Back to Journals » Clinical Epidemiology » Volume 15
Long-Term Temporal Trends in Survival Among Danish Patients with Advanced Cutaneous Melanoma: A Nationwide Follow-Up Study
Authors Pedersen AB , Johnsen SP, Horváth-Puhó E
Received 21 February 2023
Accepted for publication 10 June 2023
Published 15 June 2023 Volume 2023:15 Pages 733—742
DOI https://doi.org/10.2147/CLEP.S407060
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Irene Petersen
Alma B Pedersen,1,2 Søren P Johnsen,3,4 Erzsébet Horváth-Puhó2
1Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Clinical Epidemiology, Aarhus University, Aarhus, Denmark; 3Center for Clinical Health Services Research, Aalborg University Hospital, Aalborg, Denmark; 4Department of Clinical Medicine, Aalborg University, Aalborg, Denmark
Correspondence: Alma B Pedersen, Email [email protected]
Introduction: Population-based data on survival trends over time among patients with advanced cutaneous melanoma are lacking. We examined changes in mortality for patients diagnosed from 1980 to 2011 in a nationwide historical follow-up study using population-based medical registries from Denmark.
Material and Methods: The study population included all Danish patients with an incident diagnosis of advanced (metastatic or unresectable stage IIIA, IIIB, IIIC, or IV) cutaneous melanoma (ie, initial diagnosis for melanoma at stage III/IV) between 1980– 2011 and who were followed-up until 2013. For each patient, we randomly matched 100 individuals from the general population on sex and year of birth. Age-standardized mortality rates were calculated by calendar year of diagnosis overall, 30 days after diagnosis, and during 31 to 364 days and 0– 10 years after diagnosis. Stratified Cox’s proportional hazards regression was used to compute hazard ratios.
Results: We identified a total of 1236 patients and 123,600 comparison cohort members. We observed that the standardized mortality rates of patients with advanced melanoma dropped from the 1980s onwards, but remain high (eg, 74.3 and 248.4 per 1000 person-years in 0– 30 days and 31– 364 days after diagnosis, respectively, for patients diagnosed during 2008– 2011). Compared with the general population, patients with advanced melanoma had a 10.4-fold increased hazard of death during 0– 10 years of follow-up. The highest relative mortality was found for the first year following melanoma diagnosis. No improvements in survival compared to the general population were observed in the most recent years of the study period, thus in 2004– 2007 and 2008– 2011.
Discussion and Conclusion: Survival of patients with advanced cutaneous melanoma in Denmark improved between 1980 and 2013 but appears to have leveled off in the years leading up to more widespread introduction of newer immuno-oncology therapies.
Keywords: cohort study, general population, melanoma, mortality trend, registries
Introduction
The incidence of melanoma of the skin has increased more than any other cancer in most majority white populations during the last decades.1 Worldwide, about 325,000 new melanoma cases were estimated in 2020. The increase in melanoma incidence in early years is attributed to sun-seeking behavior, but the current trend of increased melanoma could be attributed to improvement in awareness and surveillance of skin cancer and earlier diagnosis capturing the early stage melanoma.2 There is a large geographic variation in incidence of melanoma with highest incidence in Australia/New Zealand and lowest in Northern Europe.1 Still, in the Nordic countries, melanoma incidence has risen dramatically from 1964 to 20033 and further from 2003 to 2020,4 and melanoma accounted for 6.8% of all new cancers in the Nordic countries in 2020, and 1.7% of all new cancers worldwide.5
Melanoma is by far the deadliest form of skin cancer6,7 and patients with advanced (metastasized) melanoma have historically been reported to have a very poor prognosis.8 Previous Nordic studies have reported a substantial overall improvement in the survival of melanoma patients since the 1950s, however, updated data on time trends in survival of patients with advanced melanoma are still sparse.3,9–13 The main treatments for melanoma so far have been biological therapy, chemotherapy, radiotherapy, and surgery. With the development and introduction of immunomodulatory monoclonal antibodies as a new therapeutic modality for patients with advanced melanoma, an opportunity to establish immunological therapy as the new standard of care in this patient group has been offered. Ipilimumab was the first successful immune checkpoint inhibitors introduced in Denmark and other Nordic countries in 2011, followed by BRAF gene targeted treatment with vemurafenib in 2012, and anti-PD-1 antibodies treatment with nivolumab in 2016. In addition, overall life-expectancy of the general population has improved, and previous studies had not entirely accounted for this in survival analyses of melanoma patients. Real-world evidence from newer malign melanoma treatments will take time to accumulate. Therefore, a description of advanced malign melanoma patients and an updated assessment of the long-term prognosis of these patients prior to the availability of the first of newer immunotherapeutic agents, is required as it could provide a baseline upon which to evaluate the benefits of newer treatments as they are introduced into clinical practice.
In this study, we focused on advanced melanoma (stages IIIA, IIIB, IIIC, and IV) to update current knowledge of patient characteristics and disease mortality during the years until the introduction of the first immunotherapy agent, and compare to mortality in the general population.
Materials and Methods
Study Design and Settings
The study was designed as a historical nationwide population-based longitudinal study. The source population, identified from the Danish Civil Registration System (CRS), included all persons who were residents of Denmark between 1 January 1977 and 31 December 2013, aged 18 years or older.
The Danish National Health Service provides tax-supported healthcare for the entire population, guaranteeing unfettered access to all hospitals and primary medical care.
Data Sources
Data were collected from the CRS, The Danish Cancer Registry (DCR), and the Danish National Patient Registry (DNPR).
The Civil Registration System
Since 1968, all Danish citizens are assigned a unique 10-digit civil registration number (CPR) at birth or immigration. This number encodes age and sex and is included in all public medical databases; thus, it allows unambiguous linkage between registries. The CRS maintains records on vital statistics, including births, deaths, emigration, and addresses of all Danish residents and is updated daily.14 Through the CRS, we generated a population comparison cohort and retrieved information on the vital status for both melanoma patients and the comparison cohort.
The Danish Cancer Registry
The DCR holds information on all incident diagnoses of cancer in Denmark since 1943, including information on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, and first course of cancer-directed treatment.12 Diagnoses in the DCR are coded according to a Danish version of the International Classification of Diseases, seventh revision (ICD-7), and the registry’s completeness and validity have been estimated to be 95–98%.15,16 Tumors diagnosed in the period 1978 to 2003 have been reclassified according to the 10th revision (ICD-10), therefore ICD-10 codes were used to identify incident cases of advanced cutaneous melanoma in the source population.
From the DCR, we collected information on the date of advanced cutaneous melanoma diagnosis, stage of cancer (according to the American Joint Committee on Cancer (AJCC) 7th edition staging system,17 derived from the TNM classification), and cancer spreading (according to node status and metastases, derived from the TNM classification, which was available for patients diagnosed from 2004 onwards. In addition, we collected information on the treatments given within the first four months of diagnosis, which were available before 2003.
The Danish National Patient Registry
All inpatient admissions to Danish hospitals have been recorded in the DNPR since 1977 and outpatient discharges and emergency room visits since 1995. Each record in the DNPR contains the patient’s CPR number, information on treatment procedures performed, a primary discharge diagnosis that identifies the main reason for treatment, and up to 20 secondary diagnoses.18 These are coded according to ICD-8 from 1977 to 1994 and according to ICD-10 thereafter.
From the DNPR, we collected information on treatment (surgery, chemo- and immunotherapy, and radiation therapy) given within four months of the diagnosis from 2004 onwards. In addition, we obtained data on patients’ comorbid diseases (in- and outpatient diagnosis) in the three years proceeding cancer diagnosis for the advanced melanoma cohort or index date for the general population comparison cohort and calculated the Charlson Comorbidity Index (CCI)19 adapted for administrative data.20
Study Population
Advanced Cutaneous Melanoma Cohort
From the source population, we identified all Danish patients admitted to a hospital with first-time discharge diagnosis of advanced (metastatic or unresectable stage IIIA, IIIB, IIIC, or IV) cutaneous melanoma according to AJCC 7th edition17 (ie, the patients had their first melanoma diagnosis at stage III/IV) from 1980 through 2011, as recorded in the DCR. The date of diagnosis was considered as the index date. We excluded melanoma patients with stage I and II and patients with non-skin malignant melanoma, including melanoma of female and male genital organs, Merkel cell carcinoma, uveal melanoma, and melanoma of the conjunctiva.
Matched General Population Comparison Cohort
We identified a population-based comparison cohort from the general population using the CRS. For each advanced melanoma patient, we randomly matched 100 individuals from the general population on sex and year of birth. The index date of the comparators was identical with the first-time diagnosis date of the corresponding advanced melanoma patient. To be eligible for the study, persons in the comparison cohort were not allowed to have had a hospitalization for advanced cutaneous melanoma before the admission date for the corresponding advanced cutaneous melanoma patient.
If a member of the general population cohort subsequently developed advanced melanoma, he or she contributed person-time to the general population cohort until the date of diagnosis (switching date); going forward they contributed person-time to the advanced melanoma cohort.
Both the melanoma and general population comparison cohorts were followed from the index date to 31 December 2013 to ascertain their vital status from the CRS, thus date of death or emigration. Patients who emigrated from the study population were censored at the date of emigration. For both the melanoma cohort and the general population comparison cohort, we collected information on comorbidity from the DNPR during 1977 to 2011. To have at least three years of hospitalization history before index date for all melanoma patients and the general population comparison cohort, we restricted the study population to patients diagnosed from 1980 onwards.
Patient Characteristics
Assessed patient characteristics of the melanoma patients included calendar year of melanoma diagnosis (1980–1991, 1992–2003, 2004–2007, and 2008–2011), age (at the date of diagnosis/at the index date), sex, AJCC stage of advanced melanoma IIIA, IIIB, IIIC, or IV at diagnosis from 2004 and distant stage before 2004, comorbidity at the time of diagnosis in the three years preceding diagnosis defined according to CCI and supplemented by obesity, alcoholism, alcoholism-related conditions, presence of visceral disease or brain metastasis (defined according to TNM classification [M0, M1a, M1b, and M1c] and presence of M1c [yes vs no] from 2004). In addition, treatment within the first four months of advanced melanoma diagnosis was assessed (surgery, chemo-, radiation therapy, or a combination of treatments).
Mortality
All-cause death up to 10 years following melanoma diagnosis/index date was assessed using information from the CRS covering the period from 1 January 1980 throughout 31 December 2013.
Statistical Analyses
We first examined patient and treatment characteristics of patients with advanced cutaneous melanoma. Descriptive statistics were presented for the entire melanoma cohort and the entire comparison cohort, but also according to calendar year of melanoma diagnosis.
Secondly, we computed all-cause mortality risks overall and by calendar year of diagnosis (after 30-days, 31- to 364-days, 1–5 years, 6–10 years, and 0–10 years) using the Kaplan-Meier estimator. Mortality rates were further computed as the number of deaths divided by the number of person-years at risk within the same group of subjects, expressed per 1000 person-years stratified by age. The rates were standardized to the age and sex distribution of the melanoma cohort in 2010.
We examined changes in mortality over time in patients with advanced melanoma and the matched persons from the general population using Cox’s proportional hazards regression to estimate all-cause mortality hazard ratios (HRs) and associated 95% confidence intervals (CI), both unadjusted and adjusted further for age, sex, and comorbidity before diagnosis. Finally, we investigated whether age and sex modified the analyzed association using stratification method. Log-log plots and Schoenfeld residuals were used to test the Cox assumption of proportional hazards and we found no evidence of non-proportionality.
Results
In total, 1236 persons were included in the advanced melanoma cancer cohort (Table 1). Of the included 1236 advanced melanoma patients, 526 (42.6%) were identified in the period 1980–2003 with distant stage and 710 (57.4%) were identified with stages III and IV from 2004 onwards. Most of the patients were men, 54.9% (679). Of the included patients, 22.2% (274) were 55–64 years, 21.8% (270) were 65–74 years, and 20.2% (250) were older than 75 years. Most patients were treated with surgery only (increasing from 56.5% between 1980–1991 to 80.2% between 2008–2011) within the first four months after diagnosis of advanced melanoma (Table 1). However, the surgery is unresectable including incision, excision, debridement, or laser treatment most commonly performed on the skin and subcutaneous tissue of the truncus (52%), followed by the lower limb (27%), the head and neck (11%) and the upper limb (10%). In total, 12.3% received combination treatments.
 |
Table 1 Characteristics of Patients with Advanced Melanoma Overall and According to Year of Diagnosis and for Age-, Sex-, and Index Date-Matched General Population Comparison Cohort |
For each melanoma patient, we identified 100 matched persons from the general population resulting in a comparison cohort of 123,600 persons. The general population comparison cohort was well matched on age, sex, and calendar year of melanoma diagnosis (index year) to the melanoma cohort. However, the melanoma cohort had higher comorbidity burden measured with CCI than the general population comparison cohort (Table 1). Thus, the prevalence of CCI medium and high was 18.3% in the melanoma cohort compared to 12.7% in the comparison cohort. The melanoma cohort had higher prevalence of congestive heart failure, connective tissue diseases, diabetes, other cancers, and obesity (Supplementary Table 1).
During the 10 year follow-up period, mortality risk was 74% among melanoma patients (Figure 1). Most patients died in the period between 31–364 days and between one and five years of melanoma diagnosis, 33.9% and 45.7%, respectively. The standardized mortality rate during the 10 years of follow-up was 260.6 per 1000 person-years, almost two-fold higher in the period 0–30 days and 31–364 days of diagnosis; 414.5 per 1000 person-years and 482.5 per 1000 person-years, respectively, compared with standardized mortality rates seen in the follow-up period after 364 days (Table 2).
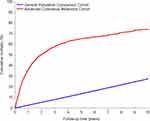 |
Figure 1 Cumulative mortality of advanced cutaneous melanoma patients and the matched general population cohort. |
Compared with the matched cohort from the general population, patients with advanced melanoma had a 10.4-fold increased HR of all-cause death during the 10 years of follow-up. We found a 12.1-fold and 18.8-fold increased hazard of death in advanced melanoma patients compared with persons from the general population during the follow-up time from 0–30 days and 31–364 days. The HRs decreased after 364 days to 10 years.
Standardized mortality rates were particularly high within 364 days of melanoma diagnosis for patients diagnosed in the period 1980–1991 and 1992–2003 – 1102.4 per 1000 person-years in 0–30 days and 1012.5 per 1000 person-years in 31–364 days for patients diagnosed during 1980–1991, and 653.2 per 1000 person-years in 0–30 days and 1015.5 per 1000 person-years in 31–364 days for patients diagnosed during 1992–2003.
No systematic improvements in long-term mortality were observed in the most recent time periods, ie, the standardized mortality rates were 88.5 per 1000 person-years in 0–30 days and 238.5 per 1000 person-years in 31–364 days for patients diagnosed during 2004–2007 compared with 74.3 per 1000 person-years in 0–30 days and 248.4 per 1000 person-years in 31–364 days for patients diagnosed during 2008–2011 (Table 3). The lack of improvement during the study period was also confirmed when stratifying according to stage (Supplementary Table 2).
Stratification on age showed variation in both standardized mortality rates and adjusted HRs by age (Supplementary Table 3). Thus, standardized mortality rates were lower in younger patients compared to older melanoma patients, whereas adjusted HRs were considerably higher in younger melanoma patients (adjusted HR was 60.9 (CI: 47.9–77.3) in group of 18–44 years and 4.3 (CI: 3.7–4.9) in group of 75+ years) compared to matched comparison cohort from the general population. Sex did not modify the association.
Discussion
Using nationwide population-based registries with long and complete follow-up, we found that short- and long-term mortality rates have dropped since the 1980s among patients with advanced melanoma but remain very high compared with the general population. Furthermore, the improvement seems to have leveled off in the most recent years of the study period, for patients diagnosed in 2004–2007 and 2008–2011.
A few studies among European cutaneous melanoma patients have previously examined time trends in mortality and found improved survival over the last decades.3,9,12,13,21 Tryggvadottir et al used the NORDCAN database to examine relative survival and excess mortality of patients diagnosed with cutaneous melanoma in the Nordic countries 1964–2003 and followed-up to the end of 2006.3 The authors found that the mortality doubled, while overall relative survival improved markedly during the study period. Hunter et al identified patients registered with cutaneous melanoma in the Northern Ireland Cancer Registry between 1984–2009.21 Five-year observed survival improved significantly from 70.5% in 1984–1988 to 82.7% in 2004–2009. When adjusting for mortality changes in the general population, five-year cause-specific survival increased from 83.6% in 1984–1988 to 89.0% in 1999–2003 after which it decreased slightly in 2004–2009 to 88.0%. Recently, Bay et al studied all skin melanoma patients registered in the DCR in 1989–2011 (n = 27,010) and performed follow-up through 2013.9 From 1989–1993 to 2009–2011, the five-year relative survival increased 12% and 6% percentage points for male and female patients, respectively. In addition, Rockberg et al examined stage-specific survival among 3554 patients diagnosed in Stockholm County Council during 2005–2012, ie, also before the wide introduction of targeted therapy. The unadjusted five-year survival varied from 91.4% for stage I to 24.6% for stage IV patients.8 Moreover, Padrik et al examined survival trends in Estonia and found that the age-adjusted five-year relative survival ratios increased significantly from 64% in 1995–1999 to 81% in 2010–2014.12 The largest survival gains were observed among patients below 50 years, those with head and neck or trunk melanomas and those with stage III cancer.12
Still, challenges have remained on how to interpret these findings of an overall improved survival, in particular in light of the steep increase in the incidence of cutaneous melanoma over the recent decades.3,4 The improved overall survival of cutaneous melanoma patients may be explained in part by increased diagnostic activities and identification of patients at earlier stage of diagnosis.22 Indeed, Hunter et al found that the overall survival trends were no longer significant when adjusting cause-specific survival for different melanoma characteristics, including Breslow depth, site, and melanoma subtype, which indicates that changes in these characteristics were the major drivers in the observed survival improvement over time.21
Large scale population-based studies on time trends in mortality among patients with advanced melanoma have so far been particularly sparse.22 Although the trend towards lower mortality rates observed in our study may to some extent have been influenced by earlier diagnosis, this mechanism obviously plays a smaller role when restricting to patients where the first diagnosis of melanoma is one of advanced melanoma, as was the case in our study. Other factors are therefore likely to have contributed to this development, including advances in surgical and oncological treatments in general,23 and more efficient implementation of evidence-based care through the establishment of and participation in national and international melanoma study groups, dissemination of clinical guidelines, and a Danish national clinical registry for melanoma used for systematic quality improvement.24
Although our study was population-based and included a large advanced cutaneous melanoma cohort with long follow-up, it also has limitations. First, the study did not include all patients with melanoma, who at some time during the course of their disease developed advanced melanoma. In contrast, it covered all patients where the melanoma diagnosis was made at a time when the cancer had already spread to other parts of the body, typically the lungs, liver, bones, brain, abdomen, and lymph nodes. This explains the relatively low number of patients identified per year although the study covered the entire Danish population and the data validity in the DCR has been examined and found to be high in terms of both completeness and quality.15 Second, the rather strict inclusion criteria resulted in mortality estimates in subgroups with limited statistical precision. Finally, detailed data on the histopathology of the melanoma and the treatment were not available, ie, only rather crude data describing the treatment provided within the first four months after diagnosis.
It should be noted that the high proportion of patients in our study where the initial treatment was surgery mainly consisted of stage III patients. There have been a few adjuvant studies for this patient group in the past 20 years but no standard adjuvant treatment during the study period. However, during the past four to five years, the availability of a wide range of novel therapeutic agents and effective combinations has transformed the treatment of melanoma. The impact of this transformation on survival at population level is yet to be assessed, but the need for further improvements in the survival of patients with advanced melanoma is evident from our study, and data on the effectiveness and safety of the new treatments are therefore highly warranted.
Conclusion
We conclude that both short- and long-term mortality rates among advanced cutaneous melanoma patients have improved substantially from 1980 to 2013. However, the improvement appeared to have reached a plateau in the years leading up to introduction of immunotherapy. The impact of the more widespread introduction of immunotherapy on survival and targeted therapies in real-life patients with advanced melanoma is yet to be shown and should be monitored closely in the coming years.
Data Sharing Statement
Additional data are not available due to Danish legislation.
Ethics Approval and Informed Consent
The study was reported to the Danish Data Protection Agency through registration at Aarhus University (record number: AU-2016-051-000001, sequential number 818).
Consent for Publication
Corresponding author confirm, on behalf of all authors that the details of any images, videos, recordings, etc can be published.
Acknowledgments
The abstract of this paper was presented at the Society for Melanoma Research (SMR) International Congress in San Francisco, CA in November 2015 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” free available on Society for Melanoma Research 2015 Congress: Society for Melanoma Research 2015 Congress (wiley.com).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising and critically reviewing the article, gave final approval of the version to be submitted for publication, and agree to take responsibility and be accountable for the contents of the article.
Funding
This work was supported by a research grant from Bristol-Myers Squibb to and administrated by Aarhus University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158(5):495–503. doi:10.1001/jamadermatol.2022.0160
2. Nazzaro G, Passoni E, Pozzessere F, Maronese CA, Marzano AV. Dermoscopy use leads to earlier cutaneous melanoma diagnosis in terms of invasiveness and size? A single-center, retrospective experience. J Clin Med. 2022;11(16):4912. doi:10.3390/jcm11164912
3. Tryggvadottir L, Gislum M, Hakulinen T, et al. Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49(5):665–672. doi:10.3109/02841861003702528
4. Tichanek F, Forsti A, Hemminki A, Hemminki O, Hemminki K. Survival in melanoma in the nordic countries into the era of targeted and immunological therapies. Eur J Cancer. 2023;186:133–141. doi:10.1016/j.ejca.2023.03.019
5. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
6. Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther. 2010;10(11):1811–1823. doi:10.1586/era.10.170
7. Böni R, Schuster C, Nehrhoff B, Burg G. Epidemiology of skin cancer. Neuro Endocrinol Lett. 2002;23:48–51.
8. McDermott D, Lebbé C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40(9):1056–1064. doi:10.1016/j.ctrv.2014.06.012
9. Bay C, Kejs AM, Storm HH, Engholm G. Incidence and survival in patients with cutaneous melanoma by morphology, anatomical site and TNM stage: a Danish Population-based Register Study 1989–2011. Cancer Epidemiol. 2015;39(1):1–7. doi:10.1016/j.canep.2014.10.010
10. Crocetti E, Mallone S, Robsahm TE, et al. Survival of patients with skin melanoma in Europe increases further: results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2179–2190. doi:10.1016/j.ejca.2015.07.039
11. Engeland A, Haldorsen T, Tretli S, et al. Prediction of cancer mortality in the Nordic countries up to the years 2000 and 2010, on the basis of relative survival analysis. A collaborative study of the five Nordic Cancer Registries. APMIS Suppl. 1995;49:1–161.
12. Padrik P, Valter A, Valter E, Baburin A, Innos K. Trends in incidence and survival of cutaneous malignant melanoma in Estonia: a population-based study. Acta Oncol. 2017;56(1):52–58. doi:10.1080/0284186X.2016.1243804
13. Rockberg J, Amelio JM, Taylor A, Jorgensen L, Ragnhammar P, Hansson J. Epidemiology of cutaneous melanoma in Sweden-Stage-specific survival and rate of recurrence. Int J Cancer. 2016;139(12):2722–2729. doi:10.1002/ijc.30407
14. Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi:10.1007/s10654-014-9930-3
15. Storm HH. Completeness of cancer registration in Denmark 1943–1966 and efficacy of record linkage procedures. Int J Epidemiol. 1988;17(1):44–49. doi:10.1093/ije/17.1.44
16. Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish cancer Registry--history, content, quality and use. Dan Med Bull. 1997;44(5):535–539.
17. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi:10.1200/JCO.2009.23.4799
18. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
20. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. doi:10.1016/s0895-4356(02)00585-1
21. Hunter HL, Dolan OM, McMullen E, Donnelly D, Gavin A. Incidence and survival in patients with cutaneous malignant melanoma: experience in a U.K. population, 1984–2009. Br J Dermatol. 2013;168(3):676–678. doi:10.1111/bjd.12046
22. Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe - a systematic review of the literature. Clin Epidemiol. 2016;8:109–122. doi:10.2147/CLEP.S99021
23. Wouters MW, Michielin O, Bastiaannet E, et al. ECCO essential requirements for quality cancer care: melanoma. Crit Rev Oncol Hematol. 2018;122:164–178. doi:10.1016/j.critrevonc.2017.12.020
24. Ellebaek E, Svane IM, Schmidt H, et al. The Danish metastatic melanoma database (DAMMED): a nation-wide platform for quality assurance and research in real-world data on medical therapy in Danish melanoma patients. Cancer Epidemiol. 2021;73:101943. doi:10.1016/j.canep.2021.101943
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


