Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Laparoscopic Hepatectomy versus Open Hepatectomy After Conversion Therapy Using Transarterial Chemoembolization or Hepatic Arterial Infusion Chemotherapy for Patients with Initially Unresectable Hepatocellular Carcinoma
Authors Yang Z, Hu Z , Fu Y , Hu D, Zhou Z, Chen M, Pan Y , Zhang Y
Received 18 April 2023
Accepted for publication 30 June 2023
Published 21 July 2023 Volume 2023:10 Pages 1157—1167
DOI https://doi.org/10.2147/JHC.S417739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Zhenyun Yang,1,2,* Zili Hu,1,2,* Yizhen Fu,1,2 Dandan Hu,1,2 Zhongguo Zhou,1,2 Minshan Chen,1,2 Yangxun Pan,1,2 Yaojun Zhang1,2
1State Key Laboratory of Oncology in South China, Sun Yat-Sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, 510060, People’s Republic of China; 2Department of Liver Surgery, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yangxun Pan; Yaojun Zhang, State Key Laboratory of Oncology in South China, Sun Yat-Sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, 510060, People’s Republic of China, Tel +86-20-87343828, Email [email protected]; [email protected]
Background: Laparoscopic hepatectomy (LH) is more advantageous than open hepatectomy (OH) for hepatocellular carcinoma (HCC). However, surgical methods of conversion resection for patients with HCC have not been compared. We aimed to compare LH with OH for HCC after conversion therapy.
Methods: We retrospectively reviewed the data of 334 patients who underwent conversion resection between January 2016 and December 2020 at Sun Yat-sen University, China. Propensity score matching (PSM) of patients in a ratio of 1:2 was conducted, and 62 patients and 121 patients who underwent LH and OH, respectively, were matched.
Results: The LH and OH groups differed at baseline in terms of ALT (P=0.008), AFP (P=0.042), largest tumor size (P=0.028), macrovascular invasion (P=0.006), BCLC stages (P=0.021), and CNLC stages (P=0.048). The incidences of postoperative complications before and after PSM were lower in the LH group than in the OH group (P=0.007 and 0.003, respectively). There were no significant differences in the overall survival (OS) and recurrence-free survival (RFS) between the two groups (P=0.79 and 0.8, respectively). According to the multivariable Cox regression analyses, the largest tumor size (P< 0.0001) and tumor number (P=0.004) were significant and independent prognostic factors of OS.
Conclusion: In our study, we found that LH is technically feasible and safe in patients after conversion therapy. Compared with OH, LH showed similar OS and RFS and was associated with fewer postoperative complications.
Keywords: laparoscopic hepatectomy, open hepatectomy, hepatocellular carcinoma, conversion therapy, propensity score matching
Introduction
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and has a poor prognosis and high level of malignancy.1 Moreover, it is the sixth most frequent malignancy and the third leading cause of cancer-related deaths worldwide.2 In China, the majority of patients with a diagnosis of HCC present with advanced, inoperable disease.3–5 However, over the past few years, treatment for patients with advanced HCC modalities has evolved rapidly.
Clinically, patients with unresectable HCC can be converted to resectable HCC through locoregional treatment strategies, such as transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC) and radiation therapy; systemic treatment, such as targeted therapy or combined with immunotherapy.6–9 In a previous study, 37 out of 157 patients with initially unresectable HCC were successfully converted to resectable disease through HAIC, and 15 out of 155 patients were converted through TACE.10 In another previous study, 15 out of 34 patients with initially unresectable HCC were successfully converted to resection through a triple combination of angiogenesis inhibitors, anti-PD-1 antibodies, and HAIC.11 Moreover, these patients who underwent safe curative resection can achieve favorable outcomes. Therefore, curative resection is essential for the prognosis of HCC patients.
It is well known that laparoscopic hepatectomy (LH) and open hepatectomy (OH) are two common surgical strategies for HCC. The choice of surgical strategy is also crucial. Comparative results of LH versus OH for HCC have been reported.12–19 On the whole, the advantages of LH include fewer complications, fewer transfusions, less blood loss, less postoperative pain and shorter hospital stays. However, for some difficult operations, OH may be a better choice. For advanced HCC patients who received conversion therapy, the choice of surgical strategy is very important. Currently, there are no data on the outcomes of patients undergoing this conversion surgery strategy.
Propensity Score Matching (PSM) is a matching method based on propensity score. The main idea is to estimate the probability of each individual receiving a treatment (propensity score), and then match individuals receiving different treatments according to this probability, such that the difference between the two groups is minimized. To overcome selection bias, we performed 1:2 propensity score (PS) matching between the LH and OH cohorts.
In this study, we aimed to use PSM analysis to compare the perioperative and long-term outcomes of LH versus OH for advanced HCC patients who received conversion therapy (HAIC or TACE).
Materials and Methods
Patient Recruitment and Selection Criteria
The study reviewed 334 patients diagnosed with HCC who underwent hepatectomy after conversion therapy at Sun Yat-sen University, China, from January 2016 to December 2020. The inclusion criteria were followed: (1) confirmed records of receiving conversion therapy, (2) confirmed diagnosis of HCC by pathologic examination of resected specimens, (3) aged from 18 or above, (4) absence of any other malignant tumor. Patients were excluded based on the following exclusion criteria: (1) Patients with any other malignant tumor; (2) Patients who discontinued treatment for personal reasons or violating treatment procedures.
Conversion Therapy Procedure
Conversion therapy, including TACE and HAIC, was used in this study. TACE and HAIC were performed according to our previously reported protocol.10 TACE cycles were performed every 6 weeks. The chemotherapy drugs were infused through the artery supplying the tumor, and embolization was performed with iodized oil. The perfusion drugs included 50 mg of epirubicin, 6 mg of mitomycin and 300 mg of carboplatin. Repeated HAIC cycles were performed every 3 weeks. On day 1 in cycle of HAIC femoral artery puncture and catheterization were performed, and patients were transferred to the inpatient ward for drug infusion via the hepatic artery. Oxaliplatin, leucovorin, and bolus fluorouracil were administered equally in both the LH and OH groups, whereas infusional fluorouracil 2400 mg/m2 was given over 23h or 46 h. After treatment initiation, the radiological response was evaluated by computed tomography (CT) or magnetic resonance imaging (MRI) performed at a baseline and every 6 weeks. HCC-specific modified RECIST (mRECIST) was used for evaluating the tumor response. Conversion therapy was stopped when radical resection could be achieved with sufficient remnant liver volume.
Data Collection
The demographic and clinical characteristics included age; sex; hepatitis B virus infection status; liver cirrhosis; hypertension; diabetes; ALT (U/L), AST (U/L), ALB (g/L), TBIL (umol/L), AFP (ng/mL), and PIVKA (mAU/mL) levels; WBC (109/L), PLT (109/L) counts; PT(s); largest tumor size (cm); tumor number; macrovascular invasion; tumor-free margin (mm); BCLC stage; and CNLC stage. The details of demographic and clinical characteristics are presented in Table 1. The blood tests and tumor burdens were measured within 5 days preoperatively.
 |
Table 1 Baseline Characteristics of the 334 Patients Included in the Present Study |
The level of difficulty of the hepatectomy was classified per the Institut Mutualiste Montsouris (IMM) classification into three levels depending on the location and extent of resection. The grading was as follows: grade I included partial resection and left lateral segmentectomy; grade II included anterolateral segmentectomy and left hepatectomy, and grade III included posterosuperior segmentectomy, right hepatectomy, right posterior sectionectomy, extended left hepatectomy, and central hepatectomy.20
Routine blood tests and CT or MRI were performed to evaluate tumor recurrence and survival status three months postoperatively, which was repeated every three months in the first and second postoperative year and every 6 months thereafter.
Overall survival (OS) was defined as the time interval from surgery to cancer-related death and recurrence-free survival (RFS) was defined as the interval from surgery to recurrence in the liver or elsewhere, or censoring at the date of the last follow-up.
Propensity Score Matching
One-to-two PSM was adopted to minimize selection bias. In this model, a caliper of 0.2 was applied. The propensity score was calculated by baseline characteristics (age and sex), preoperative blood tests (ALT and AFP), tumor characteristics (tumor size, tumor number, and macrovascular invasion), and tumor-free margin.
Statistical Analysis
Non-normally distributed data were expressed as medians and ranges. Continuous parametric variables were analyzed using the unpaired Student’s t-test, and continuous non-parametric variables were analyzed using the Mann–Whitney U-test. Categorical data were analyzed by Pearson’s correlation coefficient chi-square test with continuity correction or Fisher’s exact probability method. Forward LR-based univariate and multivariate Cox regression analyses were used to identify independent predictive variables. The OS and RFS were represented by Kaplan–Meier curves and analyzed using the Log rank test. A P value <0.05 was considered statistically significant. All analyses were performed using SPSS 25.0 software (SPSS Inc., Chicago, IL) and R version 4.0.1.
Results
Patient Characteristics in Unmatched and Matched Cohorts
We retrospectively reviewed the data of 334 patients diagnosed with HCC who underwent LH or OH after conversion therapy at Sun Yat-sen University, China, from January 2016 to December 2020. There were 63 patients in the LH group and 271 patients in the OH group (Figure 1). ALT levels (P=0.008), AFP levels (P=0.042), largest tumor size (P=0.028), macrovascular invasion (P=0.006), Barcelona clinic liver cancer (BCLC) stages (P=0.021) and China liver cancer staging (CNLC) stages (P=0.048) were the characteristics that differed between the two groups (Table 1). After propensity score matching, 62 LHs were matched and compared with 121 OHs (Figure 1). Detailed characteristics of each group after PSM are shown in Table 2. There were no significant differences between the LH and OH groups at baseline. The resected tumor’s location according to Couinaud’s classification, in the two groups before and after PSM is shown in Figures S1 and S2, respectively. Of note, there was no significant difference in the difficulty grade between the LH and OH groups (difficulty grade I/II/III: LH: 16/12/35 vs OH: 56/29/186; P=0.093) (Table 1).
 |
Table 2 Baseline Characteristics After Propensity Score Matching |
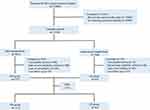 |
Figure 1 Flow diagram. Abbreviations: HCC, hepatocellular carcinoma; LH laparoscopic hepatectomy; OH, open hepatectomy. |
Operative Details in Unmatched Cohort
Operative details were compared between the two groups in the entire cohort (Table 3). The LH group had fewer cases where the Pringle maneuver was used (43 cases (68.3%) in the LH versus 251 (92.6%) in the OH group). Meanwhile, a significant difference was observed in the incidence of postoperative complications between the two groups (LH: 30.2% vs OH: 51.6%, P=0.007). Nineteen patients in the LH group had postoperative complications, two owing to biliary leakage, one owing to pleural effusion, ten owing to peritoneal encapsulated effusion, one owing to ascites, one owing to pulmonary infection, and four owing to postoperative bleeding. A total of 141 patients in the OH group experienced postoperative complications, one owing to hepatic insufficiency, two owing to biliary leakage, fifty-three owing to pleural effusion, fifty owing to peritoneal encapsulated effusion, ten owing to ascites, two owing to pulmonary infection, nine owing to wound infection, and fourteen owing to postoperative bleeding. LH group was similar to the OH group in blood loss (median 200 (range 50–2000) mL versus 300 (50–2500) mL; P=0.622), plasma transfusions (5 (7.9%) versus 27 (10%); P= 0.623), erythrocyte transfusion (4 (6.3%) versus 22 (15.4%); P=0.637), hospital stay (median 9 (range 5–15) days versus 9 (5–34) days; P=0.653) and operative time (median 154 (range 90–315) minutes versus 168 (90–420) minutes; P=0.057).
 |
Table 3 Operative Details of Patients Who Underwent LH and OH |
Operative Details in Matched Cohort
Operative details were compared between the LH group and the OH group in the matched cohort (Table 4). Similar to the unmatched cohort, the LH group was similar to the OH group in blood loss, plasma transfusions, erythrocyte transfusion, hospital stay, operative time and clamping times. LH group had fewer cases where the Pringle maneuver was used 43 (68.3%) versus 114 (94.2%) in the OH group (P<0.0001). Meanwhile, there were fewer postoperative complications in the LH group (LH: 30.2% vs OH: 51.6%, P=0.003). Nineteen patients in the LH group had incidences of postoperative complications, two owing to biliary leakage, one owing to pleural effusion, ten owing to peritoneal encapsulated effusion, one owing to ascites, one owing to pulmonary infection, and four owing to postoperative bleeding. Sixty patients in the OH group experienced postoperative complications, two owing to biliary leakage, twenty-six owing to pleural effusion, twenty-one owing to peritoneal encapsulated effusion, two owing to ascites, one owing to pulmonary infection, there owing to wound infection, and five owing to postoperative bleeding.
 |
Table 4 Operative Details of Patients Who Underwent LH and OH After Propensity Score Matching |
Tumor Response to Conversion Therapy and Patient Survival
Of the 334 patients with unresectable HCC who underwent conversion therapy, 109 (32.6%) received TACE and 225 (67.4%) received HAIC. The conversion therapy protocol rapidly reduced the tumor burden in the unmatched cohorts and matched cohorts (Figure S3). Furthermore, in the subgroup analysis that was stratified according to conversion therapy, similar results were seen (Figure S4). Additionally, parameters related to liver function, such as ALT, AST and TBIL levels, were reduced after conversion therapy, and there were significant differences between pre- and post-conversion therapy levels (P<0.0001), both in the unmatched and matched cohorts, as shown in Figures S5 and S6. Subsequently, radical resection could be achieved with sufficient remnant liver volume. There was no difference between the two groups in the long-term outcomes in the unmatched cohorts and matched cohorts (Figure 2). Furthermore, OS and RFS were similar between both groups in subgroup analysis that was stratified according to conversion therapy (Figure S7).
Univariate and Multivariable Cox Regression Analyses in All Patients
Univariate and multivariate analyses of the preoperative parameters in predicting OS were performed, as shown in Figure 3. The univariate analyses showed that AFP levels, largest tumor size, tumor number, macrovascular invasion, BCLC stage, and CNLC stage, were significant risk factors for OS. For multivariate analysis, CNLC stage was excluded because there was collinearity between the CNLC stage and other variables. The multivariate Cox proportional analysis revealed that the largest tumor size (P<0.0001) and tumor number (P=0.004), were significant and independent prognostic factors of OS (Figure 3).
 |
Figure 3 Univariate and multivariate Cox regression analyses of risk factors for overall survival before PSM. |
Discussion
It is well known that most patients with HCC are diagnosed in their advanced stages in China. For the past few years, various treatments, including HAIC, TACE, immunotherapy, targeted therapy, radiotherapy, and systemic chemotherapy, is being used to treat patients with advanced HCC, which has made conversion surgery possible. According to a randomized Phase III trial for large HCC, patients with advanced HCC were treated through TACE or HAIC, and the curative surgical resection rate was 24% in the HAIC group and 12% in TACE group.10 In general, the reported conversion rates are approximately 10–30%.21–25 In our study, of the 334 patients with unresectable HCC who underwent conversion therapy, 109 (32.6%) received TACE and 225 (67.4%) received HAIC.
Over the past few years, LH is being applied worldwide due to improved instruments and increased surgical experience. Additionally, LH showed several advantages over OH, including fewer complications, transfusions, less blood loss, and less pains.26–28 However, TACE or HAIC may cause localized inflammation leading to adhesions, which increase the level of difficulty of the surgery. Our results suggest clear advantages of LH over OH in HCC patients who received conversion therapy.
As we all know, conversion therapy may cause localized inflammation leading to adhesions, which increase the level of difficulty of the surgery. It is not hard to understand that patients undergoing conversion surgery are more difficult than those undergoing direct surgery. In a single-center real-world study, researchers clarified that patients who received conversion surgery had a higher incidence of surgery-related complications than patients who received direct surgery.29 Meanwhile, the prognosis of advanced HCC after conversion surgery is comparable to that after direct surgery. In conclusion, surgery is still the core treatment for patients with HCC to obtain the best survival benefit. On comparing the two unmatched groups, some patient and tumor characteristics showed statistically significant differences. The decision of which surgical approach should be taken is complex considering individual patient differences and the surgeon’s experience. For example, patients with worse liver function are usually selected for LH to improve their outcomes. PSM was applied to solve this unavoidable bias.
After PSM, two comparable groups of patients who underwent LH and OH for HCC were created. Complications were lesser in the LH group than in the OH group. Specifically, the incidence rates of pleural effusion and peritoneal encapsulated effusion in the LH group were lower compared to the OH group. This might be explained by the fact that in the LH group, small abdominal accesses could reduce surgery-induced injury and preserve intrahepatic vessels and lymphatic collaterals.12,30,31 Meanwhile, avoiding exposure of the abdominal tissue and organs to air reduces the chance of electrolyte imbalance and promotes reabsorption of ascites or pleural effusion.32 Although, there was no significant difference statistically in the blood loss and rates of blood transfusion, the rates of plasma transfusion and erythrocyte transfusion were lower in the LH group. This is due to the fact that laparoscopic surgery is less invasive.
Currently, LH is considered safe for the treatment of HCC.33,34 However, there was no study on the selection of the surgical approach in initially unresectable patients receiving conversion therapy. Since conversion therapy increases the difficulty of surgery, it is believed that OH might be more appropriate for these patients. This study has demonstrated that in initially unresectable patients receiving conversion therapy and conversion resection, OS and RFS were similar in the LH and OH groups, while fewer complications were noted in the LH group, indicating the safety and feasibility of LH.
This study had some limitations. Firstly, it was a retrospective study which might have introduced selection bias and further prospective studies are needed to validate our findings. Secondly, the relatively small sample size limits the generalizability of our results, and there is a risk of a type II error. Finally, a potential limitation of 1:2 matching is that many patients in the OH group were not matched and were excluded from the analysis, which can lead to a loss of information and reduce the accuracy of estimating associations between treatment and outcomes.
Conclusion
In conclusion, in initially unresectable HCC patients who received conversion therapy and conversion resection, LH is safe and technically feasible. LH showed similar OS and RFS, associated with fewer complications compared with OH.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Ethics Statement
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. This research was approved by the Ethics Committee of Sun Yat-sen University Cancer Center. Signed informed consent for the use of data for research purposes was obtained from the patients before treatment.
Patient Consent for Publication
This study was approved by the ethics committee of the Sun Yat-sen University Cancer Center (SYSUCC). Informed consent was impossible to obtain because this was a retrospective study. However, written informed consent for the use of data for research purposes was signed before each treatment.
Acknowledgments
The authors thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, interpretation, or all areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is funded by the National Natural Science Foundation of China (No: 82103566).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/s0140-6736(18)30010-2
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
5. Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160(1):99–114.e3. doi:10.1053/j.gastro.2020.04.014
6. Zhang T, Merle P, Wang H, Zhao H, Kudo M. Combination therapy for advanced hepatocellular carcinoma: do we see the light at the end of the tunnel? Hepatobiliary Surg Nutr. 2021;10(2):180–192. doi:10.21037/hbsn-2021-7
7. Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15(3):155–160. doi:10.5582/bst.2021.01091
8. Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007;14(12):3301–3309. doi:10.1245/s10434-007-9549-7
9. Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9(8):1920–1928. doi:10.1111/j.1600-6143.2009.02695.x
10. Li QJ, He MK, Chen HW, et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: a Randomized Phase III Trial. J Clin Oncol. 2022;40(2):150–160. doi:10.1200/jco.21.00608
11. Zhang J, Zhang X, Mu H, et al. Surgical Conversion for Initially Unresectable Locally Advanced Hepatocellular Carcinoma Using a Triple Combination of Angiogenesis Inhibitors, Anti-PD-1 Antibodies, and Hepatic Arterial Infusion Chemotherapy: a Retrospective Study. Front Oncol. 2021;11:729764. doi:10.3389/fonc.2021.729764
12. Deng ZC, Jiang WZ, Tang XD, Liu SH, Qin L, Qian HX. Laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 157 patients: a case controlled study with propensity score matching at two Chinese centres. Int J Surg. 2018;56:203–207. doi:10.1016/j.ijsu.2018.06.026
13. Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(7):871–880. doi:10.1002/bjs.10137
14. Kabir T, Tan ZZ, Syn NL, et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg. 2021;109(1):21–29. doi:10.1093/bjs/znab376
15. Yoon YI, Kim KH, Kang SH, et al. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: a Propensity Score Matched Analysis. Ann Surg. 2017;265(5):856–863. doi:10.1097/sla.0000000000002072
16. Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257(3):506–511. doi:10.1097/SLA.0b013e31827b947a
17. Belli G, Limongelli P, Fantini C, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96(9):1041–1048. doi:10.1002/bjs.6680
18. Belli G, Fantini C, D’Agostino A, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc. 2007;21(11):2004–2011. doi:10.1007/s00464-007-9503-6
19. Sarpel U, Hefti MM, Wisnievsky JP, Roayaie S, Schwartz ME, Labow DM. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol. 2009;16(6):1572–1577. doi:10.1245/s10434-009-0414-8
20. Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of Laparoscopic Liver Resection: proposal for a New Classification. Ann Surg. 2018;267(1):13–17. doi:10.1097/sla.0000000000002176
21. Zhu XD, Huang C, Shen YH, et al. Hepatectomy After Conversion Therapy Using Tyrosine Kinase Inhibitors Plus Anti-PD-1 Antibody Therapy for Patients with Unresectable Hepatocellular Carcinoma. Ann Surg Oncol. 2022. doi:10.1245/s10434-022-12530-z
22. Chong JU, Choi GH, Han DH, et al. Downstaging with Localized Concurrent Chemoradiotherapy Can Identify Optimal Surgical Candidates in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Ann Surg Oncol. 2018;25(11):3308–3315. doi:10.1245/s10434-018-6653-9
23. Zhu XD, Huang C, Shen YH, et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer. 2021;10(4):320–329. doi:10.1159/000514313
24. Hamaoka M, Kobayashi T, Kuroda S, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg. 2017;44:223–228. doi:10.1016/j.ijsu.2017.06.082
25. He M, Li Q, Shi M. Potential Areas of Interest in a Trial of Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin for Hepatocellular Carcinoma-In Reply. JAMA Oncol. 2019;5(12):1806–1807. doi:10.1001/jamaoncol.2019.4058
26. Cheung TT, Dai WC, Tsang SH, et al. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients With Liver Cirrhosis: a Propensity Analysis at a Single Center. Ann Surg. 2016;264(4):612–620. doi:10.1097/sla.0000000000001848
27. Kim H, Suh KS, Lee KW, et al. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc. 2014;28(3):950–960. doi:10.1007/s00464-013-3254-3
28. Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):721–727. doi:10.1002/jhbp.276
29. Wang J, Zheng Z, Wu T, et al. Hepatic Arterial Infusion Chemotherapy as a Timing Strategy for Conversion Surgery to Treat Hepatocellular Carcinoma: a Single-Center Real-World Study. J Hepatocell Carcinoma. 2022;9:999–1010. doi:10.2147/jhc.S379326
30. Brytska N, Han HS, Shehta A, Yoon YS, Cho JY, Choi Y. Laparoscopic liver resection for hepatitis B and C virus-related hepatocellular carcinoma in patients with Child B or C cirrhosis. Hepatobiliary Surg Nutr. 2015;4(6):373–378. doi:10.3978/j.issn.2304-3881.2015.04.06
31. Chen Z, Xie H, Hu M, et al. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10(9):2993–3036.
32. Frazee RC, Roberts JW, Okeson GC, et al. Open versus laparoscopic cholecystectomy. A comparison of postoperative pulmonary function. Ann Surg. 1991;213(6):651–653. doi:10.1097/00000658-199106000-00016
33. Scuderi V, Barkhatov L, Montalti R, et al. Outcome after laparoscopic and open resections of posterosuperior segments of the liver. Br J Surg. 2017;104(6):751–759. doi:10.1002/bjs.10489
34. Granito A, Bolondi L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 2017;18(2):e101–e112. doi:10.1016/s1470-2045(16)30569-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

