, a MOR was generated that is activated by G-protein-coupled inward rectifier channels, which initiate a quick K+ influx upon exposure to blue light, and photoanalgesics were developed, such as the azobenzene derivative of fentanyl.101
TRPV1 Receptors
Transient receptor potential ion vanilloid receptor 1 (TRPV1), a nonselective voltage-gated cation channel known for its role in nociception, is the most studied of the transient receptor potential ion channels.102,103 TRPV1 channels are highly expressed in pain fibers but scarcely present in the central nervous system.104–106 They are involved in the transduction of pain stimuli from the periphery towards the central nervous system.106 They are expressed in sub-populations of sensory nerve fibers within the dorsal root and TG ganglia.107
Capsaicin is a specific exogenous TRPV1 agonist. Cis-AzCA4 is the azo derivative of capsaicin that was found to be the most effective in activating TRPV1. Photoactivation of TRPV1 with this compound is reversible and has been achieved in both human embryonic kidney 293 T lymphocyte cells and C fibers. TRPV1-positive neurons of the dorsal root ganglion in mice selectively respond to photoactive TRPV1. In vivo tests demonstrated a TRPV1-mediated hyperalgesia by this photo-compound.108,109 Moreover, the first example of fusion of photo-pharmacology and lipid signaling with potential application in controlling protein–lipid interactions has been presented.109
Based primarily on the characteristics of TRPV1, a nonselective cation channel that is highly expressed in pain fibers, the molecular mechanism underlying the initiation and propagation of APs is the opening and closing of voltage-gated ion channels.110,111 When excitatory inputs to a neuron trigger a depolarization above the AP initiation threshold, voltage-gated Na+ channel quickly open and Na+ ions rush into the cell, depolarizing the membrane even further. Quaternary ammonium-azobenzene-quaternary ammonium (QAQ) is a photo-switchable compound developed on the basis of lidocaine, a local anesthetic that blocks voltage-gated channels.112,113 QAQ prevents AP firing when neurons receive excitatory inputs by blocking all voltage-gated K+, Na+, and Ca2+ channels (Figure 3D). The team of Mourot112 found that QAQ was able to enter cells through open TRPV1, enabling the targeted photosensitization of cells expressing either of these channels.113 As TRPV1 channels are heat-activated channels required for the detection of noxious heat,103 QAQ can play an inhibitory role for nociceptors that are selectively regulated by light.112,113
GABAA Receptors
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian brain. GABAA receptors are pentameric ligand-gated ion channels that are activated by GABA.114 Binding of GABA results in the opening of a Cl− selective pore. Following this interaction, Cl− influx is promoted, while postsynaptic neuron hyperpolarization and action-potential firing decrease (Figure 3E). The gabapentinoids, which are GABA analogs, are frontline therapeutic agents for neuronal pain that can act directly in the spinal cord to suppress nociception and pain aversiveness.115 In rat models of neuropathy, gabapentinoids reverse tactile and thermal allodynia.116 Thus, GABAA receptors serve as targets for anesthetic, hypnotic, and anticonvulsant drugs.114
GABAA receptors are not sensitive to light. Nevertheless, photo-switchable agents have been produced that can switch GABAergic neurons between an active and an inactive form by the application of light.117 The trans isoform of this compound was found to be capable of activating a GABAA receptor subunit, and the cis isoform was found inactive. Whole-cell patch clamp recording of HEK cells with the trans isoform also showed that exposure to light leads to conversion to its cis isoform and decreases the current amplitude. On the contrary, a dark environment could increase the current. The anesthetic activity of photo-switchable agents was investigated in albino Xenopus laevis tadpoles, and it was found that anesthesia could be achieved.117 This study demonstrated that light potentiates GABA-induced Cl− currents and that light-dependent anesthetic agents can be used effectively. These studies showed that the GABAA receptor is the target of photoanalgesia.
Immune Targets and Pathways Related to Photoanalgesia
As mentioned, Chronic pain is classified into three main categories: nociceptive, neuropathic, and nociplastic.9 A common pathogenic cause of CP is dysregulation of the immune system. Many reports showed that the immune system is strongly correlated with CP. For example, Goebel et al118 showed that IgG from fibromyalgia syndrome (FMS) patients produced sensory hypersensitivity by sensitizing nociceptive neurons. Mice treated with IgG from FMS patients displayed increased sensitivity to noxious mechanical and cold stimulation, and nociceptive fibers in skin-nerve preparations from mice treated with FMS IgG displayed an increased responsiveness to cold and mechanical stimulation. Transfer of IgG-depleted serum from FMS patients or IgG from healthy control subjects had no effect, indicating that therapies reducing IgG titers may be effective for fibromyalgia. Considering that interleukin (IL)-1β is a critical cytokine involved in creating heightened nociception associated with persistent pain, Arman et al119 used chronic constriction injury (CCI) to initiate nerve injury in rats and then quantified intrathecal IL-1β concentrations. They found that the degree of mechanical allodynia was positively correlated with the increase in the intrathecal concentration of IL-1β in CCI animals, providing a molecular biomarker of the degree of exaggerated pain. Recently, a study showed that mice exposed to four days of dim light (5 lux) at night exhibited cold hyperalgesia, and mice exhibited both cold hyperalgesia and mechanical allodynia after 28 days. This phenotype was concurrent with upregulated IL-6 and nerve growth factor mRNA expression in the medulla and elevated MOR mRNA expression in the PAG.33 In a recent study, optogenetic or chemogenetic activation of the glutamatergic neurons of the secondary visual cortex (V2MGlu) to GABAergic neurons in the anterior cingulate cortex (ACC) was performed, which inhibits local glutamatergic neurons (ACCGABA-Glu), mimicking green light-induced antinociception in both neuropathic and inflammatory pain model mice. Artificial inhibition of ACC-projecting V2MGlu neurons abolishes the antinociception induced by green light.7 This means that the V2M-ACC circuit is a potential candidate mediating green light-induced antinociceptive effects to treat immune pain. These results provide circumstantial evidence explaining how green light exerts analgesic effects in immune-related CP. Summarizing these experimental results, we believe that there is a connection among light, immunity, and pain.
Conclusion and Prospect
Chronic pain is associated with alterations in the peripheral and central nervous system, along with a decline in the quality of life.120,121 It is caused by multidimensional, dynamic interactions among biological, psychological, and social factors that reciprocally influence each other.122 As a leading cause of disability and a potential factor that affects biological processes, CP causes a heavy financial burden.9 Chronic pain affects family relationships and self-esteem of patients, and is associated with a reduced life expectancy, and leads to higher suicide rates and a higher substance abuse risk.123,124 Current methods of pain management involve pharmacotherapy using agents such as serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, nonsteroidal anti-inflammatory drugs, corticosteroids, benzodiazepines, gabapentinoids, and opioids, but these drugs cause concerning side effects, such as sedation, cardiotoxicity, ataxia, addiction, and respiratory depression.125 For this reason, there has been a growing desire to develop drugs with high efficiency and lower/no side effects and nonpharmacologic methods to manage and relieve pain. In this review, we discussed retinal photoreceptors and their functions in the photo-response. We described brain regions (including the SCN, IGL/LGN, and OPN) and circuits that ipRGCs project to, and we illustrate the evidence showing these regions are involved in photoanalgesia. In order to further explain the mechanisms underlying photoanalgesia, we introduced various potential targets associated with photoanalgesia. We aimed to provide a foundation to understand the mechanisms underlying CP and develop light-based novel analgesic treatment methods. This review may provide an opportunity to drive the field towards future translational, clinical studies and support pain drug development.
Light is a widely recognized effective analgesic which exerts its effects through the visual pathway. Numerous reports in the past decade have documented the mechanisms of pain modulation by light. Beside the therapeutic effects on pain, light of certain colors may also exacerbate clinical pain, such as fibromyalgia and migraine. For example, white, blue, amber, and red light increase pain, while green light decreases pain, probably because the photoanalgesic mechanism is different between skin and eyes:126 cutaneous application acts primarily via peripheral nerves, whereas visual application acts via the central nervous system. Red and near-infrared light are administered cutaneously with seconds to minutes of exposure, whereas green and bright white light are administered visually with much longer duration of exposure. Red and near-infrared light have large wavelengths (625−740 nm and 750−1000 nm), which allows for very high tissue penetrance.127 Because of this property, the duration of light exposure for red and infrared light treatments to cause photo-biomodulatory effects is short. Green light (500−565 nm) has a much shorter wavelength; therefore, it may be insufficient to penetrate the skin, but it exerts analgesic effects through the visual pathway. Also note that ipRGCs, which produce melanopsin, have a peak spectral sensitivity of 480 nm (blue/cyan). Bright white light, which contains light of all wavelengths in the visible spectrum, can exert analgesic effects. As green light, bright white light treatment works through the visual pathway, requiring exposure for several hours.28–31,41 However, one main difference between these two colors is the intensity at which the light is administered. Bright white light therapy is typically administered at >3000 lux and even at 5000 lux in one study.41 On the contrary, green light therapy is administered at 4–110 lux.5,6,38,40 The high tissue penetrance of red and near-infrared lights may induce thermal injury, but no adverse effects have been reported in any clinical or animal studies of visual light therapy. Based on these findings, visual light exposure can be a useful analgesic therapy. It provides a safe and effective option to reduce the physical, psychological, economic, and societal burden on patients who are beset with acute pain and CP. However, the reason why light with different characteristics leads to different analgesic effects is still unclear.
Recently, the use of light as a novel nonpharmacologic treatment for several pain syndromes has become particularly attractive to both clinicians and patients due to its noninvasiveness and lack of side effects, ultimately increasing patient compliance. Light changes the activity of live cells which generate light-sensitive proteins to play a role of abirritation. The novel cross-integration discipline of optogenetics has been established; as the name suggests, optogenetics is the integration of two fields: optics and genetics (the optics part is associated with illumination with a specific light spectrum, whereas the genetics part is associated with the expression of the modified opsin protein in cells). Based on this technology, photo-pharmacology has come into being. Photo-pharmacology deals with photo-responsive agents that exert analgesic effects when a switch occurs between two (cis–trans) or more isoforms. So far, targets for these agents range from ion channels and GPCRs to transporters, enzymes, and lipids.128 Hence, the biological functions can be controlled by acting on native receptors or acceptors.108,128,129 The photo-pharmacology principle holds an advantage of selectivity because light can be delivered with a high precision in terms of its intensity and wavelength for adjustable dosing.108,128,129 The possibility to alter the activity of a drug by light offers several advantages, such as reducing off-target, systemic side effects or even drug resistance,108 which provides a powerful potential for clinical transformation.
In the past few decades, nanosized polymeric micelles have emerged as promising drug delivery carriers due to their outstanding characteristics, including high drug loading capacity, long circulation in the bloodstream, and passive targeting capability based on the enhanced permeability and retention effect.130 An ideal nanocarriers combines efficient and stable drug encapsulation while possessing the unique features of releasing substances or molecules upon application of a specific external stimulus. In typical systems, conformational changes of nanocarriers are stimulated by classical triggers such as pH131 and temperature.132 Nevertheless, these systems often suffer from spatial restrictions and/or inefficient disruption/swelling of the nanocarrier wall. Aiming to address this problem, researchers have invented a kind of capsule whose wall has been doped with irradiation dyes, and its properties can be reversibly altered by applying a specific wavelength stimulus.133 The applications of this technology include the treatment of many diseases, including pain. For example, a novel concept has been introduced to fabricate a two-photon-sensitive and sugar-targeting nanocarrier which can contain a clinical anticancer drug, doxorubicin, to be released in a controlled manner by changing the irradiation time.134 Another anti-cancer study based on similar principles and techniques is that by Meng and coworkers,135 who invented a chitosan-based nanocarrier which exhibits a dual response to pH and light. Optical control nanocarrier technology is also widely used in ophthalmic diseases. The aim is to employ a delivery method to deliver a photosensitizing compound selectively to the target tissue.131,136 Liu and coworkers137 explored a light-sensitive nanocarrier for simultaneous triggered antibiotic release. Another important research field of optically controlled nanocarrier technology is nanocarrier-mediated RNA interference therapeutics,138,139 which has become a promising way to treat numerous human diseases caused by genetic factors. These studies have proved that light can be used to treat clinical diseases and reveal a broad prospect for phototherapy.
Pain is associated with both psychological and biological changes and with alterations in both the peripheral and the central nervous system. In the future, we can pay more attention to (i) research the long-loop circuit of photoanalgesic, (ii) fully explain the mechanism of photoanalgesia, including the photoreceptors in the eyes and their connections with pain-related brain regions, such as the TG system and PAG, and (iii) identify the specific pain receptors on the body surface. At present, the underlying mechanisms of photoanalgesia have yet to be fully elucidated. Pain is a subjective feeling accompanied by substantial or potential tissue damage, and the pathological mechanism is influenced by central and peripheral factors. When we try to find the targets of photoanalgesia, perhaps it can be considered from the comorbidity of pain and emotion, such as comorbid depression and CP140–142 or pain on opiate addiction.143 It suggests that when we look for targets of pain, we should not only focus on the physiological molecules related to pain, but also broaden our horizons to the targets of psychological diseases, such as brain-derived nerve factor (BDNF),144,145 serotonin (5-hydroxytryptamine; 5-HT),146–148 and dopamine.149,150 Considering these factors together may aid in elucidating the mechanisms underlying photoanalgesia.
The promising results from preclinical studies of phototherapy in animals and clinical studies in patients reveal that light can modulate pain. Light may provide more possibilities for disease treatment in the future.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 82274383, 81974553 and 82004078), the Special Funding for Taishan Scholars Project (no. tsqn202211137), the Natural Science Foundation of Shandong Province (nos. ZR2020ZD17 and ZR2021LZY018), the Chinese Medicine and Brain Science Youth Scientific Research Innovation Team, Shandong University of Traditional Chinese Medicine (no. 22202101), High Level Key Disciplines of Traditional Chinese Medicine: Basic Theory of Traditional Chinese Medicine, National Administration of Traditional Chinese Medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
1. LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–454. doi:10.1038/nrn3743
2. Shen J, Tower J. Effects of light on aging and longevity. Ageing Res Rev. 2019;53:100913. doi:10.1016/j.arr.2019.100913
3. Fernandez DC, Fogerson PM, Lazzerini Ospri L, et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell. 2018;175(1):71–84.e18. doi:10.1016/j.cell.2018.08.004
4. Hu Z, Mu Y, Huang L, et al. A visual circuit related to the periaqueductal gray area for the antinociceptive effects of bright light treatment. Neuron. 2022;110(10):1712–1727 e1717. doi:10.1016/j.neuron.2022.02.009
5. Tang Y-L, Liu A-L, S-S L, et al. Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons in the ventrolateral geniculate nucleus. Sci Transl Med. 2022;14(674):eabq6474. doi:10.1126/scitranslmed.abq6474
6. Martin LF, Patwardhan AM, Jain SV, et al. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: a preliminary one-way cross-over clinical trial. Cephalalgia. 2021;41(2):135–147. doi:10.1177/0333102420956711
7. Cao P, Zhang M, Ni Z, et al. Green light induces antinociception via visual-somatosensory circuits. Cell Rep. 2023;42(4):112290. doi:10.1016/j.celrep.2023.112290
8. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
9. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi:10.1016/S0140-6736(21)00393-7
10. Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007.
11. Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4
12. Rice ASC, Smith BH, Blyth FM. Pain and the global burden of disease. Pain. 2016;157(4):791–796. doi:10.1097/j.pain.0000000000000454
13. Disease GBD. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858.
14. Varrassi G, Muller-Schwefe G, Pergolizzi J, et al. Pharmacological treatment of chronic pain - The need for CHANGE. Curr Med Res Opin. 2010;26(5):1231–1245. doi:10.1185/03007991003689175
15. Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–e283. doi:10.1016/j.bja.2019.03.023
16. Knotkova H, Hamani C, Sivanesan E, et al. Neuromodulation for chronic pain. Lancet. 2021;397(10289):2111–2124. doi:10.1016/S0140-6736(21)00794-7
17. Xie Z, Fan T, An J, et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem Soc Rev. 2020;49(22):8065–8087. doi:10.1039/D0CS00215A
18. Racz E, Prens EP. Phototherapy of Psoriasis, a Chronic Inflammatory Skin Disease. Adv Exp Med Biol. 2017;996:287–294.
19. Faulkner SM, Dijk DJ, Drake RJ, Bee PE. Adherence and acceptability of light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuropsychiatric illness: a systematic review. Sleep Health. 2020;6(5):690–701. doi:10.1016/j.sleh.2020.01.014
20. Pjrek E, Friedrich ME, Cambioli L, et al. The Efficacy of Light Therapy in the Treatment of Seasonal Affective Disorder: a Meta-Analysis of Randomized Controlled Trials. Psychother Psychosom. 2020;89(1):17–24. doi:10.1159/000502891
21. Lin YP, Su YH, Chin SF, Chou YC, Chia WT. Light-emitting diode photobiomodulation therapy for non-specific low back pain in working nurses: a single-center, double-blind, prospective, randomized controlled trial. Medicine. 2020;99(32):e21611. doi:10.1097/MD.0000000000021611
22. Yeh SW, Hong CH, Shih MC, Tam KW, Huang YH, Kuan YC. Low-Level Laser Therapy for Fibromyalgia: a Systematic Review and Meta-Analysis. Pain Physician. 2019;22(3):241–254.
23. de Andrade ALM, Bossini PS, Do Canto De Souza ALM, Sanchez AD, Parizotto NA. Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Lasers Med Sci. 2017;32(4):865–872. doi:10.1007/s10103-017-2186-x
24. Tan P, He L, Huang Y, Zhou Y. Optophysiology: illuminating cell physiology with optogenetics. Physiol Rev. 2022;102(3):1263–1325. doi:10.1152/physrev.00021.2021
25. Corbo J. Optogenetics. Nat Biotechnol. 2022;40(10):1431. doi:10.1038/s41587-022-01501-0
26. Walch JM, Rabin BS, Day R, Williams JN, Choi K, Kang JD. The effect of sunlight on postoperative analgesic medication use: a prospective study of patients undergoing spinal surgery. Psychosom Med. 2005;67(1):156–163. doi:10.1097/01.psy.0000149258.42508.70
27. Noseda R, Copenhagen D, Burstein R. Current understanding of photophobia, visual networks and headaches. Cephalalgia. 2019;39(13):1623–1634. doi:10.1177/0333102418784750
28. Burgess HJ, Park M, Ong JC, Shakoor N, Williams DA, Burns J. Morning Versus Evening Bright Light Treatment at Home to Improve Function and Pain Sensitivity for Women with Fibromyalgia: a Pilot Study. Pain Med. 2017;18(1):116–123. doi:10.1093/pm/pnw160
29. Burgess HJ, Rizvydeen M, Kimura M, et al. An Open Trial of Morning Bright Light Treatment Among US Military Veterans with Chronic Low Back Pain: a Pilot Study. Pain Med. 2019;20(4):770–778. doi:10.1093/pm/pny174
30. Burns JW, Gerhart J, Rizvydeen M, Kimura M, Burgess HJ. Morning Bright Light Treatment for Chronic Low Back Pain: potential Impact on the Volatility of Pain, Mood, Function, and Sleep. Pain Med. 2020;21(6):1153–1161. doi:10.1093/pm/pnz235
31. Hu ZF, Mu YM, Huang L, et al. A visual circuit related to the periaqueductal gray area for the antinociceptive effects of bright light treatment. Neuron. 2022;110(10):1712.
32. Naber WC, Fronczek R, Haan J, et al. The biological clock in cluster headache: a review and hypothesis. Cephalalgia. 2019;39(14):1855–1866. doi:10.1177/0333102419851815
33. Bumgarner JR, Walker WH, Liu JA, Walton JC, Nelson RJ. Dim Light at Night Exposure Induces Cold Hyperalgesia and Mechanical Allodynia in Male Mice. Neuroscience. 2020;434:111–119. doi:10.1016/j.neuroscience.2020.03.022
34. Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 2011;52(11):7852–7858. doi:10.1167/iovs.11-7604
35. Martenson ME, Halawa OI, Tonsfeldt KJ, et al. A possible neural mechanism for photosensitivity in chronic pain. Pain. 2016;157(4):868–878. doi:10.1097/j.pain.0000000000000450
36. Khanna R, Patwardhan A, Yang X, et al. Development and Characterization of An Injury-free Model of Functional Pain in Rats by Exposure to Red Light. J Pain. 2019;20(11):1293–1306. doi:10.1016/j.jpain.2019.04.008
37. Serrage H, Heiskanen V, Palin WM, et al. Under the spotlight: mechanisms of photobiomodulation concentrating on blue and green light. Photochem Photobiol Sci. 2019;18(8):1877–1909. doi:10.1039/c9pp00089e
38. Cao P, Zhang M, Ni Z, et al. Green light induces antinociception via visual-somatosensory circuits. Cell Reports. 2023;42(4):112290.
39. Noseda R, Bernstein CA, Nir -R-R, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain. 2016;139(Pt 7):1971–1986. doi:10.1093/brain/aww119
40. Ibrahim MM, Patwardhan A, Gilbraith KB, et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain. 2017;158(2):347–360. doi:10.1097/j.pain.0000000000000767
41. Leichtfried V, Matteucci Gothe R, Kantner-Rumplmair W, et al. Short-term effects of bright light therapy in adults with chronic nonspecific back pain: a randomized controlled trial. Pain Med. 2014;15(12):2003–2012. doi:10.1111/pme.12503
42. Nir -R-R, Lee AJ, Huntington S, et al. Color-selective photophobia in ictal vs interictal migraineurs and in healthy controls. Pain. 2018;159(10):2030–2034. doi:10.1097/j.pain.0000000000001303
43. Martin L, Porreca F, Mata EI, et al. Green Light Exposure Improves Pain and Quality of Life in Fibromyalgia Patients: a Preliminary One-Way Crossover Clinical Trial. Pain Med. 2021;22(1):118–130. doi:10.1093/pm/pnaa329
44. Lamb TD. Why rods and cones? Eye. 2016;30(2):179–185. doi:10.1038/eye.2015.236
45. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi:10.1126/science.1067262
46. Grossniklaus HE, Geisert EE, Nickerson JM. Introduction to the Retina. Prog Mol Biol Transl Sci. 2015;134:383–396.
47. Kawamura S, Tachibanaki S. Molecular bases of rod and cone differences. Prog Retin Eye Res. 2022;90:101040. doi:10.1016/j.preteyeres.2021.101040
48. Lipton RB, Serrano D, Buse DC, et al. Improving the detection of chronic migraine: development and validation of Identify Chronic Migraine (ID-CM). Cephalalgia. 2016;36(3):203–215. doi:10.1177/0333102415583982
49. Wilkins AJ, Haigh SM, Mahroo OA, Plant GT. Photophobia in migraine: a symptom cluster? Cephalalgia. 2021;41(11–12):1240–1248. doi:10.1177/03331024211014633
50. Noseda R, Bernstein CA, Nir RR, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain. 2016;139(Pt 7):1971–1986.
51. Bernstein CA, Nir RR, Noseda R, et al. The migraine eye: distinct rod-driven retinal pathways’ response to dim light challenges the visual cortex hyperexcitability theory. Pain. 2019;160(3):569–578. doi:10.1097/j.pain.0000000000001434
52. Sakai D, Tomita H, Maeda A. Optogenetic Therapy for Visual Restoration. Int J Mol Sci. 2022;23(23):15041. doi:10.3390/ijms232315041
53. Mure LS, Vinberg F, Hanneken A, Panda S. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science. 2019;366(6470):1251–1255. doi:10.1126/science.aaz0898
54. Hu J, Shi Y, Zhang J, et al. Melanopsin retinal ganglion cells mediate light-promoted brain development. Cell. 2022;185(17):3124–3137.e15. doi:10.1016/j.cell.2022.07.009
55. Yates D. Shining a light on pain. Nat Rev Neurosci. 2022;23(5):253. doi:10.1038/s41583-022-00590-9
56. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–245. doi:10.1038/nn.2475
57. Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–349. doi:10.1002/cne.20970
58. Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi:10.1038/nature01761
59. Orlowska-Feuer P, Allen AE, Storchi R, Szkudlarek HJ, Lewandowski MH. The contribution of inner and outer retinal photoreceptors to infra-slow oscillations in the rat olivary pretectal nucleus. Eur J Neurosci. 2016;43(6):823–833. doi:10.1111/ejn.13184
60. Schoonderwoerd RA, de Torres Gutiérrez P, Blommers R, et al. Inhibitory responses to retinohypothalamic tract stimulation in the circadian clock of the diurnal rodent Rhabdomys pumilio. FASEB J. 2022;36(8):e22415. doi:10.1096/fj.202200477R
61. Patton AP, Hastings MH. The suprachiasmatic nucleus. Curr Biol. 2018;28(15):R816–R822. doi:10.1016/j.cub.2018.06.052
62. Coomans CP, Ramkisoensing A, Meijer JH. The suprachiasmatic nuclei as a seasonal clock. Front Neuroendocrinol. 2015;37:29–42. doi:10.1016/j.yfrne.2014.11.002
63. De Pablo-Fernández E, Courtney R, Warner TT, Holton JL. A Histologic Study of the Circadian System in Parkinson Disease, Multiple System Atrophy, and Progressive Supranuclear Palsy. JAMA Neurol. 2018;75(8):1008–1012. doi:10.1001/jamaneurol.2018.0640
64. Ono D, Mukai Y, Hung CJ, Chowdhury S, Sugiyama T, Yamanaka A. The mammalian circadian pacemaker regulates wakefulness via CRF neurons in the paraventricular nucleus of the hypothalamus. Sci Adv. 2020;6(45). doi:10.1126/sciadv.abd0384
65. Jones JR, Chaturvedi S, Granados-Fuentes D, Herzog ED. Circadian neurons in the paraventricular nucleus entrain and sustain daily rhythms in glucocorticoids. Nat Commun. 2021;12(1):5763. doi:10.1038/s41467-021-25959-9
66. Ziegler KA, Ahles A, Dueck A, et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science. 2023;381(6655):285–290. doi:10.1126/science.abn6366
67. Sapède D, Cau E. The pineal gland from development to function. Curr Top Dev Biol. 2013;106:171–215.
68. Hussain SA-R, Al-Khalifa II, Jasim NA, Gorial FI. Adjuvant use of melatonin for treatment of fibromyalgia. J Pineal Res. 2011;50(3). 267–271. doi:10.1111/j.1600-079X.2010.00836.x
69. Siah KTH, Wong RKM, Ho KY. Melatonin for the treatment of irritable bowel syndrome. World J Gastroenterol. 2014;20(10):2492–2498. doi:10.3748/wjg.v20.i10.2492
70. Liu W, Jiang H, Liu X, et al. Melatonin Abates TMJOA Chronic Pain by MT2R in Trigeminal Ganglion Neurons. J Dent Res. 2022;101(1):111–119. doi:10.1177/00220345211026551
71. Yang C, Kang F, Huang X, et al. Melatonin attenuates bone cancer pain via the SIRT1/HMGB1 pathway. Neuropharmacology. 2022;220:109254. doi:10.1016/j.neuropharm.2022.109254
72. Wang Y, Pan Q, Tian R, et al. Repeated oxytocin prevents central sensitization by regulating synaptic plasticity via oxytocin receptor in a chronic migraine mouse model. J Headache Pain. 2021;22(1):84. doi:10.1186/s10194-021-01299-3
73. Zhang B, Qiu L, Xiao W, et al. Reconstruction of the Hypothalamo-Neurohypophysial System and Functional Dissection of Magnocellular Oxytocin Neurons in the Brain. Neuron. 2021;109(2). doi:10.1016/j.neuron.2020.10.032.
74. Gamal-Eltrabily M, Espinosa de Los Monteros-Zúñiga A, Manzano-García A, Martínez-Lorenzana G, Condés-Lara M, González-Hernández A. The Rostral Agranular Insular Cortex, a New Site of Oxytocin to Induce Antinociception. J Neurosci. 2020;40(29):5669–5680. doi:10.1523/JNEUROSCI.0962-20.2020
75. Rashidi M, Maier E, Dekel S, et al. Peripartum effects of synthetic oxytocin: the good, the bad, and the unknown. Neurosci Biobehav Rev. 2022;141:104859. doi:10.1016/j.neubiorev.2022.104859
76. Hoshiyama E, Tatsumoto M, Iwanami H, et al. Postpartum migraines: a long-term prospective study. Intern Med. 2012;51(22):3119–3123. doi:10.2169/internalmedicine.51.8542
77. Grewen KM, Davenport RE, Light KC. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology. 2010;47(4):625–632. doi:10.1111/j.1469-8986.2009.00968.x
78. Eliava M, Melchior M, Knobloch-Bollmann HS, et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron. 2016;89(6):1291–1304. doi:10.1016/j.neuron.2016.01.041
79. Liu Y, Li A, Bair-Marshall C, et al. Oxytocin promotes prefrontal population activity via the PVN-PFC pathway to regulate pain. Neuron. 2023;111(11):1795–1811.e7. doi:10.1016/j.neuron.2023.03.014
80. Palus K, Chrobok L, Kepczynski M, Lewandowski MH. Enkephalin and neuropeptide-Y interaction in the intergeniculate leaflet network, a part of the mammalian biological clock. Neuroscience. 2017;343:10–20. doi:10.1016/j.neuroscience.2016.11.034
81. Oh SW, Harris JA, Ng L, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207–214. doi:10.1038/nature13186
82. Nguyen E, Grajales-Reyes JG, Gereau RW, Ross SE. Cell type-specific dissection of sensory pathways involved in descending modulation. Trends Neurosci. 2023;46(7):539–550. doi:10.1016/j.tins.2023.04.002
83. Soleimani AH, Dehghani A, Abbasnejad M, Esmaeili-Mahani S, Raoof M, Lobbezoo F. Apelin signalling in the periaqueductal grey matter alleviates capsaicin-evoked pulpal nocifensive behaviour and capsaicin-induced spatial learning and memory impairments in rat. Int Endod J. 2023;56(8):968–979. doi:10.1111/iej.13930
84. Kuroda K, Fujiwara A, Takeda Y, Kamei C. Effects of narcotics, including morphine, on visual evoked potential in rats. Eur J Pharmacol. 2009;602(2–3):294–297. doi:10.1016/j.ejphar.2008.11.048
85. May PJ, Warren S. Pupillary light reflex circuits in the Macaque Monkey: the olivary pretectal nucleus. Brain Struct Funct. 2020;225(1):305–320. doi:10.1007/s00429-019-02003-7
86. Allen AE, Brown TM, Lucas RJ. A distinct contribution of short-wavelength-sensitive cones to light-evoked activity in the mouse pretectal olivary nucleus. J Neurosci. 2011;31(46):16833–16843. doi:10.1523/JNEUROSCI.2505-11.2011
87. Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019;18(8):795–804. doi:10.1016/S1474-4422(19)30185-1
88. Di Stefano G, De Stefano G, Leone C, et al. Concomitant continuous pain in patients with trigeminal neuralgia is associated with trigeminal nerve root atrophy. Cephalalgia. 2020;40(13):1502–1510. doi:10.1177/0333102420949206
89. Jeon HJ, Han SR, Park MK, Yang KY, Bae YC, Ahn DK. A novel trigeminal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):149–158. doi:10.1016/j.pnpbp.2012.03.002
90. Liu Y-J, Y-L L, Fang Z-H, et al. NMDARs mediate peripheral and central sensitization contributing to chronic orofacial pain. Front Cell Neurosci. 2022;16:999509. doi:10.3389/fncel.2022.999509
91. Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149(2):235–242. doi:10.1016/j.pain.2010.02.004
92. Berndt A, Lee SY, Wietek J, et al. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci U S A. 2016;113(4):822–829. doi:10.1073/pnas.1523341113
93. Boyden ES. Optogenetics and the future of neuroscience. Nat Neurosci. 2015;18(9):1200–1201. doi:10.1038/nn.4094
94. Rost BR, Schneider-Warme F, Schmitz D, Hegemann P. Optogenetic Tools for Subcellular Applications in Neuroscience. Neuron. 2017;96(3):572–603. doi:10.1016/j.neuron.2017.09.047
95. Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017;38(1):5–19. doi:10.1016/j.it.2016.10.001
96. Serafini RA, Estill M, Pekarskaya EA, et al. Tianeptine promotes lasting antiallodynic effects in a mouse model of neuropathic pain. Neuropsychopharmacology. 2023;48(11):1680–1689. doi:10.1038/s41386-023-01645-w
97. Palmer CB, Meyrath M, Canals M, Kostenis E, Chevigné A, Szpakowska M. Atypical opioid receptors: unconventional biology and therapeutic opportunities. Pharmacol Ther. 2022;233:108014. doi:10.1016/j.pharmthera.2021.108014
98. Hofmann KP, Scheerer P, Hildebrand PW, et al. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem Sci. 2009;34(11):540–552. doi:10.1016/j.tibs.2009.07.005
99. Cosentino C, Alberio L, Gazzarrini S, et al. Optogenetics. Engineering of a light-gated potassium channel. Science. 2015;348(6235):707–710. doi:10.1126/science.aaa2787
100. Alberio L, Locarno A, Saponaro A, et al. A light-gated potassium channel for sustained neuronal inhibition. Nat Methods. 2018;15(11):969–976. doi:10.1038/s41592-018-0186-9
101. Schönberger M, Trauner D. A photochromic agonist for μ-opioid receptors. Angew Chem Int Ed Engl. 2014;53(12):3264–3267. doi:10.1002/anie.201309633
102. Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6(5):357–372. doi:10.1038/nrd2280
103. Abbas MA. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem Biol Interact. 2020;330:109178. doi:10.1016/j.cbi.2020.109178
104. Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat Methods. 2008;5(4):299–302. doi:10.1038/nmeth.1190
105. Cavanaugh DJ, Chesler AT, Jackson AC, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31(13):5067–5077. doi:10.1523/JNEUROSCI.6451-10.2011
106. Campbell JN, Stevens R, Hanson P, et al. Injectable Capsaicin for the Management of Pain Due to Osteoarthritis. Molecules. 2021;26(4):778. doi:10.3390/molecules26040778
107. Liu D-L, Wang W-T, Xing J-L, S-J H. Research progress in transient receptor potential vanilloid 1 of sensory nervous system. Neurosci Bull. 2009;25(4):221–227. doi:10.1007/s12264-009-0506-2
108. Lerch MM, Hansen MJ, van Dam GM, Szymanski W, Feringa BL. Emerging Targets in Photopharmacology. Angew Chem Int Ed Engl. 2016;55(37):10978–10999. doi:10.1002/anie.201601931
109. Frank JA, Moroni M, Moshourab R, Sumser M, Lewin GR, Trauner D. Photoswitchable fatty acids enable optical control of TRPV1. Nat Commun. 2015;6(1):7118. doi:10.1038/ncomms8118
110. Roux B. Ion channels and ion selectivity. Essays Biochem. 2017;61(2):201–209. doi:10.1042/EBC20160074
111. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29(1):355–384. doi:10.1146/annurev-cellbio-101011-155833
112. Mourot A, Tochitsky I, Kramer RH. Light at the end of the channel: optical manipulation of intrinsic neuronal excitability with chemical photoswitches. Front Mol Neurosci. 2013;6:5. doi:10.3389/fnmol.2013.00005
113. Mourot A, Fehrentz T, Le Feuvre Y, et al. Rapid optical control of nociception with an ion-channel photoswitch. Nat Methods. 2012;9(4):396–402. doi:10.1038/nmeth.1897
114. Masiulis S, Desai R, Uchański T, et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature. 2019;565(7740):454–459. doi:10.1038/s41586-018-0832-5
115. Bannister K, Qu C, Navratilova E, et al. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain. 2017;158(12):2386–2395. doi:10.1097/j.pain.0000000000001040
116. Zhang G-F, Chen S-R, Jin D, Huang Y, Chen H, Pan H-L. α2δ-1 Upregulation in Primary Sensory Neurons Promotes NMDA Receptor-Mediated Glutamatergic Input in Resiniferatoxin-Induced Neuropathy. J Neurosci. 2021;41(27):5963–5978. doi:10.1523/JNEUROSCI.0303-21.2021
117. Stein M, Middendorp SJ, Carta V, et al. Azo-propofols: photochromic potentiators of GABA(A) receptors. Angew Chem Int Ed Engl. 2012;51(42):10500–10504. doi:10.1002/anie.201205475
118. Goebel A, Krock E, Gentry C, et al. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 2021;131(13). doi:10.1172/JCI144201.
119. Arman A, Deng F, Goldys EM, Liu G, Hutchinson MR. In vivo intrathecal IL-1beta quantification in rats: monitoring the molecular signals of neuropathic pain. Brain Behav Immun. 2020;88:442–450. doi:10.1016/j.bbi.2020.04.009
120. Clauw DJ, Essex MN, Pitman V, Jones KD. Reframing chronic pain as a disease, not a symptom: rationale and implications for pain management. Postgrad Med. 2019;131(3):185–198. doi:10.1080/00325481.2019.1574403
121. Cauda F, Palermo S, Costa T, et al. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neuroimage Clin. 2014;4:676–686. doi:10.1016/j.nicl.2014.04.007
122. Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):168–182. doi:10.1016/j.pnpbp.2018.01.017
123. Davis KD, Aghaeepour N, Ahn AH, et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol. 2020;16(7):381–400. doi:10.1038/s41582-020-0362-2
124. Kirtley OJ, Rodham K, Crane C. Understanding suicidal ideation and behaviour in individuals with chronic pain: a review of the role of novel transdiagnostic psychological factors. Lancet Psychiatry. 2020;7(3):282–290. doi:10.1016/S2215-0366(19)30288-3
125. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
126. Cheng K, Martin LF, Slepian MJ, Patwardhan AM, Ibrahim MM. Mechanisms and Pathways of Pain Photobiomodulation: a Narrative Review. J Pain. 2021;22(7):763–777. doi:10.1016/j.jpain.2021.02.005
127. Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye Brain. 2011;3:49–67. doi:10.2147/EB.S21391
128. Broichhagen J, Frank JA, Trauner D. A roadmap to success in photopharmacology. Acc Chem Res. 2015;48(7):1947–1960. doi:10.1021/acs.accounts.5b00129
129. Velema WA, Szymanski W, Feringa BL. Photopharmacology: beyond proof of principle. J Am Chem Soc. 2014;136(6):2178–2191. doi:10.1021/ja413063e
130. Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55(3):403–419. doi:10.1016/S0169-409X(02)00226-0
131. Ashrafizadeh M, Hushmandi K, Mirzaei S, et al. Chitosan-based nanoscale systems for doxorubicin delivery: exploring biomedical application in cancer therapy. Bioeng Transl Med. 2023;8(1):e10325. doi:10.1002/btm2.10325
132. Pasparakis G, Alexander C. Sweet talking double hydrophilic block copolymer vesicles. Angew Chem Int Ed Engl. 2008;47(26):4847–4850. doi:10.1002/anie.200801098
133. Achilleos DS, Hatton TA, Vamvakaki M. Light-regulated supramolecular engineering of polymeric nanocapsules. J Am Chem Soc. 2012;134(13):5726–5729. doi:10.1021/ja212177q
134. Sun L, Yang Y, Dong CM, Wei Y. Two-photon-sensitive and sugar-targeted nanocarriers from degradable and dendritic amphiphiles. Small. 2011;7(3):401–406. doi:10.1002/smll.201001729
135. Meng L, Huang W, Wang D, Huang X, Zhu X, Yan D. Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules. 2013;14(8):2601–2610. doi:10.1021/bm400451v
136. Onugwu AL, Nwagwu CS, Onugwu OS, et al. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J Control Release. 2023;354:465–488. doi:10.1016/j.jconrel.2023.01.018
137. Liu G, Mao S, Kim JH. A Mature-Tomato Detection Algorithm Using Machine Learning and Color Analysis. Sensors. 2019;19(9):56.
138. Wang J, He X, Shen S, Cao Z, Yang X. ROS-Sensitive Cross-Linked Polyethylenimine for Red-Light-Activated siRNA Therapy. ACS Appl Mater Interfaces. 2019;11(2):1855–1863. doi:10.1021/acsami.8b18697
139. Deng X, Liang S, Cai X, et al. Yolk-Shell Structured Au Nanostar@Metal-Organic Framework for Synergistic Chemo-photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2019;19(10):6772–6780. doi:10.1021/acs.nanolett.9b01716
140. Zhu X, Tang HD, Dong WY, et al. Distinct thalamocortical circuits underlie allodynia induced by tissue injury and by depression-like states. Nat Neurosci. 2021;24(4):542–553. doi:10.1038/s41593-021-00811-x
141. Zhou W, Jin Y, Meng Q, et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. 2019;22(10):1649–1658. doi:10.1038/s41593-019-0468-2
142. Kai Y, Li Y, Sun T, et al. A medial prefrontal cortex-nucleus acumens corticotropin-releasing factor circuitry for neuropathic pain-increased susceptibility to opioid reward. Transl Psychiatry. 2018;8(1):100. doi:10.1038/s41398-018-0152-4
143. Hipólito L, Wilson-Poe A, Campos-Jurado Y, et al. Inflammatory Pain Promotes Increased Opioid Self-Administration: role of Dysregulated Ventral Tegmental Area μ Opioid Receptors. J Neurosci. 2015;35(35):12217–12231. doi:10.1523/JNEUROSCI.1053-15.2015
144. Cavaleri D, Moretti F, Bartoccetti A, et al. The role of BDNF in major depressive disorder, related clinical features, and antidepressant treatment: insight from meta-analyses. Neurosci Biobehav Rev. 2023;149:105159. doi:10.1016/j.neubiorev.2023.105159
145. Zhang K, Wang F, Zhai M, et al. Hyperactive neuronal autophagy depletes BDNF and impairs adult hippocampal neurogenesis in a corticosterone-induced mouse model of depression. Theranostics. 2023;13(3):1059–1075. doi:10.7150/thno.81067
146. Haleem DJ. Targeting Serotonin1A Receptors for Treating Chronic Pain and Depression. Curr Neuropharmacol. 2019;17(12):1098–1108. doi:10.2174/1570159X17666190811161807
147. Israelyan N, Del Colle A, Li Z, et al. Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology. 2019;157(2). doi:10.1053/j.gastro.2019.04.022.
148. Zhou W, Jin Y, Meng Q, et al. Publisher Correction: a neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. 2019;22(11):1945. doi:10.1038/s41593-019-0522-0
149. Bekhbat M, Li Z, Mehta ND, et al. Functional connectivity in reward circuitry and symptoms of anhedonia as therapeutic targets in depression with high inflammation: evidence from a dopamine challenge study. Mol Psychiatry. 2022;27(10):4113–4121. doi:10.1038/s41380-022-01715-3
150. Matini T, Haghparast A, Rezaee L, Salehi S, Tehranchi A, Haghparast A. Role of Dopaminergic Receptors Within the Ventral Tegmental Area in Antinociception Induced by Chemical Stimulation of the Lateral Hypothalamus in an Animal Model of Orofacial Pain. J Pain Res. 2020;13:1449–1460. doi:10.2147/JPR.S255250
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.