Back to Journals » Cancer Management and Research » Volume 12
Long Non-Coding RNA TMPO-AS1 Promotes Cervical Cancer Cell Proliferation, Migration, and Invasion by Regulating miR-143-3p/ZEB1 Axis
Authors Gang X, Yuan M, Zhang J
Received 7 August 2019
Accepted for publication 22 December 2019
Published 3 March 2020 Volume 2020:12 Pages 1587—1599
DOI https://doi.org/10.2147/CMAR.S226409
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Xiaoqing Gang, Mengmeng Yuan, Juxin Zhang
Department of Gynecology, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, Henan, People’s Republic of China
Correspondence: Juxin Zhang
Department of Gynecology, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, No. 7 Weiwu Road, Jinshui District, Zhengzhou, Henan 450003, People’s Republic of China
Tel +86-138 3818 4667
Email [email protected]
Objective: Long non-coding RNAs (lncRNAs) have been identified as important players in tumorigenesis. LncRNA TMPO antisense RNA 1 (TMPO-AS1) has been shown to be involved in several tumors. However, the functional role and the underlying mechanism of TMPO-AS1 in regulating cervical cancer cell behavior remain unclear.
Materials and Methods: Expression of TMPO-AS1, miR-143-3p, and ZEB1 were examined by qRT-PCR and Western blot. Cell proliferation, migration and invasion were evaluated using CCK-8 assay and Transwell migration and invasion assays, respectively. Luciferase reporter assay was performed to investigate the interaction miR-143-3p and TMPO-AS1 or ZEB1.
Results: TMPO-AS1 was highly expressed in cervical cancer cells. Furthermore, TMPO-AS1 overexpression significantly promoted C-33A cell proliferation, migration, and invasion. In contrast, TMPO-AS1 silencing inhibited SiHa cell proliferation, migration, and invasion. Mechanistically, TMPO-AS1 acted as a sponge of miR-143-3p to elevate expression of zinc finger E-box binding homeobox 1 (ZEB1), a target of miR-143-3p, and thereby promoted C-33A cell proliferation, migration, and invasion. Further assays showed that TMPO-AS1 knockdown inhibited cervical cancer cell tumorigenesis in vivo.
Conclusion: TMPO-AS1 promotes cervical cancer cell proliferation, migration, and invasion by regulating the miR-143-3p/ZEB1 axis.
Keywords: cervical cancer, TMPO-AS1, miR-143-3p, ZEB1
Introduction
Cervical cancer contributes to the second-highest number of deaths in female cancers with high risks of morbidity and mortality.1 Currently, the diagnosis and therapy of cervical cancer are still challenging. Therefore, searching for the ideal tumor markers and therapeutic targets is urgently needed to improve the diagnosis and treatment of cervical cancer.
Long non-coding RNAs (lncRNAs) are commonly defined as non-protein-coding RNA transcripts with lengths exceeding 200 nucleotides and have emerged as pivotal regulators in various cellular processes.2 Considerable evidence indicates that lncRNAs are deregulated and may exhibit tumor-suppressive and oncogenic functions.3 With regard to cervical cancer, increasing data have highlighted the importance of lncRNAs in cervical cancer in terms of prognosis and tumor progression, invasion and metastasis, apoptosis, and radio-resistance.1,4
TMPO antisense RNA 1 (TMPO-AS1), a lncRNA located at chromosome 12, have been recently shown to be involved in several cancers. Huang et al5 reported that TMPO-AS1 was upregulated in PCa tissue samples and promoted PCa cell proliferation and migration. TMPO-AS1 could also affect the prognosis of patients with lung adenocarcinoma through regulating cell cycle and cell adhesion.6 With regard to cervical cancer, Liu et al7 showed that TMPO-AS1 was highly expressed in the cervical cancer tissues when compared with the adjacent tissues according to chip analysis. However, the expression of TMPO-AS1 in cervical cancer cells and its exact role in cervical cancer remain unclear.
Extensive studies have shown that lncRNAs exert roles by acting as competitive endogenous RNAs (ceRNAs) to segregate microRNAs (miRNAs) away from target mRNAs.8 Our bioinformatics analysis revealed that TMPO-AS1 harbors putative binding sites of miR-143-3p. Existing evidence showed that miR-143-3p was lowly expressed in cervical cancer tissues and cell lines and exerted tumor-suppressive effects in cervical cancer.9–11 Furthermore, our bioinformatics analysis showed that zinc finger E‑box binding homeobox 1 (ZEB1) harbors a miR-143-3p binding sequence in the 3ʹ-untranslated region (3ʹ-UTR) of its mRNA. ZEB1 is generally believed to exert intrinsic oncogenic functions and foster cancer cell migration, invasion, and metastasis.12 Thus, in this study, we tested the hypothesis that TMPO-AS1 elevates ZEB1 expression by competitively sponging miR-143-3p, and thereby affects cervical cancer cell behaviors.
Materials and Methods
Cell Lines and Culture
Four cervical cancer cell lines (HeLa, SiHa, CaSki, C-33A) and human papillomavirus (HPV)-immortalized cervical epithelial cells (H8) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin in humidified air at 37°C with 5% CO2.
Cell Transfection
To overexpress TMPO-AS1, the full-length TMPO-AS1 was cloned into pcDNA 3.1 (Invitrogen, Carlsbad, CA USA), generating pcDNA3.1-TMPO-AS1, with an empty pcDNA3.1 vector used as a control. The specific siRNAs for TMPO-AS1 and ZEB1 (si-TMPO-AS1 and si-ZEB1), scramble siRNA control, miR‐143-3p mimic, miR‐143-3p inhibitor and their negative controls (mimic NC and inhibitor NC) were synthesized and purchased from GenePharma (Shanghai, China). The si-TMPO-AS1, si-ZEB1, and scramble control were used at a final concentration of 50 nM. The sequences of these siRNAs were as follows: si-TMPO-AS1: sense, 5ʹ-GAGCCGAACUACGAACCAATT-3ʹ and antisense, 5ʹ-UUGGUUCGUAGUUCGGCUCTT-3ʹ; si-ZEB1: sense, 5ʹ-UGCAUUUGCAGAUUGAGGCUGAUCA-3ʹ and antisense, 5ʹ-UGAUCAGCCUCAAUCUGCAAAUGCA-3; a scrambled siRNA negative control: sense, 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ and antisense, 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ. C-33A or SiHa cells were transfected with these siRNAs, miRNAs, or plasmids using Lipofectamine 3000 (Invitrogen) according to the instructions. Following 48 h of transfection, cells were harvested for quantitative real-time PCR (qRT-PCR) analysis to examine the knockdown or overexpression efficiencies.
Cell Viability Assay
Cell viability was determined using the CCK-8 kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Briefly, following transfection, C-33A or SiHa cells were harvested and seeded into 96-well plates at a density of 5×103 cells/well. After 48 h of incubation, 10 μL of CCK-8 solution was added to each well. Following incubation for 4 h at 37°C, the optical density (OD) values at 450 nm were detected by an enzyme-labeled analyzer and were normalized to control well.
Transwell Migration and Invasion Assay
Following transfection, cells were suspended in 200 μL of serum-free medium and added into the upper chambers of a 24-well Transwell plate with 8.0 μm pore which was pre-coated with Matrigel membrane. Subsequently, 500 μL of complete medium containing 10% FBS was added into the lower chambers as a chemoattractant. Following 24 h of incubation, the cells in the upper layer of the membrane were gently wiped off with a cotton swab. Cells that invaded through the membrane were fixed with methanol and stained with crystal violet. Then the invaded cells were observed under a microscope and the number of invaded cells was counted from five randomly selected fields. The Transwell migration assay did not require the addition of Matrigel.
Luciferase Reporter Assay
Dual-luciferase reporter assays were performed to explore the direct interactions between TMPO-AS1 and miR-143-3p as well as miR-143-3p and the 3ʹ-UTR of ZEB1 mRNA. Briefly, the fragments of TMPO-AS1 and 3ʹ-UTR of ZEB1 containing the predicted wild-type (WT) or mutated (Mut) binding sites of miR-143-3p were amplified, purified, and inserted into the pMIR-GLO Luciferase vector (Promega, Madison, WI, USA). Subsequently, we co-transfected the pMIR-GLO-WT-TMPO-AS1 or pMIR-GLO-Mut-TMPO-AS1 with miR-143-3p mimics or mimic NC into HEK293 cells using Lipofectamine 3000. The pMIR-GLO-WT-ZEB1 or pMIR-GLO-Mut-ZEB1 was transfected into HEK293 cells in a similar way. The pRL-TK plasmid expressing Renilla luciferase was co-transfected into HEK293 cells as the internal control. Luciferase activities were measured at 48 h post-transfection using the Dual-Luciferase Reporter Assay System (Promega) and normalized to the Renilla luciferase activity.
Animal Experiments
Five-week-old female athymic BALB/c mice were randomly divided into three groups (n=4 per group), namely, control, LV-sh NC (lentiviruses bearing scramble shRNA), and LV-sh TMPO-AS1 (lentiviruses bearing TMPO-AS1 shRNA). SiHa cells were infected with LV-sh TMPO-AS1 or LV-sh NC. At 24 h after infection, infected cells (1 × 106) stably expressing LV-sh TMPO-AS1 or LV-sh NC were subcutaneously injected into the mice. The mice in the control group received a subcutaneous injection of untreated SiHa cells. Tumor volume (V, mm3) was monitored every 1 week after injection using a caliper and calculated (π/6 × minor axis2 × major axis). Animals were sacrificed under anesthesia with pentobarbital (50 mg/kg body weight, intraperitoneally) at 5 weeks post-injection and tumor tissues were separated for determination of TMPO-AS1, miR-143-3p, and ZEB1 expression. This study was approved by the Ethics Committee of People’s Hospital of Zhengzhou University.
RNA Extraction and qRT-PCR Analysis
Total RNA was extracted from cells or tumors using TRIzol reagent (Invitrogen) and was reverse transcribed into cDNAs using the Reverse Transcription Kit (Takara). qRT-PCR was performed to amplify the cDNA template through using SYBR Premix Dimmer Eraser kit (Takara) on the ABI7900 system (Applied Biosystem). The relative expression of candidate genes was calculated by the 2−ΔΔCt method and normalized to the internal control 18S (for TMPO-AS1 and miR-143-3p) or GAPDH (for ZEB1).
Western Blot
Total protein was extracted from the cells or tumors using the radioimmunoprecipitation assay lysis buffer (Beyotime, Shanghai, China) and quantified with the bicinchoninic acid kit. Then the protein samples were loaded and separated using 10% SDS-PAGE gel electrophoresis and then transferred to PVDF membranes. After that, the membranes were blocked with 5% skim milk and then incubated with the following primary antibodies against ZEB1 (1:1000; Santa Cruz Biotechnology) and GAPDH (1:1000; Santa Cruz Biotechnology) overnight at 4°C. Subsequently, the membranes were washed with TBST three times and incubated with the horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2000; Santa Cruz Biotechnology) at room temperature for 2 h. Blots were examined by an Enhanced Chemiluminescence (ECL) Detection kit (Pierce Biotechnology, USA). Image-Pro Plus 6.0 software was used to analyze the band intensity.
Statistical Analysis
Statistical analyses were performed with SPSS 21.0 (SPSS, Chicago, Illinois, USA), and the values were presented as the mean ± standard deviation (SD). The Student’s t-test was used to analyze differences between the two groups. One-way ANOVA was used to analyze differences among three or more groups. P<0.05 was considered statistically significant.
Results
TMPO-AS1 Overexpression Promoted C-33A Cell Proliferation, Migration, and Invasion
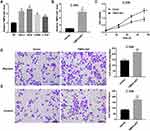
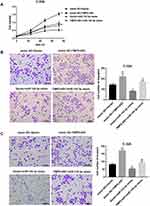
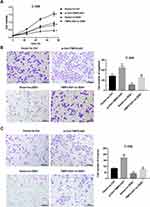
TMPO-AS1 expression was markedly higher in cervical cancer cell lines (HeLa, SiHa, CaSki, C-33A) than that in the HPV-immortalized cervical epithelial cells line H8 (Figure 1A). To explore the potential role of TMPO-AS1 in regulating cervical cancer cell behaviors, we overexpressed TMPO-AS1 in C-33A cell line that expresses relatively lower TMPO-AS1 expression. The overexpression efficiency was confirmed by qRT-PCR (Figure 1B). Importantly, CCK-8 assay indicated that the cell viability of cells transfected with pcDNA3.1- TMPO-AS1 was enhanced (Figure 1C). Transwell migration and invasion assays demonstrated that the number of migrated (Figure 1D) and invaded cells (Figure 1E) in cells transfected with pcDNA3.1- TMPO-AS1 was significantly more than that in those transfected with vector control. These data indicated that TMPO-AS1 overexpression promoted C-33A cell proliferation, migration, and invasion.
TMPO-AS1 Silencing Inhibited SiHa Cell Proliferation, Migration, and Invasion
To further clarify the functional role of TMPO-AS1 in regulating cervical cancer cell behaviors, we silenced TMPO-AS1 in SiHa cells. The silencing efficiency was confirmed by qRT-PCR (Figure 2A). Data revealed that TMPO-AS1 silencing significantly inhibited cell proliferation (Figure 2B), migration (Figure 2C), and invasion (Figure 2D) in SiHa cells.
TMPO-AS1 Acted as a ceRNA by Sponging miR-143-3p to Elevate ZEB1 Expression
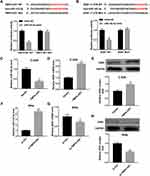
Luciferase reporter assay showed that miR-143-3p mimic caused a marked reduction in luciferase activity in TMPO-AS1 WT reporter, whereas had no obvious effect in TMPO-AS1 Mut reporter. These data suggested the direct binding between TMPO-AS1 and miR-143-3p (Figure 3A). Furthermore, miR-143-3p mimic significantly decreased the luciferase activity of ZEB1 WT reporter (Figure 3B), indicating the direct binding between miR-143-3p and ZEB1 3ʹUTR.
We then determined the effect of TMPO-AS1 overexpression and silencing on the expression of miR-143-3p and ZEB1. Data revealed that TMPO-AS1 overexpression significantly decreased miR-143-3p expression (Figure 3C), whereas increased ZEB1 mRNA (Figure 3D) and protein (Figure 3E) levels in C-33A cells. In contrast, TMPO-AS1 knockdown notably increased miR-143-3p expression (Figure 3F), whereas decreased ZEB1 mRNA (Figure 3G) and protein (Figure 3H) levels in SiHa cells.
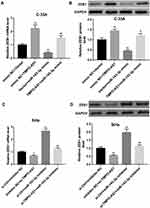
In addition, miR-143-3p mimic significantly decreased ZEB1 mRNA (Figure 4A) and protein levels (Figure 4B) in C-33A cells, whereas miR-143-3p inhibitor increased mRNA (Figure 4C) and protein levels (Figure 4D) of ZEB1 in SiHa cells. More importantly, TMPO-AS1 overexpression effectively restored the miR-143-3p mimic-mediated downregulation of ZEB1 mRNA (Figure 4A) and protein levels (Figure 4B) in C-33A cells. Moreover, TMPO-AS1 silencing significantly eliminated the miR-143-3p inhibitor-mediated elevation of ZEB1 mRNA (Figure 4C) and protein levels (Figure 4D) in SiHa cells. Taken together, these results indicated that TMPO-AS1 acted as a sponge for miR-143-3p to elevate ZEB1 expression.
miR-143-3p Mimic and ZEB1 Silencing Impaired the TMPO-AS1 Overexpression-Mediated Promotion of C-33A Cell Proliferation, Migration, and Invasion
We then sought to elucidate whether miR-143-3p and its target ZEB1 are involved in the regulation of cervical cancer cell behavior mediated by TMPO-AS1 overexpression. In contrast with TMPO-AS1 overexpression, miR-143-3p mimic significantly inhibited C-33A cell proliferation (Figure 5A), migration (Figure 5B), and invasion (Figure 5C). Of note, miR-143-3p mimic effectively abrogated the TMPO-AS1 overexpression-mediated promotion of C-33A cell proliferation (Figure 5A), migration (Figure 5B), and invasion (Figure 5C). Furthermore, ZEB1 silencing notably inhibited C-33A cell proliferation (Figure 6A), migration (Figure 6B), and invasion (Figure 6C), which was opposite to the effect by TMPO-AS1 overexpression. Importantly, ZEB1 silencing, similar to miR-143-3p mimic, efficiently impaired the TMPO-AS1 overexpression-mediated promotion of C-33A cell proliferation (Figure 6A), migration (Figure 6B), and invasion (Figure 6C). Collectively, these data indicated that TMPO-AS1 overexpression promoted C-33A cell proliferation, migration, and invasion, via regulating the miR-143-3p/ZEB1 axis.
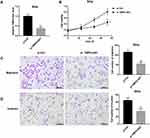
TMPO-AS1 Knockdown Inhibited Cervical Cancer Cell Tumorigenesis in vivo
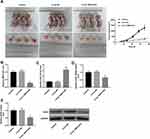
The tumor volume in SiHa cells-induced mouse xenograft models of cervical cancer was significantly decreased by TMPO-AS1 knockdown (Figure 7A). Furthermore, TMPO-AS1 expression was observably downregulated in tumors in the LV-sh TMPO-AS1 group (Figure 7B). Moreover, miR-143-3p expression in tumors formed by LV-sh TMPO-AS1-infected SiHa cells was markedly downregulated when compared with the LV-sh NC group (Figure 7C). In contrast, ZEB1 mRNA and protein levels were notably downregulated in tumors formed by LV-sh TMPO-AS1-infected SiHa cells in comparison with the LV-sh NC group (Figure 7D and E).
Discussion
The present study provided evidence for the first time that TMPO-AS1 overexpression promoted C-33A cell proliferation, migration, and invasion. In contrast, TMPO-AS1 silencing inhibited SiHa cell proliferation, migration, and invasion. Mechanistically, TMPO-AS1 acted as a ceRNA by sponging miR-143-3p to elevate ZEB1 expression, and thereby promoted C-33A cell proliferation, migration, and invasion. Further in vivo assay showed that TMPO-AS1 knockdown inhibited cervical cancer cell tumorigenesis.
Abundant evidence has identified aberrant lncRNAs with important roles in the pathogenesis of multiple malignancies including cervical cancer.1,13 Recent studies revealed that lncRNAs that are dysregulated in cervical cancer can be used as biomarkers in cervical cancer prognosis for metastasis and provide a novel treatment for cervical cancer.14 The alteration in the expression of these lncRNAs not only has obvious effects on the biological behaviors such as proliferation, migration and invasion of cervical cancer cells, but is closely related to the tumor size, angiogenesis, Federation of Gynecology and Obstetrics (FIGO) stage, lymph node metastasis and poor prognosis of cervical cancer.1,15 Thus, regulation of certain lncRNAs can represent a novel therapeutic strategy in the treatment of cervical cancer. For instance, Zhang et al15 recently reported that lncRNA SOX21 antisense RNA 1 (SOX21-AS1) was significantly upregulated in both cervical cancer tissues and cell lines and high expression of SOX21-AS1 was correlated with FIGO stage, lymph node metastasis and depth of cervical invasion. They also found that SOX21-AS1 promoted cervical cancer progression and could be used as a potential prognostic biomarker for patients with cervical cancer. Also, Chen et al16 demonstrated that lncRNA X–inactive specific transcript (XIST) contributed to progression of cervical cancer by sponging miR-140-5p. Here, we detected lncRNA TMPO-AS1 expression in cervical cancer cells and found that TMPO-AS1 was highly expressed in cervical cancer cells. Furthermore, TMPO-AS1 overexpression and knockdown experiments revealed that TMPO-AS1 promoted cervical cancer cell proliferation, migration, and invasion. Our findings indicated that TMPO-AS1 functioned as an oncogenic lncRNA in cervical cancer.
LncRNAs control tumorigenesis mainly by regulating the expression levels of certain oncogenes or tumor suppressors.17 It is well-established that lncRNAs can function as ceRNAs or molecular sponges to segregate miRNAs away from target mRNAs.8 For example, lncRNA SBF2 antisense RNA 1 (SBF2-AS1) promoted the progression of cervical cancer by regulating miR-361-5p/FOXM1 axis;18 lncRNA TPT1 antisense RNA 1 (TPT1-AS1) facilitated cell growth and metastasis in cervical cancer by functioning as an endogenous sponge for miR-324-5p.19 The cytoplasm is the main location for ceRNA action.20 TMPO-AS1 was predominantly localized in the cytoplasm in PCa cells,5 indicating the post-transcriptional regulation of TMPO-AS1. Accordingly, we explored whether TMPO-AS1 functioned as a ceRNA in regulating miR-143-3p. By using bioinformatics analysis and luciferase activity reporter, we confirmed the direct interaction between miR-143-3p and TMPO-AS1 or ZEB1.
Recent studies showed that miR-143-3p was lowly expressed in cervical cancer tissues and cell lines and exerted tumor-suppressive effects in cervical cancer.9–11 ZEB1 is generally believed to exert intrinsic oncogenic functions and foster cell migration, invasion, and metastasis in cervical cancer.8,12,21 miRNAs regulate gene expression through post-transcriptional pattern by directly targeting 3ʹ-UTR with paired bases, leading to translational repression or target degradation.22 Our results here showed that ZEB1 served as a target molecule of miR-143-3p, which was consistent with previous studies in hepatocellular carcinoma.17 Furthermore, the inhibition of ZEB1 mRNA and protein levels by miR-143-3p mimic was counteracted by TMPO-AS1 overexpression, whereas the induction of ZEB1 expression by miR-143-3p inhibitor was abrogated by TMPO-AS1 silencing, indicating that TMPO-AS1 acted as a sponge of miR-143-3p and prevented it from binding to ZEB1 mRNA, thereby elevating ZEB1 mRNA and protein levels. Further rescue experiments showed that miR-143-3p mimic and ZEB1 silencing impaired the effect of TMPO-AS1 overexpression on cancer cell behavior, indicating that TMPO-AS1 promoted cervical cancer cell growth via regulating miR-143-3p/ZEB1 axis.
Conclusions
In conclusion, our findings demonstrate for the first time that lncRNA TMPO-AS1 functions as an oncogenic lncRNA in cervical cancer, and indicate that TMPO-AS1/miR-143-5p/ZEB1 regulatory pathway plays an important role in cervical cancer. Our study suggests that inhibition of TMPO-AS1 might serve as a promising strategy for the treatment of cervical cancer clinically.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Peng L, Yuan X, Jiang B, Tang Z, Li GC. LncRNAs: key players and novel insights into cervical cancer. Tumour Biol. 2016;37(3):2779–2788. doi:10.1007/s13277-015-4663-9
2. Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46.
3. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981. doi:10.1158/0008-5472.CAN-16-2634
4. Dong J, Su M, Chang W, Zhang K, Wu S, Xu T. Long non-coding RNAs on the stage of cervical cancer (review). Oncol Rep. 2017;38(4):1923–1931. doi:10.3892/or.2017.5905
5. Huang W, Su X, Yan W, et al. Overexpression of AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate. 2018;78(16):1248–1261. doi:10.1002/pros.v78.16
6. Peng F, Wang R, Zhang Y, et al. Differential expression analysis at the individual level reveals a lncRNA prognostic signature for lung adenocarcinoma. Mol Cancer. 2017;16(1):98. doi:10.1186/s12943-017-0666-z
7. Liu Y, Yang Y, Li L, et al. LncRNA SNHG1 enhances cell proliferation, migration, and invasion in cervical cancer. Biochem Cell Biol. 2018;96(1):38–43. doi:10.1139/bcb-2017-0188
8. Zhu H, Zeng Y, Zhou CC, Ye W. SNHG16/miR-216-5p/ZEB1 signal pathway contributes to the tumorigenesis of cervical cancer cells. Arch Biochem Biophys. 2018;637:1–8. doi:10.1016/j.abb.2017.11.003
9. Liu M, Jia J, Wang X, Liu Y, Wang C, Fan R. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol Ther. 2018;19(5):391–399. doi:10.1080/15384047.2018.1423921
10. Chen X, Xiong D, Yang H, et al. Long noncoding RNA OPA-interacting protein 5 antisense transcript 1 upregulated SMAD3 expression to contribute to metastasis of cervical cancer by sponging miR-143-3p. J Cell Physiol. 2019;234(4):5264–5275. doi:10.1002/jcp.27336
11. Yang J, Jiang B, Hai J, Duan S, Dong X, Chen C. Long noncoding RNA opa-interacting protein 5 antisense transcript 1 promotes proliferation and invasion through elevating integrin alpha6 expression by sponging miR-143-3p in cervical cancer. J Cell Biochem. 2019;120(1):907–916. doi:10.1002/jcb.27454
12. Caramel J, Ligier M, Puisieux A. Pleiotropic roles for ZEB1 in cancer. Cancer Res. 2018;78(1):30–35. doi:10.1158/0008-5472.CAN-17-2476
13. Aalijahan H, Ghorbian S. Long non-coding RNAs and cervical cancer. Exp Mol Pathol. 2019;106:7–16. doi:10.1016/j.yexmp.2018.11.010
14. Shi D, Zhang C, Liu X. Long noncoding RNAs in cervical cancer. J Cancer Res Ther. 2018;14(4):745–753. doi:10.4103/jcrt.JCRT_669_17
15. Zhang X, Zhao X, Li Y, Zhou Y, Zhang Z. Long noncoding RNA SOX21-AS1 promotes cervical cancer progression by competitively sponging miR-7/VDAC1. J Cell Physiol. 2019;234(10):17494–17504.
16. Chen X, Xiong D, Ye L, et al. Up-regulated lncRNA XIST contributes to progression of cervical cancer via regulating miR-140-5p and ORC1. Cancer Cell Int. 2019;19:45. doi:10.1186/s12935-019-0744-y
17. Chen L, Yao H, Wang K, Liu X. Long non-coding RNA MALAT1 regulates ZEB1 expression by sponging miR-143-3p and promotes hepatocellular carcinoma progression. J Cell Biochem. 2017;118(12):4836–4843. doi:10.1002/jcb.v118.12
18. Gao F, Feng J, Yao H, Li Y, Xi J, Yang J. LncRNA SBF2-AS1 promotes the progression of cervical cancer by regulating miR-361-5p/FOXM1 axis. Artif Cells Nanomed Biotechnol. 2019;47(1):776–782. doi:10.1080/21691401.2019.1577883
19. Jiang H, Huang G, Zhao N, et al. Long non-coding RNA TPT1-AS1 promotes cell growth and metastasis in cervical cancer via acting AS a sponge for miR-324-5p. J Exp Clin Cancer Res. 2018;37(1):169. doi:10.1186/s13046-018-0846-8
20. Bai Y, Long J, Liu Z, et al. Comprehensive analysis of a ceRNA network reveals potential prognostic cytoplasmic lncRNAs involved in HCC progression. J Cell Physiol. 2019. doi:10.1002/jcp.28522
21. Chen G, Huang P, Xie J, Li R. microRNA211 suppresses the growth and metastasis of cervical cancer by directly targeting ZEB1. Mol Med Rep. 2018;17(1):1275–1282. doi:10.3892/mmr.2017.8006
22. Sun X, Dai G, Yu L, Hu Q, Chen J, Guo W. miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2. Sci Rep. 2018;8(1):606. doi:10.1038/s41598-017-18739-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.