Back to Journals » Journal of Pain Research » Volume 17
Long-Term Follow-Up of Ultrasound-Guided Glossopharyngeal Nerve Block Treatment for Glossopharyngeal Neuralgia: A Retrospective Clinical Study of 43 Cases
Authors You S, Qin X, Tong L, Feng Z
Received 4 October 2023
Accepted for publication 20 February 2024
Published 6 March 2024 Volume 2024:17 Pages 913—921
DOI https://doi.org/10.2147/JPR.S437609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rushna Ali
Shaohua You,1,* Xiaoyan Qin,2,* Li Tong,3 Zeguo Feng1
1Department of Pain Medicine, The First Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 2Department of Clinical Laboratory, Shijingshan Teaching Hospital of Capital Medical University, Beijing Shijingshan Hospital, Beijing, 100049, People’s Republic of China; 3Department of Anesthesiology, The First Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zeguo Feng, Department of Pain Medicine, The First Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China, Email [email protected]; Li Tong, Department of Anesthesiology, The First Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China, Email [email protected]
Background: Glossopharyngeal neuralgia (GPN) is a rare chronic neuropathic pain disorder that significantly impacts quality of life. Ultrasound-guided glossopharyngeal nerve blocks (UGPNB) have gained popularity due to their various advantages. However, there have been no studies reporting the long-term outcomes of UGPNB in a larger cohort of GPN patients.
Aim: This study aims to evaluate the efficacy and safety of UGPNB in patients with GPN.
Methods: We reviewed the electronic medical records of patients with GPN who received UGPNB at the Department of Pain Medicine of the First Medical Center, PLA General Hospital between June 1, 2011, and June 1, 2022. The effect of UGPNB was evaluated using the Barrow Neurological Institute (BNI) scale. Improvement was defined as a reduction in pain category by comparing pain categories before and after therapy. Recovery was defined as achieving BNI I after treatment. Patients who responded to treatment but then regressed to the category before therapy were considered to have experienced pain relapse.
Results: A total of 43 patients with GPN who received UGPNB were included in the analysis. At discharge, 35 (81.4%) patients experienced pain improvement after treatment, and among them, 13 (30.2%) patients achieved recovery. After discharge, 13 patients (37.1%) out of the 35 effective patients experienced pain relapse at different time intervals: 0.5, 0.7, 1, 1, 3, 3, 4, 12, 15, 36, 45, 63, and 96 months. The cumulative recurrence-free survival rates were 88.85% at month 1, 82.83% at month 3, 77.04% at month 12, 70.31% at month 36, and 54.66% at month 120. Among the 13 patients who experienced relapse, four patients received a second UGPNB treatment, and pain improved in two patients (50%). No severe adverse reactions were documented.
Conclusion: UGPNB is an effective, repeatable, safe, and minimally invasive treatment for patients with GPN. It may be preferable to consider UGPNB before undergoing invasive intracranial surgery or neurodestructive methods.
Keywords: ultrasound-guided glossopharyngeal nerve block, glossopharyngeal neuralgia, efficacy, safety
Introduction
Glossopharyngeal neuralgia (GPN) is a painful condition associated with the cranial nerve (CN) IX that is characterized by unilateral, brief, and stabbing pains. These pains are typically localized in the ear, base of the tongue, tonsillar fossa, and/or beneath the angle of the jaw. They can be triggered by swallowing, talking, coughing, chewing, or yawning but may also occur spontaneously with abrupt onset and termination.1,2 Despite being a rare cause of craniofacial pain, representing only 0.2% to 1.3% of facial pain syndromes, GPN has an estimated overall incidence of 0.2 cases per 100,000 people each year3,4 significantly impacting both the quality of life and socioeconomic functioning of patients.5 The International Classification of Headache Disorders, 3rd edition, categorizes GPN into classical, secondary, and idiopathic types,1 with classical GPN often being attributed to neurovascular compression, secondary GPN resulting from an underlying disease, and idiopathic GPN having an unknown etiology. In rare cases, GPN can present in conjunction with vagal symptoms such as cough, hoarseness, syncope, and/or bradycardia—a condition some authors have termed vagoglossopharyngeal neuralgia.6,7
Current first-line medical treatments for GPN include carbamazepine or oxcarbazepine, with their side effects being the primary reason for treatment discontinuation.8,9 In scenarios where oral medications are not suitable, microvascular decompression (MVD) is regarded as a viable treatment choice for classical GPN,5,10,11 although it comes with potential complications such as hearing loss, dystaxia, and cerebrospinal fluid leakage.5,10 If exploratory surgery fails to identify a causative vessel, sectioning cranial nerve IX along with the upper rootlets of cranial nerve X may be considered12, risking adverse outcomes like dysphagia and vocal cord paralysis.13 For patients who cannot tolerate, or choose not to undertake, invasive treatments, and for those with classical or idiopathic GPN where conservative therapies have failed, minimally invasive options such as Gamma Knife radiosurgery (GKR)14,15, radiofrequency thermocoagulation (RFT)16 and pulsed radiofrequency (PRF),17 could be offered. Although these interventions are less invasive and relatively simple to perform, they still pose risks including larynx numbness, dysesthesia, dysphagia, and choking coughs. These interventional treatments are minimally invasive, and relatively simple to be performed. However, all these minimally invasive approaches present risks of larynx numbness, dysesthesia, dysphagia, and choking coughs. Currently, there is no ideal treatment for GPN. The key is to develop a treatment that is easy, safe, nondestructive, repeatable, and has a fairly satisfactory rate of pain relief.
Ultrasound-guided nerve blocks have gained popularity compared to landmark techniques due to various advantages. These include real-time visualization of the passage of the block needle, visualization of vessels, and the requirement of a lesser volume of local anesthetics.18 In a retrospective study conducted by Liu et al, involving 12 patients with GPN, the effective rates of ultrasound-guided glossopharyngeal nerve blocks (UGPNB) were found to be 83.3% at discharge, 83.3% at 6 months, 58.3% at 1 year, and 33.3% at 18 months, respectively.19 In a prospective study by Preet et al, with a small sample size, it was reported that patients with GPN who underwent UGPNB experienced significantly lower pain intensities and reduced interference with their quality of life. However, by the end of 90 days, the numerical pain scale scores were statistically similar between the control and intervention groups.20 However, there is currently no study reporting the long-term outcomes of UGPNB in a larger cohort of patients with GPN. Therefore, we conducted a retrospective analysis of data on GPN patients who underwent UGPNB between June 2011 and June 2022. Our objective was to determine the initial efficacy and safety of UGPNB, as well as its long-term efficacy.
Methods
Patients
We conducted a review of the electronic medical records of patients diagnosed with GPN who underwent UGPNB treatment at the Department of Pain Medicine, First Medical Center, PLA General Hospital, between June 1, 2011, and June 1, 2022. These data were exclusively collected for this study and have not been previously published or utilized in any other research. Approval for this study was granted by the Ethics Committee of the First Medical Center, PLA General Hospital, with informed consent waived. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki (WMA Declaration of Helsinki, 2013). The study posed minimal risk, and all individuals’ information was anonymized. A single-group retrospective pretest and posttest design was employed in this study.
The diagnosis of GPN was based on the International Classification of Headache Disorders (ICHD)-3 beta criteria.1 All these patients underwent a preoperative evaluation, including maxillofacial region CT of and brain magnetic resonance imaging (MRI). The demographic information of patients was depicted in Table 1.
 |
Table 1 Demographic Data of Patients in the Study |
The inclusion criteria were 1) received UGPNB treatment in our center; 2) Diagnosed with GPN in accordance with the International Classification of Headache Disorders 3rd edition. The exclusion criteria were 1) history of operation for GPN such as MVD, GKR, RFT, PRF; 2) patients with abnormal coagulation function; 3) patients with local anesthetic allergy; 4) Mental illness evaluated by a psychiatrist; 5) Incomplete medical records. General and clinical information of the patients is shown in Supplementary Table 1.
Ultrasound-Guided Glossopharyngeal Nerve Block
Before the surgery, the patient signs an informed consent form. The patient is placed in a supine position with the head turned in the opposite direction to maintain neck extension. The skin on one side of the neck is sterilized, and a linear ultrasound probe (WISONIC, linear probe 4–15 MHz) is placed vertically on the skin for transverse scanning of the neck. The neck scan starts from the base to identify the common carotid artery (CCA) and internal jugular vein (IJV), followed by confirmation using color Doppler. The CCA is traced upwards, identifying the bifurcation of the internal carotid artery (ICA) and external carotid artery (ECA). Subsequently, the ICA is traced to the submandibular area. A 25-gauge needle with a length of 35 mm is then used for ultrasound-guided lateral puncture. The needle is advanced to the posterior position of the ICA, and the syringe is aspirated to verify proper placement within the vessel (Figure 1). If the ICA or needle trajectory within the vessel is confirmed, hydrodissection is used to separate the vessel. A solution of 0.2% ropivacaine (3–5 mL) is injected into the posterior region behind the ICA. Severe adverse reactions such as local anesthetic toxicity or injection of drugs into the bloodstream should be immediately addressed during or after the procedure, while milder reactions such as hoarseness should be observed. The patient receives injections every other day,21 and the treatment response is evaluated the next morning. Once the patient achieves satisfactory sedation or if the treatment response is unsatisfactory, the treatment can be discontinued and the patient can be discharged. In the first injection, 2 mg of betamethasone sodium phosphate and 5 mg of dexamethasone disodium phosphate are added to the 0.2% ropivacaine solution.
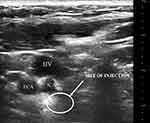 |
Figure 1 Ultrasound image of the neck demonstrating ICA, ECA and IJV at submandibular region. Abbreviations: ICA, internal carotid artery; ECA, external carotid artery; IJV, internal jugular vein. |
Definitions of Outcome
The treatment effect was evaluated using the Barrow Neurological Institute scale (BNI) which includes the following classifications: class I - no pain and no medication, class II - occasional pain that does not require medication, class IIIa - no pain but continued medication, class IIIb - pain controlled with medication, class IV - some pain that is not adequately controlled with medication, and class V - severe pain with no relief.14,22 Improvement was defined as a reduction in pain category when comparing the pain categories before and after the therapy. Recovery was defined as achieving BNI class I after the treatment. Patients who initially responded to treatment but later returned to their previous pain category were considered to have experienced pain relapse.
Data Collection
All eligible patients were enrolled based on the predetermined inclusion and exclusion criteria. Baseline demographic characteristics of each patient, including gender, age, pain distribution side, disease duration, baseline BNI pain intensity classification, analgesic dosage, and presence of concurrent diseases, were collected. The number of UGPNB, the BNI pain intensity classification after treatment, as well as perioperative complications and side effects such as neck hematoma, dysphagia, vocal cord paralysis, larynx numbness, dysesthesia, dysphagia, and coughing, were also recorded. To ensure high medical quality, regular follow-ups were conducted via telephone, and all data were meticulously documented in electronic records.
Statistical Analysis
Prior to the study, no statistical power calculation was conducted and sample sizes were based on the available data. All data manipulation and statistical analysis were performed using SPSS software (SPSS version 23.0, SPSS Inc.). The Shapiro–Wilk test was used to determine the normality of data distribution before statistical analysis. Continuous data that followed a normal distribution were described as mean ± standard deviation (SD), while non-normally distributed data were expressed as median (interquartile range). A Kaplan-Meier plot was utilized to present recurrence-free survival curves. A value of P < 0.05 was considered to indicate a statistically significant difference.
Results
Baseline Characteristics
Between 1 June 2011 and 1 June 2022, a total of 44 consecutive patients with GPN underwent UGPNB treatment at the department of Pain Medicine of the First Medical Center, PLA General Hospital. One patient was excluded from this study according to the exclusion criteria because of incomplete medical record. Thus, a total of 23 males and 20 females were included, with a median age of 58 years old (IQR: 52–68, range 38–84). At admission, the median (IQR) duration of pain in these patients was 6 (12, 25) months. In all, 22 patients had left GPN, and 21 had right GPN. Prior to UGPNB, all patients had BNI scores greater than IIIa. 16 patients were accompanied by more than one underlying disease, mostly hypertension and diabetes. All the patients were using one or more drugs, mostly carbamazepine or oxcarbazepine. The follow-up duration ranged from 3 to 144 months, with a median length of 70 months (IQR, 39, 119). During the period of follow-up, 1 patient died due to esophageal varices and 1 patient were lost owing to lack of telephone contact. Demographic information is displayed in Table 1.
Primary Outcome
Forty-three patients completed a total of 107 UGPNB treatments (range: 1–8). At the time of hospital discharge, 35 patients (81.4%) reported an improvement of pain after treatment, and among them, 13 patients (30.2%) completely recovered (BNI I). Eight patients (18.6%) did not respond to UGPNB treatment. Among these, 6 patients recovered after undergoing MVD, 1 patient recovered after receiving acupuncture, and 1 patient’s condition improved after oral administration of traditional Chinese medicine (Supplementary Table 2).
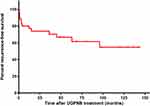
Thirteen out of the 35 patients (37.1%) who experienced pain relief upon treatment, reported pain relapse at various timepoints, including at month 0.5, 0.8, 1, 1, 3, 3, 4, 12, 15, 36, 45, 63, and 96 (Supplementary Table 2). The cumulative recurrence-free survival of the 35 effective patients after discharge is shown in a Kaplan-Meier actuarial curve in Figure 2. The cumulative recurrence-free survival rates after discharge were 88.85% at month 1, 82.83% at month 3, 77.04% at month 12, 70.31% at month 36, and 54.66% at month 120. The median follow-up time of the 35 effective patients was 48 months (IQR, 12 months–96 months), with an unreached median recurrence-free time.
Among the 13 patients with recurrent pain, 4 patients recovered after undergoing MVD treatment, 1 patient showed improvement after MVD treatment, 3 patients improved after RFT, 1 patient recovered after PRF, and 4 patients chose to undergo a second UGPNB treatment. Among these patients who received a second UGPNB treatment, 2 reported satisfactory pain relief. One patient who did not respond to the second UGPNB treatment sought MVD treatment and eventually recovered, while another patient failed to follow up due to a lack of telephone contact (as shown in Figure 3). Treatment outcomes are presented in Supplementary Table 3.
Complications
Five patients (11.6%) experienced temporary hoarseness, and 3 patients (6.98%) had difficulty with drinking and swallowing. No specific treatment was administered for these adverse reactions. However, by the following day, the adverse reactions had significantly improved, and by the third day, they had completely disappeared without any lasting effects. Other patients did not report any significant adverse reactions during the treatment period. Details of the treatment complications are provided in Supplementary Table 2.
Discussion
This retrospective study reports on the clinical characteristics, efficacy, and safety of UGPNB treatment in 43 patients with GPN between June 1, 2011 and June 1, 2022. At discharge, 81.4% of patients showed improvement after treatment, and 30.2% of patients fully recovered. However, after discharge, 37.1% of patients experienced pain relapse within months. Among the 13 patients with recurrent pain, 4 chose to undergo a second UGPNB treatment, and 2 of these patients (50%) experienced satisfactory pain relief. No serious adverse reactions were observed or documented during the study.
GPN occurs mainly in adults, with a higher incidence in females and individuals over the age of 50,23,24 which aligns with the findings of our study. In a study by Liu et al18, the efficacy of UGPNB in 12 GPN patients was reported, with an effective rate of 83.3% at discharge, 83.3% at 6 months, 58.3% at 1 year, and 33.3% at 18 months. The pain relief observed at discharge and within one year was similar to our results. However, it is important to note that differences in study population, underlying diseases, environmental factors, and sample size may have influenced the variation in pain relief rates after 1 year. The initial pain relief rate of UGPNB treatment was also comparable to the success rate of RFT treatment, which ranged from 78.8% to 82.1% for GPN.16,24 Another study conducted by Jia et al demonstrated that PRF could provide early pain relief with an efficacy rate of 93.3%.25 Overall, these studies concluded that minimally invasive treatments such as RFT, and PRF are effective in reducing GPN-related pain. However, these treatments can have adverse effects, such as hoarseness and difficulties with drinking and swallowing. It is worth noting that the duration of adverse reactions caused by UGPNB is significantly shorter compared to RFT and PRF. The adverse reactions caused by RFT and PRF can last for 2–3 weeks and 12.9 ± 5.1 weeks, respectively, significantly impacting patients’ quality of life.16,25 In our study, the duration of adverse reactions caused by UGPNB was only 1–2 days, minimizing the impact on patients’ quality of life.
In 1970, Laha and Jannetta effectively employed the use of a microscope to perform MVD as a therapeutic intervention for trigeminal neuralgia (TN) and hemifacial spasm (HFS).26 Since then, MVD has unequivocally emerged as the preferred treatment modality for these particular ailments.27 In the context of a literature review, the documented range of pain relief rates achieved through MVD treatment for GPN varied from 50% to 100%. Nevertheless, the author posits that these findings appear to be notably impacted by variables such as the quality of equipment utilized, the proficiency of the surgeon, and the implementation of neurophysiological monitoring during the surgical procedure.28 Additionally, in our study, 52.3% of patients who experienced no relief or a recurrence of pain after UGPNB opted for MVD, which resulted in high levels of pain relief (Figure 2), consistent with findings reported by Teton et al.10 This could be attributed to the fact that the MVD surgeries conducted in this study were exclusively carried out within the neurosurgery department of various esteemed tertiary hospitals situated in Beijing, China, renowned for their exceptional medical infrastructure. However, the surgical risks associated with MVD encompass the potential for injury to the penetrating artery, vasospasm, and angulation. These risks have the potential to give rise to more severe short-term complications that pose a threat to life, such as postoperative ischemia and bleeding.29 Based on our follow-up, most patients are hesitant to choose MVD due to the perception that craniotomy is complex and expensive. Despite the relatively lower efficacy of UGPNB in providing pain relief when compared to MVD, its inherent safety and simplicity as a surgical procedure suggest its potential as a viable first-line treatment approach for GPN.
The present study also proposes that 13 out of 35 effective patients (37.1%) experienced pain relapse at various time points after discharge: 0.5, 0.7, 1, 1, 3, 3, 4, 12, 15, 36, 45, 63, and 96 months. Therefore, it can be speculated that effective patients are more likely to experience recurrence within 3 months, and the likelihood of recurrence significantly reduces after this period. However, no similar findings were observed in the other two retrospective studies on PRF and RFT treatment of GPN.16,25 Hence, this speculation requires confirmation through larger prospective research samples.
In this study, a total of 13 patients (30.2%) experienced pain recurrence, and out of those, 4 patients (30.8%) were willing to undergo a second UGPNB treatment. Among the four patients, two (50.0%) reported satisfactory pain relief after the second procedure. These results indicate that although there is a certain recurrence rate after UGPNB treatment, patients can still opt for UGPNB again in case of recurrence, and for some patients, the treatment can still be effective. Therefore, UGPNB demonstrates the characteristic of being repeatable.
The glossopharyngeal nerve exits the brain through the jugular foramen, alongside the vagus nerve and accessory nerve. After leaving the jugular foramen, the glossopharyngeal nerve descends with the vagus nerve, accessory nerve, internal jugular vein (IJV), and internal carotid artery (ICA). When administering a GPN block, the close proximity of the glossopharyngeal nerve and vital blood vessels poses an inherent risk of injury or inadvertent injection into the IJV and ICA. Consequently, the risks of vessel puncture with local anesthetic intoxication, hematoma formation, and even upper airway obstruction should be carefully considered when performing UGPNB. Furthermore, a GPN block may lead to an inadvertent block of the closely adjacent vagus nerve, making the bilateral use of this technique prohibitively risky.30
Ultrasound can provide better visualization, resulting in more accurate blocks with higher success rates, lower incidence of neuropathy or accidental vessel puncture, shorter procedure times, and increased patient satisfaction. Compared to CT and C-arm X-ray, ultrasound is readily available at bedside in most modern facilities, without the need for ionizing radiation. Additionally, physicians are becoming increasingly comfortable and confident in their use of ultrasound.31 An ultrasound-guided block of the distal branch of the glossopharyngeal nerve in the parapharyngeal space is commonly used to treat oral and lingual diseases or pain resulting from surgery.32,33 However, if the block is positioned lower than the glossopharyngeal nerve trunk, the ultrasound-guided parapharyngeal block may be less effective in treating classical GPN conditions. For ultrasound-guided glossopharyngeal nerve trunk blocks, the styloid process often serves as a localization marker. The small acoustic window and the presence of bones in close proximity can obstruct the view during ultrasound imaging. To overcome this, we propose using the midpoint of the line connecting the mastoid and mandibular angle as the puncture point, with the posterior ICA as the target area for blocking the nerve trunk of the glossopharyngeal nerve.18 This puncture method allows for clear identification of the internal carotid artery and vein using color Doppler imaging. Real-time ultrasound monitoring enables dynamic observation of the drug diffusion range, allowing for timely adjustment of the needle position and improvement in the success rate of blocking.
There were several strengths to this study. First, based on our knowledge, this retrospective study reports the largest sample size and longest follow-up of patients with GPN undergoing UGPNB treatment. Second, the inclusion of long-term follow-up is essential. To minimize the rates of loss to follow-up, we recorded the phone numbers of patients and multiple family members. As a result, the rate of lost to follow-up in this study was low, allowing for a more accurate assessment of the efficacy and safety of UGPNB in patients with GPN.
Limitation
This study is subject to several limitations. First, it shares the inherent limitations of retrospective studies. Additionally, due to the low incidence of GPN, the cohort size was small, and there was a lack of a control arm or parallel comparison group. Furthermore, our study did not include a stratified analysis on the effect of UGPNB treatment for classic and idiopathic GPN, nor did it account for population stratification. In light of these limitations, we look forward to future randomized controlled studies with a larger sample size and a longer follow-up period to confirm these findings.
Conclusion
UGPNB is an effective, repeatable, safe, and minimally invasive treatment for GPN patients, possibly making it a preferable treatment to invasive intracranial surgery or neurodestructive methods.
Data Sharing Statement
All data extracted or analyzed during this study are included in this article and its Supplementary Information.
Acknowledgments
We thank Xiaoyan Qin for polishing the English.
Disclosures
The authors declare no conflicts of interest in this work.
References
1. Arnold M. Headache classification committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
2. Wu L, Xiong J, Huang Y, et al. Case report: trigeminal neuralgia misdiagnosed as glossopharyngeal neuralgia. Front Neurol. 2023;14:1079914. doi:10.3389/fneur.2023.1079914
3. Ma Y, Li Y-F, Wang Q-C, et al. Neurosurgical treatment of glossopharyngeal neuralgia: analysis of 103 cases. J Neurosurg. 2016;124(4):1088–1092. doi:10.3171/2015.3.JNS141806
4. Han A, Montgomery C, Zamora A, et al. Glossopharyngeal neuralgia: epidemiology, risk factors, pathophysiology, differential diagnosis, and treatment options. Health Psychol Res. 2022;10(3):36042. doi:10.52965/001c.36042
5. Du T, Ni B, Shu W, et al. Neurosurgical choice for glossopharyngeal neuralgia: a benefit-harm assessment of long-term quality of life. Neurosurgery. 2020;88(1):131–139. doi:10.1093/neuros/nyaa325
6. Burfield L, Ahmad F, Adams J. Glossopharyngeal neuralgia associated with cardiac syncope. BMJ Case Rep. 2016;2016. doi:10.1136/bcr-2015-214104
7. Chalissery AJ, Gaughan M, Haughton G, et al. Teaching video neuroimages: vagoglossopharyngeal neuralgia mimicking a seizure. Neurology. 2018;90(13):e1179. doi:10.1212/WNL.0000000000005211
8. Blumenfeld A, Nikolskaya G. Glossopharyngeal neuralgia. Curr Pain Headache Rep. 2013;17(7):343. doi:10.1007/s11916-013-0343-x
9. Nishie H, Sakuta Y, Nakatsuka H. A case of glossopharyngeal neuralgia successfully treated with levetiracetam. JA Clin Rep. 2023;9(1):5. doi:10.1186/s40981-023-00596-x
10. Teton ZE, Holste KG, Hardaway FA, et al. Pain-free survival after vagoglossopharyngeal complex sectioning with or without microvascular decompression in glossopharyngeal neuralgia. J Neurosurg. 2019;132(1):232–238. doi:10.3171/2018.8.JNS18239
11. Zheng W, Zhao P, Song H, et al. Prognostic factors for long-term outcomes of microvascular decompression in the treatment of glossopharyngeal neuralgia: a retrospective analysis of 97 patients. J Neurosurg. 2021;2021;1–8.
12. Ordonez-Rubiano EG, Garcia-Chingate CC, Rodriguez-Vargas S, et al. Microvascular decompression for a patient with a glossopharyngeal neuralgia: a technical note. Cureus. 2017;9(7):e1494. doi:10.7759/cureus.1494
13. Ni B, Hu Y, Du T, et al. Reoperation after failed microvascular decompression for glossopharyngeal neuralgia. Acta Neurochir. 2020;162(11):2783–2789. doi:10.1007/s00701-020-04383-w
14. Pommier B, Touzet G, Lucas C, et al. Glossopharyngeal neuralgia treated by Gamma Knife radiosurgery: safety and efficacy through long-term follow-up. J Neurosurg. 2018;128(5):1372–1379. doi:10.3171/2017.3.JNS162542
15. Kano H, Urgosik D, Liscak R, et al. Stereotactic radiosurgery for idiopathic glossopharyngeal neuralgia: an international multicenter study. J Neurosurg. 2016;125(Suppl 1):147–153. doi:10.3171/2016.7.GKS161523
16. Song L, He L, Pei Q, et al. CT-guided percutaneous radiofrequency thermocoagulation for glossopharyngeal neuralgia: a retrospective clinical study of 117 cases. Clin Neurol Neurosurg. 2019;178:42–45. doi:10.1016/j.clineuro.2019.01.013
17. Zhu Q, Wang S, Chen R, et al. Continuous radiofrequency thermocoagulation under CT-guidance for glossopharyngeal neuralgia: two case reports. Medicine. 2018;97(24):e11079. doi:10.1097/MD.0000000000011079
18. Punj J, Sundaram S. Ultrasound-guided glossopharyngeal nerve block: description of a new technique. J Anaesthesiol Clin Pharmacol. 2021;37(3):483–485. doi:10.4103/joacp.JOACP_138_19
19. Liu Q, Zhong Q, Tang G, et al. Ultrasound-guided glossopharyngeal nerve block via the styloid process for glossopharyngeal neuralgia: a retrospective study. J Pain Res. 2019;12:2503–2510. doi:10.2147/JPR.S214596
20. Singh PM, Dehran M, Mohan VK, et al. Analgesic efficacy and safety of medical therapy alone vs combined medical therapy and extraoral glossopharyngeal nerve block in glossopharyngeal neuralgia. Pain Med. 2013;14(1):93–102. doi:10.1111/pme.12001
21. Ma Y, Li B, Sun L, et al. A prospective randomized comparison of the efficacy of standard antiviral therapy versus ultrasound-guided thoracic paravertebral block for acute herpes zoster. Ann Med. 2022;54(1):369–378. doi:10.1080/07853890.2022.2031267
22. Rogers CL, Shetter AG, Fiedler JA, et al. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013–1019. doi:10.1016/S0360-3016(00)00513-7
23. Rozen TD. Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin. 2004;22(1):185–206. doi:10.1016/S0733-8619(03)00094-X
24. Wang X, Tang Y, Zeng Y, et al. Long-term outcomes of percutaneous radiofrequency thermocoagulation for glossopharyngeal neuralgia: a retrospective observational study. Medicine. 2016;95(48):e5530. doi:10.1097/MD.0000000000005530
25. Jia Y, Shrestha N, Wang X, et al. The long-term outcome of CT-guided pulsed radiofrequency in the treatment of idiopathic glossopharyngeal neuralgia: a retrospective multi-center case series. J Pain Res. 2020;13:2093–2102. doi:10.2147/JPR.S259994
26. Laha RK, Jannetta PJ. Glossopharyngeal neuralgia. J Neurosurg. 1977;47(3):316–320. doi:10.3171/jns.1977.47.3.0316
27. Park JS, Ahn YH. Glossopharyngeal neuralgia. J Korean Neurosurg Soc. 2023;66(1):12–23. doi:10.3340/jkns.2022.0178
28. Chen J, Sindou M. Vago-glossopharyngeal neuralgia: a literature review of neurosurgical experience. Acta Neurochirurgica. 2014;157(2):311–321. doi:10.1007/s00701-014-2302-7
29. Wang L, Liu Q, Dong X, et al. Comparative analysis of MVD and RHZ in the treatment of primary glossopharyngeal neuralgia: a clinical report on 61 cases. Front Neurol. 2023;14:1.
30. Azman J, Stopar Pintaric T, Cvetko E, et al. Ultrasound-guided glossopharyngeal nerve block: a Cadaver and a Volunteer Sonoanatomy Study. Reg Anesth Pain Med. 2017;42(2):252–258. doi:10.1097/AAP.0000000000000561
31. Nagdev A, Dreyfuss A, Martin D, et al. Principles of safety for ultrasound-guided single injection blocks in the emergency department. Am J Emerg Med. 2019;37(6):1160–1164. doi:10.1016/j.ajem.2019.03.045
32. Sirohiya P, Kumar V, Yadav P, et al. Ultrasound-guided glossopharyngeal nerve block at pharyngeal wall level in a patient with carcinoma tongue. Indian J Palliat Care. 2020;26(1):140–141. doi:10.4103/IJPC.IJPC_132_19
33. Ahmed SA, Omara AF. The effect of glossopharyngeal nerve block on post-tonsillectomy pain of children; randomized controlled trial. Anesth Pain Med. 2019;9(2):e90854. doi:10.5812/aapm.90854
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.