Back to Journals » OncoTargets and Therapy » Volume 14
lncRNA RAET1K Promotes the Progression of Acute Myeloid Leukemia by Targeting miR-503-5p/INPP4B Axis
Authors Li L, Wan D, Li L, Qin Y, Ma W
Received 9 November 2020
Accepted for publication 27 November 2020
Published 18 January 2021 Volume 2021:14 Pages 531—544
DOI https://doi.org/10.2147/OTT.S291123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alberto Bongiovanni
Li Li,1 Dingming Wan,1 Lin Li,2 Yang Qin,1 Wang Ma3
1Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou City, Henan Province 450052, People’s Republic of China; 2Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou City, Henan Province 450052, People’s Republic of China; 3Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou City, Henan Province 450052, People’s Republic of China
Correspondence: Wang Ma
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, No. 1 East Jianshe Road, Zhengzhou City, Henan Province 450052, People’s Republic of China
Email [email protected]
Background: Although long non-coding RNA (lncRNA) RAET1K has been observed to be abnormally expressed in patients with various cancers, its role and molecular mechanism in acute myeloid leukemia (AML) remain unclear.
Methods: The expression of RAET1K and miR-503-5p in bone marrow tissues and cell lines was detected by qRT-PCR. Cell proliferation was evaluated by cell counting kit-8 and 5-ethynyl-20-deoxyuridine (EdU) staining assay. Cell invasion and migration were detected by transwell assay. Cell apoptosis was evaluated by flow cytometry. The relationship between RAET1K and miR-503-5p, as well as miR-503-5p and INPP4B, was determined by luciferase reporter assay and RNA immunoprecipitation (RIP) assay. In addition, the tumorigenesis of leukemia cells was evaluated by using a xenograft mouse model in vivo.
Results: RAET1K was significantly upregulated and miR-503-5p was markedly downregulated in bone marrow tissues and cell lines (HL-60 and THP-1). Silencing of RAET1K (si-RAET1K) and overexpression of miR-503-5p inhibited cell proliferation, migration, and invasion but promoted apoptosis of HL-60 and THP-1 cells. RAET1K functioned as a sponge of miR-503-5p, and miR-503-5p inhibitor obviously attenuated the effect of si-RAET1K on AML progression in vitro. INPP4B was identified as a target of miR-503-5p, and INPP4B overexpression obviously reversed the effect of miR-503-5p mimics on cell proliferation, migration, invasion, and apoptosis of HL-60 and THP-1 cells in vitro. Knockdown of RAET1K effectively inhibited the tumorigenesis of leukemia cells in vivo.
Conclusion: Our results demonstrated that RAET1K/miR-503-5p/INPP4B axis contributed to AML progression, suggesting that RAET1K might be a potential target for the treatment of AML.
Keywords: acute myeloid leukemia, RAET1K, miR-503-5p, INPP4B, tumorigenesis
Introduction
Acute myeloid leukemia (AML), similarly to most other human malignant tumors, is a highly heterogeneous disease.1 Approximately 40% of the younger and 10–20% of the older adults with AML can be cured with current standard chemotherapy.2 Despite recent progresses in treating AML, the long-term survival of AML patients is still poor due to the rapid development of resistance and the high rates of relapse after the treatment.3 Hence, the identification of specific and novel therapeutic targets against AML is more and more urgent, which may improve the clinical outcomes of AML.
In recent years, a class of non-coding RNAs with more than 200 nucleotides in length and lack protein-encoding functions, long non-coding RNAs (lncRNAs), have been demonstrated to be dysregulated in a large number of human cancers.4,5 LncRNAs play crucial roles in many pathophysiological and biological processes including protein translation, RNA splicing, and epigenetic modification.6 For example, PANDAR is upregulated in cervical cancer tissues and high expression of PANDAR promotes the proliferation of cervical cancer cells.7 The level of SNHG7 is highly overexpressed in gastric cancer tissues, and overexpression of SNHG7 enhances cell proliferation and inhibits apoptosis of gastric cancer cells, and also promotes the tumorigenesis of gastric cancer cells in vivo.8 TUBA4B is significantly downregulated in epithelial ovarian cancer tissues and overexpression of TUBA4B effectively reduces the proliferation of cancer cell line SKOV3.9 Retinoic acid early transcript 1K (RAET1K) is a recently identified lncRNA, has only been revealed to be highly elevated, and plays an essential role in hepatocellular carcinoma and lung adenocarcinoma.10,11 However, the function of RAET1K in most other human cancers remains unclear and our study aims to explore its role in AML progression.
MicroRNAs (miRNAs) are emerged as a group of small non-coding RNAs approximately containing 18–25 nucleotides and commonly bind to the 3′ untranslated region (3′‐UTR) of their target mRNAs.12 Increasing pieces of evidence have demonstrated that those abnormally expressed miRNAs are closely associated with the progression of AML. For example, miR-34a promotes apoptosis of AML cells by directly targeting the 3′‐UTR of HMGB1.13 MiR-130a is significantly downregulated in AML patients and inhibition of miR-130a significantly induces apoptosis of AML cells.14 MiR-126 is overexpressed in all of the AML cell lines, and knockdown of miR-126 significantly promoting cell death by inducing apoptosis.15 MiR-503-5p, a mature miRNA derived from 5ʹ ends of pre-miR-503, has been reported to regulate cell proliferation, transformation, migration, and invasion in different types of cancers such as hepatocellular carcinoma,16 lung cancer,17 and ovarian cancer.18 These reports suggest that miR-503-5p is involved in the development of various human cancers including AML. However, the regulatory axis of lncRNAs-miR-503-5p in AML has not been well studied.
It has been reported that inositol polyphosphate-4-phosphatase type II (INPP4B) exhibits a potent carcinogenesis in various human cancers including breast cancer,19 laryngeal cancer,20 and melanoma.21 Recently, INPP4B is found to be aberrantly overexpressed and emerged as an independent indicator of poor prognosis in AML patients.22 In addition, one previous study found that overexpression of INPP4B effectively enhanced the potential of colony formation and promote proliferation in AML cell lines, and also recapitulated the chemotherapy resistance in patients with AML.23 However, the regulatory networks involved in INPP4B in AML are lack.
In the present study, we explored the function of RAET1K in AML in detail. Specifically, we found that RAET1K was significantly upregulated in bone marrow tissues of AML patients and relevant cell lines. Furthermore, by performing a series of function assays, we revealed that downregulation of RAET1K effectively inhibited proliferation, the ability of cell invasion and migration, and promoted apoptosis of AML cell lines in vitro, and also suppressed the tumorigenesis of leukemia cells in vivo by sponging miR-503-5p to elevate the level of INPP4B. Taken together, our study suggested that RAET1K might be potentially regarded as an effective target for developing anti-cancer agents in AML.
Materials and Methods
The Collection of Clinical Samples
A total of 35 bone marrow tissues were obtained from 35 patients with AML by surgery at the First Affiliated Hospital of Zhengzhou University between 2017 and 2019. In addition, 30 bone marrow tissues were obtained from healthy volunteers and used as negative control. All participants have provided written informed consents and this study was approved by the human Ethics Committee of the First Affiliated Hospital of Zhengzhou University and conducted in accordance with the Declaration of Helsinki.
Cell Culture
Leukemia cell lines (HL-60 and THP1) and human stromal cell line HS were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplementing with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) at 37°C with 5% CO2.
Cell Transfection
The RAET1K smart silencer (si-RAET1K) and its negative control si-NC were obtained from RiboBio (siBDM1999A, Guangzhou, China). The sequence of si-RAET1K: sense: 5ʹ-CCAAGUGGUGACUUCACCATT-3ʹ, antisense: 5ʹ-UGGGUAAGGGCCCACCUUGTT-3ʹ. The short hairpin sh-RAET1K and negative control sh-NC plasmids were constructed by Genewiz, Inc. The sequence of short hairpin sh-RAET1K was as follows: 5ʹ-ATTGGGAGCTGTGGTCAATTA-3ʹ. For manipulation of miR-503-5p levels, miR-503-5p mimic (overexpression of miR-503-5p), miR-503-5p inhibitor (knockdown of miR-503-5p), and corresponding negative controls (miR-NC and inhibitor NC) were purchased from RiboBio (Guangzhou, China). To overexpress RAET1K and INPP4B, the cDNA sequences of RAET1K and INPP4B were amplified and inserted into the Expression Vector pcDNA3.1 to generate pc-RAET1K and pcDNA-INPP4B, and the empty vector pcDNA3.1 was used as negative control. Cell transfection was performed by using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
RNA Isolation and qRT-PCR
Total RNA was extracted from bone marrow tissues and cultured cells by using Trizol reagent (Thermo Fishier Scientific). qRT-PCR experiments were performed by using SYBR Green PCR Master Mix (Applied Biosystems) under an ABI 7900 Real-Time PCR System (Applied Biosystems). GAPDH acted as the endogenous control to normalize the expression of INPP4B, and U6 snRNA for RAET1K, miR-503-5p. The expression level of RAET1K, miR-503-5p, and INPP4B was calculated based on the 2−ΔΔCt method. The primers used are shown in Table 1.
 |
Table 1 The Primers Used in the Study |
CCK-8 Assay
Cell viability was evaluated by using the cell counting kit-8 (Solarbio) as previously reported.24 In brief, approximately 2 × 103/well cells were seeded into 96-well plates. After transfection for 24, 48, 72, and 96 h, 10 μL of CCK8 solution was added and incubated for another 2 h. The absorbance at 450 nm was detected by a microplate reader.
5-Ethynyl-20-Deoxyuridine (EdU) Staining Assay
Cell proliferation was detected by using the EdU Staining Proliferation Kit (Abcam). Briefly, cells were seeded into 96-well plates and 100 μL of fresh medium containing 50 μM EdU was added and incubated for 3 h. Then, cells were washed with PBS and re-stained by 10 μg/mL Hoechst 33,342 solution for 20 min. Finally, proliferative cells were observed under the Olympus fluorescence microscope.
Apoptosis Analysis
Cell apoptosis was analyzed by using the Annexin V-FITC Apoptosis Kit (BD Biosciences) according to the manufacturer’s instructions. Approximately 5 x 105 cells were seeded into 6-well plates and cultured to logarithmic growth phase. Cells were re-suspended in 195 μL of Annexin V-FITC binding solution, subsequently, 5 μL of Annexin V-FITC and 10 μL of PI staining solution were added and incubated for 12 min in the dark. Finally, cell apoptosis was analyzed using flow cytometry (BD Biosciences, USA).
Cell Invasion and Migration
Cell invasion and migration were evaluated by using the transwell assay as previously described.25 For migration assay, 1×105 cells were seeded into the upper chamber of a transwell insert (pore size, 8 μm) in RPMI-1640 medium supplemented with 1% FBS, and the medium containing 10% FBS was added into the lower chamber. For invasion assay, the matrix gel was covered on the lower surface of the chamber and the other steps were the same as the migration assay. After incubation for 24 h, the migrated or invaded cells were fixed with crystal violet, then captured and counted under a microscope.
Luciferase Reporter Assay
The putative binding sites between RAET1K and miR-503-5p, miR-503-5p and INPP4B were predicted by searching StarBase (http://starbase.sysu.edu.cn) and TargetScan (http://www.targetscan.org/vert_72/), respectively. The sequences of wild type RAET1K (WT-RAET1K), mutant type RAET1K (MUT-RAET1K), wild type 3ʹ-UTR of INPP4B (WT-INPP4B), and mutant type 3ʹ-UTR of INPP4B (MUT-INPP4B) containing the putative binding site with miR-503-5p were amplified and cloned into the pmirGLO vector (Promega). Then, luciferase reporter vectors were co-transfected with miR-503-5p mimics or miR-NC into HL-60 and THP-1 cells by using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, the luciferase activity was detected under a dual-luciferase reporter assay system (Promega).
RNA Immunoprecipitation (RIP) Assay
RIP assay was carried out by using the Magna RIP RNA-Binding Protein IP Kit (Millipore, USA) as previously reported. Briefly, HL-60 and THP-1 cells were lysed with RIPA lysis buffer, and the supernatant was mixed with RIP buffer containing a magnetic bead conjugated with human anti-Ago2 antibody (Cell Signaling, Danvers, MA, USA) or negative control mouse immunoglobulin G (IgG, Millipore, USA). The enriched RNA was submitted to qRT-PCR analysis to determine the expression levels of targets.
Western Blot
Total protein was extracted from bone marrow tissues and cultured cells using the RIPA lysis buffer (Beyotime, China). Approximately 25 µg proteins were separated by 12% SDS-PAGE and then transferred into PVDF membranes. After blocking with 5% lipid-free milk solution, the membranes were incubated with primary antibodies anti-INPP4B (1:1000; #ab14768, Abcam) and anti-GAPDH (1:2000; Boster, Wuhan, China) overnight at 4°C. Subsequently, the membranes were exposed to horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 2 h. Finally, the protein signals of targets were detected by using the ECL Kit (Millipore), and relative protein levels were quantified by using ImageJ software.
Xenograft Tumor Model
The NOD/SCID nude mice (5-week old) were maintained under the specific pathogen-free conditions. HL-60 cells stably transfected with sh-RAET1K, miR-503-5p inhibitor, and co-transfected with sh-RAET1K and miR-503-5p inhibitor were injected subcutaneously into the right flank of nude mice (n = 8). Tumor volume of each mouse from different groups was evaluated every 1 week for 5 weeks according to the following formula: length × width2/2 (length: tumor length; width: tumor width). On day 35, mice were sacrificed by cervical dislocation and xenograft tumors were weighted and then photographed with a digital camera. All animal experiments were performed according to the National Institutes of Health guide for the care and use of laboratory animals, following the protocols approved by the Institutional Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Immunohistochemistry (IHC) Assay
The xenograft tumors were embedded in paraffin and cut into 4-μm thickness sections. The sections were incubated with the antibody against Ki67 (1:200, Abcam) and detected by the Leica TCS SP8X confocal microscope as previously described.26
Statistical Analysis
Data were presented as mean ± standard deviation (SD) method by the SPSS19.0 statistical software. The overall survival of patients with AML was evaluated by using the Kaplan-Meier curve, and the difference in overall survival was determined by log-rank method. The difference between the two groups was determined by the Student’s t-test and that among multiple groups by one-way analysis of variance (ANOVA). Statistical significance was set at P < 0.05.
Results
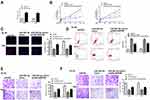
The Correlation Between RAET1K and miR-503-5p in AML
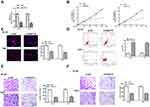
To study the involvement of RAET1K and miR-503-5p during AML progression, a total of 35 bone marrow tissues and 30 control tissues were collected from AML patients and healthy volunteers. The results of qRT-PCR assay showed that the expression levels of RAET1K in the bone marrow tissues from AML patients were significantly higher than those from healthy volunteers (p = 0.0082, Figure 1A). Meanwhile, an obvious decrease in the levels of miR-503-5p in the bone marrow tissues from AML patients was observed compared with that from healthy volunteers (p = 0.0075, Figure 1B). By performing Pearson correlation analysis, we found that there was a significantly negative correlation between the expression of RAET1K and miR-503-5p in 35 bone marrow tissues of AML patients (p = 0.0066, Figure 1C). In addition, we also found that the expression levels of RAET1K were significantly upregulated and miR-503-5p was downregulated in AML cell lines (HL-60 and THP-1) compared with that in human stromal cell line HS (p = 0.0081, Figure 1D and E). Moreover, all 35 AML patients were divided into two groups based on the median expression value of RAET1K and Kaplan-Meier curve indicated that patients with high expression of RAET1K had a poor prognosis compared with that with low RAET1K levels (p = 0.0059, Figure 1F). These results suggested that RAET1K and miR-503-5p might play important roles in AML.
Silencing of RAET1K Inhibited Proliferation, Invasion, Migration and Promoted Apoptosis of Leukemia Cells in vitro
To determine the function of RAET1K in AML, si-RAET1K was transfected into HL-60 and THP-1 cells to silence the expression of RAET1K. The transfection efficiency was evaluated by qRT-PCR and the result showed that the mRNA levels of RAET1K in the si-RAET1K group were significantly lower than that in the si-NC group in two cells (p = 0.00059, Figure 2A). By performing CCK-8 assay (Figure 2B) and EdU staining assay (Figure 2C), we found that silencing of RAET1K markedly inhibited cell proliferation of two cells compared with si-NC. The apoptosis of two cells in the si-RAET1K group was significantly higher than the si-NC group (p = 0.0081, Figure 2D). In addition, silencing of RAET1K effectively decreased the ability of cell migration and invasion of two cells compared with si-NC (p = 0.0087, Figure 2E and F). These results revealed that silencing of RAET1K inhibited proliferation, invasion, migration and promoted apoptosis of leukemia cells in vitro.
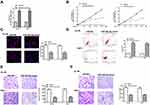
Overexpression of miR-503-5p Inhibited Proliferation, Invasion and Migration of Leukemia Cells in vitro
To determine the function of miR-503-5p in AML, HL-60, and THP-1 cells were transfected with miR-503-5p mimics or the negative control (miR-NC). qRT-PCR assay revealed that miR-503-5p mimics significantly increased the expression of miR-503-5p compared with miR-NC in two cells (p = 0.00085, Figure 3A). The results of CCK-8 (Figure 3B) and EdU staining assay (Figure 3C) showed that overexpression of miR-503-5p effectively inhibited the proliferation of two cells compared with miR-NC. Compared with miR-NC, miR-503-5p mimics significantly enhanced cell apoptosis of two cells (p = 0.0085, Figure 3D). Meanwhile, transwell assay revealed that miR-503-5p mimics markedly decreased the migration and invasion capacities of two cells compared with miR-NC (p = 0.0075, Figure 3E and F). These results demonstrated overexpression of miR-503-5p inhibited proliferation, invasion, migration and promoted apoptosis of leukemia cells in vitro.
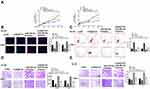
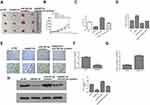
RAET1K Was a Sponge of miR-503-5p
To explore the relationship between RAET1K and miR-503-5p, Starbase online database was used and we found that there was a putative binding site between RAET1K and miR-503-5p (Figure 4A). To confirm the prediction, luciferase reporter assay was carried out and the results showed that miR-503-5p mimics significantly decreased the relative luciferase activity of WT-RAET1K, while exhibited no obvious change in MUT RAET1K in two cells (p = 0.0069, Figure 4B). In addition, RIP assay was also performed and the results showed that RAET1K and miR-503-5p were both enriched preferentially in miRNPs containing anti-Ago2 antibody compared with immune-precipitates containing anti-IgG antibody in two cells (p = 0.0053, Figure 4C). Moreover, silencing of RAET1K significantly increased the expression of miR-503-5p (p = 0.0069, Figure 4D), and overexpression of RAET1K obviously decreased the expression of miR-503-5p in two cells (p = 0.0083, Figure 4E). These data suggested that RAET1K was a sponge of miR-503-5p.
Downregulation of miR-503-5p Reversed Si-RAET1K Induced Protective Role in AMI Progression in vitro
To explore whether the oncogenic role of RAET1K in AML was mediated by miR-503-5p, HL-60, and THP-1 cells were transfected with si-NC, si-RAET1K, miR-503-5p inhibitor or co-transfected with si-RAET1K and miR-503-5p inhibitor. Cell proliferation was evaluated by CCK-8 assay (Figure 5A) and EdU staining assay (Figure 5B), and the results showed that si-RAET1K decreased the growth of two cells compared with si-NC (p = 0.0075), and miR-503-5p inhibitor obviously elevated cell proliferation in two cells (p = 0.0048), while co-transfection of si-RAET1K and miR-503-5p inhibitor partly eliminated the inhibitory effect of si-RAET1K on cell proliferation in two cells (p = 0.038). Flow cytometry assay revealed that si-RAET1K promoted the apoptosis of two cells (p = 0.0058), and miR-503-5p inhibitor significantly decreased the apoptosis (p = 0.028), while co-transfection of si-RAET1K and miR-503-5p inhibitor obviously reversed the effect of si-RAET1K on cell apoptosis in two cells (p = 0.039) (Figure 5C). Cell migration and invasion were evaluated by transwell assay (Figure 5D and E), and the results indicated that si-RAET1K significantly inhibited the migration and invasion abilities of two cells (p = 0.0084), and miR-503-5p inhibitor obviously enhanced migration and invasion abilities of two cells (p = 0.046), while co-transfection of si-RAET1K and miR-503-5p inhibitor partly eliminated the inhibitory effect of si-RAET1K on invasion and migration in two cells. These results suggested that the effect of RAET1K in AML was partly mediated by miR-503-5p.
INPP4B Was a Target of miR-503-5p
Then, Targetscan was applied to predict the potential targets of miR-503-5p, and the results suggested that INPP4B might be a target of miR-503-5p (Figure 6A). Meanwhile, luciferase reporter assay revealed that miR-503-5p mimics significantly decreased the relative luciferase activity of WT-INPP4B, while exhibited no obvious change in MUT-INPP4B in two cells (p = 0.0058, Figure 6B). In addition, miR-503-5p mimics decreased the expression of INPP4B both at mRNA level and protein level in two cells (p = 0.0038), and miR-503-5p inhibitor increased the expression of INPP4B in two cells compared with inhibitor NC (p = 0.0068, Figure 6C and D). Moreover, silencing of RAET1K markedly decreased the expression of INPP4B in two cells (p = 0.0068, Figure 6E). A significant upregulation of INPP4B in the bone marrow tissues of AML patients was observed compared with that of healthy controls (p = 0.0051, Figure 6F). There was also an obviously negative correlation between the expression levels of miR-503-5p and INPP4B in 35 bone marrow tissues of AML patients (p = 0.00075, Figure 6G). These results demonstrated that INPP4B was a target of miR-503-5p.
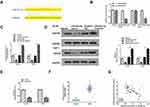
Overexpression of INPP4B Reversed miR-503-5p Mimics Induced Protective Role in AMI Progression in vitro
To explore whether miR-503-5p affected AML progression through INPP4B, HL-60 and THP-1 cells were transfected with miR-503-5p mimics, miR-NC, or co-transfected with miR-503-5p mimics and pcDNA-INPP4B (overexpression of INPP4B). The results of qRT-PCR assay demonstrated that overexpression of INPP4B significantly elevated the expression of INPP4B compared with negative control pcDNA-NC in two cells (p = 0.0069, Figure 7A). By performing CCK-8 assay (Figure 7B) and EdU staining assay (Figure 7C), we found that miR-503-5p mimics significantly inhibited cell proliferation (p = 0.0068), while co-transfection of miR-503-5p mimics and pcDNA-INPP4B attenuated the inhibitory effect of miR-503-5p mimics on proliferation. For cell apoptosis, the apoptotic rate in miR-503-5p mimics group was higher than that in the miR-NC group (p = 0.0065), while co-transfection of miR-503-5p mimics and pcDNA-INPP4B obviously reversed the effect of miR-503-5p mimics on apoptosis in two cells (p = 0.0058, Figure 7D). In addition, miR-503-5p mimics decreased the abilities of migration and invasion in two cells (p = 0.0059), while co-transfection of miR-503-5p mimics and pcDNA-INPP4B significantly attenuated miR-503-5p mimics induced inhibition on cell migration and invasion (p = 0.041, Figure 7E and F). These results revealed that the effect of miR-5-3-5p in AML development was partly through INPP4B.
Downregulation of RAET1K Suppressed the Tumorigenesis of Leukemia Cells Through Regulating miR-503-5p/INPP4B Axis in vivo
To determine the oncogenic role of RAET1K in vivo, a xenograft mouse model was applied through subcutaneously injection with HL-60 cells which were stably transfected with sh-RAET1K, sh-NC (negative control), miR-503-5p inhibitor, or co-transfected with sh-RAET1K and miR-503-5p inhibitor. After 5 weeks, mice were sacrificed and the representative images of subcutaneous tumor from four groups were observed (Figure 8A). Meanwhile, both tumor weight and tumor volume were significantly decreased in the sh-RAET1K group (p = 0.0068), and increased in the miR-503-5p inhibitor group (p = 0.0054), while co-transfection of sh-RAET1K and miR-503-5p inhibitor efficiently reversed the inhibitory effect of sh-RAET1K on tumor weight and volume (p = 0.031, Figure 8B and C). Meanwhile, compared with the sh-NC group, the average survival days were longer in the sh-RAET1K group (p = 0.0042), and decreased in the miR-503-5p inhibitor group (p = 0.039), while co-transfection of sh-RAET1K and miR-503-5p inhibitor efficiently reversed the effect of sh-RAET1K on average survival time (p = 0.038, Figure 8D). In addition, the results of Ki-67 staining assay in tumor tissues showed that sh-RAET1K obviously decreased cell proliferation, and miR-503-5p inhibitor enhanced cell proliferation, while co-transfection of sh-RAET1K and miR-503-5p inhibitor efficiently reversed the inhibitory effect of sh-RAET1K on proliferation in vivo (Figure 8E). Moreover, the expression of RAET1K in vivo was decreased (p = 0.0045, Figure 8F), and the expression of miR-503-5p was increased in the sh-RAET1K group (p = 0.0049, Figure 8G). In addition, the expression of INPP4B in tumor tissues was also assessed by Western blot and the results indicated that sh-RAET1K significantly reduced the expression of INPP4B compared with sh-NC (p = 0.0053), and miR-503-5p inhibitor elevated the expression of INPP4B compared with sh-NC (p = 0.034), while co-transfection of sh-RAET1K and miR-503-5p inhibitor obviously attenuated the inhibitory effect of sh-RAET1K on INPP4B expression (p = 0.041, Figure 8H). These results revealed that downregulation of RAET1K could effectively inhibit the tumorigenesis of leukemia cells through regulating miR-503-5p/INPP4B axis in vivo.
Discussion
In this study, we found for the first time that the expression levels of RAET1K were highly overexpressed during AML. Knockdown of RAET1K significantly decreased cell viability and proliferative capacity, inhibited invasion and migration abilities, while enhanced apoptotic rate of AML cell lines, and even suppressed the tumorigenesis of leukemia cells in a xenograft mouse model. Taken together, RAET1K promoted the progression of AML through directly sponging miR-503-5p and then upregulate INPP4B both in vitro and in vivo.
In the last decades, more and more lncRNAs have been observed to be abnormally expressed during AML progression and many of them have been elucidated clearly. For example, H19 has been demonstrated to promote cell proliferation of AML cells through regulating Wnt/β-catenin signaling pathway.27 LINP1 is significantly overexpressed in AML patients at diagnosis, and knockdown of LINP1 obviously suppresses glucose uptake and the survival of AML cells by modulating HNF4α/AMPK/WNT5A signaling pathway.28 ANRIL is upregulated in the bone marrow samples of AML patients, and ANRIL knockdown effectively inhibits cell proliferation, migration, and invasion while enhances the apoptosis of AML cells.29 KCNQ1OT1 contributes to the development and chemoresistance in AML by modulating Tspan3 level.30 High levels of SNHG3 predict a poor outcome for AML, and downregulation of SNHG3 has been identified to effectively suppress cell growth of AML cells.31 RAET1K has been only studied in hepatocellular carcinoma,10 and lung adenocarcinoma,11 so we explored the potential role of RAET1K in AML. Interestingly, our study revealed a function of RAET1K in AML and provided that RAET1K might be a novel diagnostic and therapeutic target for AML, which even might contribute to identify and develop new anti-cancer agents.
LncRNAs have been found to function their roles by directly sponging miRNAs to silence the expression of target miRNAs in eukaryotic cells.32 Previous studies explained different biological functions of RAET1K in cancers by sponging their target miRNAs. For example, downregulation of RAET1K blocks the cell cycle arrest of lung adenocarcinoma cells through sponging miR-133a-5p to downregulate its level, and overexpression and miR-135a-5p exhibits an opposite effect of RAET1K knockdown.11 In our study, we found that miR-503-5p was downregulated in bone marrow samples of AML patients and two cell lines, and an obviously negative correlation between RAET1K and miR-503-5p level in bone marrow tissues of AML patients was observed. Hence, we speculated miR-503-5p might be a target of RAET1K and the function of RAET1K in AML might be mediated by miR-503-5p. Then, Starbase database was used to predict their interaction, and the prediction suggested that there was putative binding site between RAET1K and miR-503-5p. MiR-503-5p has been revealed to play essential roles in human diseases such as cervical cancer,33 neuroblastoma,34 gastric cancer,35 and so on. These studies above suggested that miR-503-5p might also play important roles in AML. Here, we found that overexpression of miR-503-5p exhibited a similar effect to si-RAET1K in AML progression in vitro, which was, miR-503-5p mimics inhibited cell growth, invasion, migration, and promoted apoptosis of AML cells. In addition, miR-503-5p inhibitor significantly reversed the protective role of si-RAET1K in AML progression both in vitro and in vivo.
To further investigate the mechanism of RAET1K in AML, Targetscan was applied and the prediction suggested that INPP4B might be a direct target of miR-503-5p. Moreover, INPP4B was upregulated in AML patients and cell lines as expected. Previous studies found that INPP4B levels were increased during AML progression and might be potentially considered as a new player in the chemo-resistance of AML.36 Increasing pieces of evidence demonstrated that INPP4B could function as direct targets of miRNAs to participate in the development of different types of cancers, such as miR-937 in lung cancer,37 miR-1290 in colorectal cancer,38 and miR-181 in breast cancer.39 To determine whether INPP4B acted as a target of miR-503-5p to affect the progression of AML, rescue experiments were performed and the results revealed that overexpression of INPP4B could effectively attenuate the inhibitory effect of miR-503-5p mimics on cell proliferation, invasion, migration, and apoptosis of AML cells in vitro. These data suggested that the effect of miR-503-5p in AML was partly mediated by INPP4B. In addition, previous studies have reported that miR-503-5p played essential roles in human diseases through regulating a series of targeted mRNAs such as WEE1 in hepatocellular carcinoma,16 VEGF-A in colon cancer,40 CDCA4 in osteosarcoma,41 and so on. These well-known targeted genes whether mediated the function of miR-503-5p in AML should be explored in the future.
Conclusion
In summary, our study revealed a novel regulatory network of lncRNA-miRNA-mRNA in AML progression: RAET1K promoted cell proliferation, invasion, migration and inhibited apoptosis of leukemia cells during AML development by targeting miR-503-5p/INPP4B axis, suggesting that RART1K might be a potential therapeutic target for AML.
Abbreviations
lncRNA, long non-coding RNA; AML, acute myeloid leukemia; lncRNAs, long non-coding RNAs; RAET1K, retinoic acid early transcript 1K.
Funding
There is no funding to report.
Disclosure
The authors confirmed they have no conflicts of interest in this work.
References
1. Bullinger L, Döhner K, Döhner H. Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol. 2017;35(9):934–946. doi:10.1200/JCO.2016.71.2208
2. Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol. 2017;18(3):17. doi:10.1007/s11864-017-0456-2
3. Döhner H, Weisdorf DJ, Bloomfield CD, Longo DL. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi:10.1056/NEJMra1406184
4. Wang WT, Ye H, Wei PP, et al. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J Hematol Oncol. 2016;9:117.
5. Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15:43.
6. Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci. 2019;44:33–52.
7. Huang HW, Xie H, Ma X, Zhao F, Gao Y. Upregulation of LncRNA PANDAR predicts poor prognosis and promotes cell proliferation in cervical cancer. Eur Rev Med Pharmacol Sci. 2017;21:4529–4535.
8. Wang MW, Liu J, Liu Q, et al. LncRNA SNHG7 promotes the proliferation and inhibits apoptosis of gastric cancer cells by repressing the P15 and P16 expression. Eur Rev Med Pharmacol Sci. 2017;21:4613–4622.
9. Zhu FF, Zheng FY, Wang HO, Zheng JJ, Zhang Q. Downregulation of lncRNA TUBA4B is associated with poor prognosis for epithelial ovarian cancer. Pathol Oncol Res. 2018;24:419–425.
10. Zhou Y, Huang Y, Hu K, Zhang Z, Yang J, Wang Z. HIF1A activates the transcription of lncRNA RAET1K to modulate hypoxia-induced glycolysis in hepatocellular carcinoma cells via miR-100-5p. Cell Death Dis. 2020;11(3):176. doi:10.1038/s41419-020-2366-7
11. Zheng C, Li X, Ren Y, Yin Z, Zhou B. Long noncoding RNA RAET1K enhances CCNE1 expression and cell cycle arrest of lung adenocarcinoma cell by sponging miRNA-135a-5p. Front Genet. 2020;10:1348. doi:10.3389/fgene.2019.01348
12. Orso F, Quirico L, Dettori D, et al. Role of miRNAs in tumor and endothelial cell interactions during tumor progression. Semin Cancer Biol. 2020;60:214–224. doi:10.1016/j.semcancer.2019.07.024
13. Liu L, Ren W, Chen K. MiR-34a promotes apoptosis and inhibits autophagy by targeting HMGB1 in acute myeloid leukemia cells. Cell Physiol Biochem. 2017;41(5):1981–1992. doi:10.1159/000475277
14. Ding C, Chen S-N, Macleod RAF, et al. MiR-130a is aberrantly overexpressed in adult acute myeloid leukemia with t(8;21) and its suppression induces AML cell death. Ups J Med Sci. 2018;123(1):19–27. doi:10.1080/03009734.2018.1440037
15. Ding Q, Wang Q, Ren Y, Zhu HQ, Huang Z. MicroRNA-126 attenuates cell apoptosis by targeting TRAF7 in acute myeloid leukemia cells. Biochem Cell Biol. 2018;96(6):840–846. doi:10.1139/bcb-2018-0017
16. Jiang SP, Li ZR. MiR-503-5p regulates cell epithelial-to-mesenchymal transition, metastasis and prognosis of hepatocellular carcinoma through inhibiting WEE1. Eur Rev Med Pharmacol Sci. 2019;23:2028–2037.
17. Sun Y, Li L, Xing S, et al. miR-503-3p induces apoptosis of lung cancer cells by regulating p21 and CDK4 expression. Cancer Biomarkers. 2017;20(4):597–608. doi:10.3233/CBM-170585
18. Sun Q, Li Q, Xie F. <p>LncRNA-MALAT1 regulates proliferation and apoptosis of ovarian cancer cells by targeting miR-503-5p. Oncotargets Ther. 2019;12:6297–6307. doi:10.2147/OTT.S214689
19. Gasser JA, Inuzuka H, Lau AW, Wei W, Beroukhim R, Toker A. SGK3 mediates INPP4B-dependent PI3K signaling in breast cancer. Mol Cell. 2014;56(4):595–607. doi:10.1016/j.molcel.2014.09.023
20. Min JW, Kim KI, Kim H-A, et al. INPP4B-mediated tumor resistance is associated with modulation of glucose metabolism via hexokinase 2 regulation in laryngeal cancer cells. Biochem Biophys Res Commun. 2013;440(1):137–142. doi:10.1016/j.bbrc.2013.09.041
21. Chen L, Cao Y, Rong D, Wang Y, Cao Y. MicroRNA-605 functions as a tumor suppressor by targeting INPP4B in melanoma. Oncol Rep. 2017;38(2):1276–1286. doi:10.3892/or.2017.5740
22. Rijal S, Fleming S, Cummings N, et al. Inositol polyphosphate 4-phosphatase II (INPP4B) is associated with chemoresistance and poor outcome in AML. Blood. 2015;125(18):2815–2824. doi:10.1182/blood-2014-09-603555
23. Dzneladze I, He R, Woolley JF, et al. INPP4B overexpression is associated with poor clinical outcome and therapy resistance in acute myeloid leukemia. Leukemia. 2015;29:1485–1495.
24. Wang Y, Zhou Q, Ma JJ. High expression of lnc-CRNDE presents as a biomarker for acute myeloid leukemia and promotes the malignant progression in acute myeloid leukemia cell line U937. Eur Rev Med Pharmacol Sci. 2018;22:763–770.
25. Li G, Zheng P, Wang H, Ai Y, Mao X. Long non-coding RNA TUG1 modulates proliferation, migration, and invasion of acute myeloid leukemia cells via regulating miR-370-3p/MAPK1/ERK. Onco Targets Ther. 2019;12:10375–10388.
26. Hao X, Shin MS, Zhou JX, et al. Histologic and molecular characterizations of megakaryocytic leukemia in mice. Leuk Res. 2006;30(4):397–406. doi:10.1016/j.leukres.2005.08.021
27. Zhao TT, Liu X. LncRNA-H19 inhibits apoptosis of acute myeloid leukemia cells via targeting miR-29a-3p. Eur Rev Med Pharmacol Sci. 2019;23:224–231.
28. Shi J, Dai R, Chen Y, Guo H, Han Y, Zhang Y. LncRNA LINP1 regulates acute myeloid leukemia progression via HNF4α/AMPK/WNT5A signaling pathway. Hematol Oncol. 2019;37(4):474–482. doi:10.1002/hon.2651
29. Wang C-H, Li Q-Y, Nie L, Ma J, Yao C-J, Chen F-P. LncRNA ANRIL promotes cell proliferation, migration and invasion during acute myeloid leukemia pathogenesis via negatively regulating miR-34a. Int J Biochem Cell Biol. 2020;119:105666. doi:10.1016/j.biocel.2019.105666
30. Sun H, Sun Y, Chen Q, Xu Z. LncRNA KCNQ1OT1 contributes to the progression and chemoresistance in acute myeloid leukemia by modulating Tspan3 through suppressing miR-193a-3p. Life Sci. 2020;241:117161. doi:10.1016/j.lfs.2019.117161
31. Peng L, Zhang Y. lncRNA SNHG3 facilitates acute myeloid leukemia cell growth via the regulation of miR-758-3p/SRGN axis. J Cell Biochem. 2020;121:1023–1031.
32. Akhade VS, Pal D, Kanduri C. Long noncoding RNA: genome organization and mechanism of action. Adv Exp Med Biol. 2017;1008:47–74.
33. Ran W, Zeng YH, Ma XJ, Liao P, Liu XL. [The effect of miR-503-5p on the proliferation, invasion, migration and epithelial interstitium of cervical cancer HeLa cells via targeting E2 F3]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2020;51:178–184.
34. De Mariano M, Stigliani S, Moretti S, et al. A genome-wide microRNA profiling indicates miR-424-5p and miR-503-5p as regulators of ALK expression in neuroblastoma. Oncotarget. 2017;8:56518–56532.
35. Cai X, Nie J, Chen L, Yu F. Circ_0000267 promotes gastric cancer progression via sponging MiR-503-5p and regulating HMGA2 expression. Mol Genet Genomic Med. 2020;8:e1093.
36. Recher C. INPP4B, a new player in the chemoresistance of AML. Blood. 2015;125:2738–2739.
37. Zhang L, Zeng D, Chen Y, et al. miR-937 contributes to the lung cancer cell proliferation by targeting INPP4B. Life Sci. 2016;155:110–115.
38. Ma Q, Wang Y, Zhang H, Wang F. miR-1290 contributes to colorectal cancer cell proliferation by targeting INPP4B. Oncol Res. 2018;26:1167–1174.
39. Strotbek M, Schmid S, Sánchez-González I, Boerries M, Busch H, Olayioye MA. miR-181 elevates Akt signaling by co-targeting PHLPP2 and INPP4B phosphatases in luminal breast cancer. Int J Cancer. 2017;140:2310–2320.
40. Wei L, Sun C, Zhang Y, Han N, Sun S. miR-503-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-A. Gene Ther. 2020.
41. Li J, Zhang F, Li H, et al. Circ_0010220-mediated miR-503-5p/CDCA4 axis contributes to osteosarcoma progression tumorigenesis. Gene. 2020;763:145068.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.