Back to Journals » Infection and Drug Resistance » Volume 16
L. pneumophila Infection Diagnosed by tNGS in a Lady with Lymphadenopathy
Authors Li S, Tong J, Li H, Mao C, Shen W, Lei Y, Hu P
Received 16 April 2023
Accepted for publication 27 June 2023
Published 6 July 2023 Volume 2023:16 Pages 4435—4442
DOI https://doi.org/10.2147/IDR.S417495
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Shiying Li,1 Jin Tong,2 Hu Li,1 Chenxue Mao,3 Wei Shen,1 Yu Lei,1 Peng Hu1
1Department of Infectious Diseases, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Respiratory Medicine, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, People’s Republic of China; 3Department of Laboratory Diagnosis, ChongQing KingMed Center for Clinical Laboratory Co., Ltd, Chongqing, 400050, People’s Republic of China
Correspondence: Peng Hu, Department of Infectious Diseases, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, The Second Affiliated Hospital, Chongqing Medical University, 74# Linjiang Road, Chongqing, 400010, People’s Republic of China, Email [email protected]; [email protected]
Abstract: We report a case of a 34-year-old lady with multiple joint pain. Autoimmune diseases were considered first with a positive result of anti-Ro antibody and her right knee joint cavity effusion. Later, bilateral interstitial changes in her lungs and mediastinal lymphadenopathy were found after chest CT scanning. Empirical quinolone therapy was given although pathological examinations of blood, sputum and bronchoalveolar lavage fluid (BALF) did not find anything. Finally, Legionella pneumophila was identified by target next-generation sequencing (tNGS) detection. This case highlighted the timely use of tNGS, a new tool with fast speed, high accuracy and effective cost, could help to identify atypical infection and start an early therapy.
Keywords: case report, Legionella pneumophila, targeted next-generation sequencing, tNGS, precision treatment, levofloxacin
Introduction
Legionella pneumophila is a common atypical pathogen that can cause pneumonia clinically resembling other bacterial pneumonias.1–3 It is thought to be responsible for about 2–15% community acquired pneumonia (CAP) which requires hospitalization.4,5 Symptoms of infected people range from mild disease to severe pneumonia such as acute respiratory distress syndrome (ARDS) and multiple-organ dysfunction syndrome (MODS).6 The term Legionellosis refers to the whole disease spectrum after infecting with Legionella spp, including milder form of illness, acute pneumonia (Legionnaire’s disease) and Pontiac fever (PF). Legionella pneumophila is the most common cause.7
Legionellosis is a reportable disease in the USA, Canada, Europe, Japan, New Zealand, Singapore, and Australia.8 In China, all provinces (including Taiwan) except Tibet have been reported this disease.9 Legionella pneumophila was first discovered in 1976 at Philadelphia.8 Since then, a brand-new bacterium designated Legionella pneumophila was known. Its family consists of 58 species and three subspecies. All Legionella bacteria have been gradually isolated from aqueous environments. About 30 species cause infection in people, mainly by attacking the lower respiratory tract.
Legionella spp are Gram-negative bacteria with strict growth requirements.10,11 Infection occurs when the bacteria are inhaled or aspirated. Patients with Legionella pneumonia often have a non-specific epidemiological history (eg, travel history, bathing, and cruises) and non-specific clinical symptoms (eg, slow pulse, gastrointestinal symptoms, and/or muscle pain, as well as with involvement of the kidneys and/or nervous system).10 Along with laboratory tests that lack specificity, early diagnosis is not easy.
Culture of Legionella is often time-consuming, and easily contaminated.12 Thus, Legionella’s diseases are often diagnosed by non-culture method in clinic. However, Legionella spp are difficult to detect by Gram staining as they are small coccobacilli to short rod in shape.13 Cytology detection may help because patients with Legionellosis typically produce thin watery sputum that contains few neutrophils. The detection of anti-Legionella antibodies is another common method. However, most patients develop anti-Legionella antibodies around 3 weeks after the onset of the disease, which resulted in delaying of diagnosis.14
The emergence of metagenomic next-generation sequencing (mNGS) has markedly improved the rate of early diagnosis of Legionellosis, especially for the unspecific ones.15 However, mNGS is expensive and greatly influenced by human genes, and it is not possible to conduct DNA and RNA dual process detection at the same time. Therefore, we adopted a more rapid, economic, and accurate technology, target next-generation sequencing (tNGS), to achieve an early diagnosis of respiratory infection, whose target detection was of only 153 pathogens (Supplementary Table 1) but responsible for more than 95% of the respiratory infection.16 tNGS in our study can detect 65 bacteria, 68 viruses (25 DNA viruses and 43 RNA viruses), 14 fungi, and 6 mycoplasmas/chlamydia. Here, we report the first case of Legionella pneumophila identified by tNGS in Chongqing, China.
Case Description
A 34-year-old young lady was admitted to the Department of Rheumatology and Immunology of the 2nd affiliated hospital of Chongqing Medical University (CQMU), Chongqing, China, on May 6, 2021, due to “limb joint pain for more than one month” (Figure 1). More than a month before admission, the patient developed bilateral symmetrical pains in her wrists, elbows, knees, and ankles without obvious causes, along with tenderness. These symptoms were heavier in the morning with stiffness, and slight relieving at afternoon. The involved joints were intermittent swelling, without wandering or joint deformity. During the course of disease, she had no fever, cough, expectoration, abdominal pain, and diarrhea. This lady was with a height of 160cm, and a weight of 55kg (BMI 21.48kg/m2). She was a salesperson, working at a supermarket with central air conditioning. And a dog was raised at the lady’s home. She had married without a child. She had no history of smoking or alcohol consumption. There was an allergic history to cephalosporin about 10 years ago. She was born and living in Chongqing for a long time, and she had not gone to other places for a recent year. Physical examination of her whole body showed no abnormal findings.
 |
Figure 1 The clinical course of the patient (schematic). (Red color: During hospitalization, green color: After discharge). |
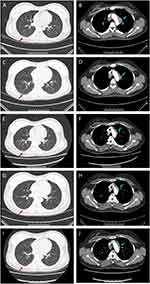
After admission, firstly, the lady’s anti-Ro antibody was positive (++), Sjogren’s syndrome was suspected. However, no abnormality was found in the parotid gland and bilateral submandibular glands. Therefore, lip mucosa biopsy was performed to further exclude the diagnosis of Sjogren’s syndrome. At the same time, ultrasonography of muscle and bone showed that there was some effusion in the joint cavity of the right knee joint. Along with the symptoms of joint pain, rheumatoid arthritis should be considered. Next day, the negative results of anti-cyclic citrullinated peptide (CCP) and rheumatoid factor (RF) helped decrease the possibility of rheumatoid arthritis’ diagnosis. Enhanced CT scanning of chest (Figure 2) unexpectedly showed bilateral pulmonary interstitial inflammation, with multiple lymphadenopathies, thus pneumonia was considered (Figure 2A and B). Although the patient had no typical symptoms like fever, cough, expectoration, or shortness of breath, levofloxacin was given empirically with elevated neutrophils (Figure 3A). At the same time, the relevant etiological examination was further arranged; however, these results were successively showed negative (Supplementary Table 2).
On the third day, the lady’s joint pain was still obvious, so she underwent an intra-articular puncture, fluid extraction, and 1 mL of Diprospan injection into the intra-articular cavity for anti-inflammatory treatment. The pathological results of lip mucosa came back and showed that no lymphocyte aggregation was found. Till then, the diagnosis of Sjogren’s syndrome was completely excluded. And the patient’s joint pain relived. One week later, a re-examination of chest CT scanning showed that the inflammation of both lungs was smaller, but lymphadenopathies were basically the same as compared to before (Figure 2C and D). After consultation from the respiratory department, a transfer was recommended to clarify the nature of lymphadenopathy. Doppler ultrasonography of superficial lymph nodes detected several enlarged lymph nodes on the right clavicle, with clear boundaries, and the largest one was about 1.6 x 0.7cm.
On the eighth day, the patient underwent a bronchoscopy plus endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) operation under general anesthesia. During the operation, a small amount of purulent secretion was found in the patient’s trachea and bilateral main bronchus, which was lavaged, washed out, and sent to culture. The lavage fluid in the lower left lobe was sent for exfoliative cytology and Xpert MTB/RIF detection. Microscopically, it can be seen that the bilateral bronchial mucosa of each leaf and segment of this patient was normal, the lumen was unobstructed. And no neoplasm, bleeding, or necrosis tissues were found. Then, the ultrasonic bronchoscope was replaced. EBUS-TBNA exploration showed that the lymph nodes in group N4, N7, R10, and L11 were swollen and fused. The lymph nodes in groups N4, N7, R10, and L11 were punctured with 21G puncture needles for 1, 3, 2, and 3 times, respectively. Necrotic chips and strip tissues were extracted and sent to exfoliated cytology, tNGS and pathological biopsy detection. The whole operation process was smooth.
The results of exfoliated cytology showed a few lymphocytes and neutrophils (Figure 3B–E). Pathologist reported squeezed lymphocytes and a few epithelial-like cells (Figure 3F and G). Immunohistochemistry analysis was CK (-), Vim (+), Ki-67 (+) 1%, CEA nidus (+), CK7 (-), and anti-acid staining (-). Xpert MTB/RIF was negative. However, tNGS reported 1225 reads of Legionella pneumophila in the samples of lymph node (Supplementary Table 3). Soon a multidisciplinary team (MDT) including experts in hematology, pathology, rheumatism immunity, respiration, and infection discussed the exact cause of this young lady’s hospitalization. Finally, with all the information collected, especially the tNGS results, agreement was made that the patient’s condition can be rationally explained by the infection of Legionella pneumophila. Thus, levofloxacin continued. By the 15th day, joint pain of the patient was completely relieved, so she was discharged from the hospital with the advice of another week’s oral levofloxacin taking.
A month later, not only did the interstitial changes in both lungs were further improved by re-examination of chest CT scanning, but also the enlarged lymph nodes began to shrink (Figure 2E and F). On the 59th day, the patient was followed up at the outpatient department. The symptoms of joint pain did not recur, the interstitial changes of both lungs on chest CT were further improved, and the enlarged lymph nodes were further reduced (Figure 2G and H). During the last follow-up of 141th day, the joint pain still did not recur. The interstitial lesions in both lungs disappeared completely on chest CT, and the enlarged lymph nodes became much smaller (Figure 2I and J).
Discussion
To our knowledge, this is the first case report of Legionella’ disease confirmed by tNGS, which played an important role in diagnosis. The manifestation of this young lady was quite atypical, which undoubtedly increased the difficulty of diagnosis. In clinics, commonly seen Legionellosis is an atypical pneumonia.17,18 Typical cases manifest a series of distinct clinical syndromes (like fever, cough, sputum, and dyspnea) and radiographic changes, which are similar to pneumococcal pneumonia.2,19–21 Fever (67–100%) is usually present except in some patients with immunosuppression. Gastrointestinal and neurological manifestations in patients with pneumonia should suggest Legionellosis, including diarrhea (19–47%), nausea, vomiting (9–25%), abdominal pain, headache (17–43%), obtundation, seizures, and focal neurological findings (38–53%).19,20 About 50% patients have cough (41–92%) with purulent sputum. Less common symptoms include myalgia and arthralgia (20–43%).
The young lady in our case came to hospital with chief complaint of joint pain. And the first positive result was anti-Ro antibody (++). Therefore, autoimmune disorders especially rheumatoid arthritis and Sjogren’s syndrome should be considered first. However, the detections of rheumatoid arthritis-related antibodies (anti-CCP, RF) were all negative. Color Doppler ultrasound of parotid and bilateral submandibular glands and pathological biopsy of labial mucosa showed no abnormalities. As a result, the above two diagnoses were excluded. A follow-up chest CT examination found interstitial changes in both lungs and multiple lymphadenopathies. CAP was considered. But the patient had no respiratory symptoms such as fever, cough, expectoration, and shortness of breath. Meanwhile, the relevant pathogenic tests were all negative. After a week of empirical antibiotics treatment with quinolones, chest CT showed that bilateral pneumonia was absorbed a bit, but lymphadenopathy did not improve. At that time, the causes of lymphadenopathy should be excluded from lymphoma, lymph node tuberculosis, and other diseases. Subsequent mediastinal lymph node punctures did not support the diagnosis of lymphoma and tuberculosis, but found the pathogen of Legionella pneumophila. Diagnosis was finally confirmed with a great help of tNGS results, along with the recovering of patients’ condition after being treated with levofloxacin.
For the specific treatment of Legionellosis, macrolides, doxycycline, and quinolones are the first-line antibiotics that have been widely used in clinic. It is worth mentioning that β-lactams and aminoglycosides are ineffective.22 For the young lady in this case, although the pathogen of CAP was not clear at the beginning, we empirically selected quinolones who can not only cover Streptococcus pneumoniae and Haemophilus influenzae but also attack mycoplasma, chlamydia, and other atypical bacteria. Then, this choice was a proven correct choice by the results of tNGS test and satisfied achieved result.
mNGS is an effective new tool in clinic to help the diagnosis of infectious disease, especially some rare infection and emerging infectious disease. However, not everyone can afford it. Meanwhile, tNGS test has similar advantages of fast detection speed, broad coverage, and high accuracy as mNGS, but its cost is only a quarter. More important, for tNGS test, DNA and RNA dual process detection can be conducted in one experiment, which could not be achieved by mNRG test. In this case, we observed that this new technique is effective in detecting hard-to-culture, atypical, and rare pathogens. Of course, shortcomings exist as tNGS test cannot detect unknown new pathogenic microorganisms. There was another interesting exploration of culture-free phylogenetic analysis of Legionella pneumophila.23 In any case, we believe that our brave attempt of tNGS test in pathogen identification will guide clinical diagnosis and treatment, and then greatly benefit patients.
Data Sharing Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding authors.
Ethic Statement
This study was approved by the Ethics Committee of Chongqing Medical University in accordance with the principles stated in the Declaration of Helsinki, and written informed consent was provided by the patient to have the case details and any accompanying images published included in this article. Approval from the Ethics Committee of Chongqing Medical University was required to publish the case details.
Acknowledgments
We thank Chongqing KingMed Diagnostics for tNGS detection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Chongqing Talents Project (cstc2021ycjh-bgzxm0150), the General Program of Chongqing Natural Science Foundation (CSTB2022NSCQ-MSX0901), and Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University (kryc-yq-2204).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cunha BA. Clinical features of legionnaires’ disease. Semin Respir Infect. 1998;13:116–127.
2. Mulazimoglu L, Yu VL. Can Legionnaires disease be diagnosed by clinical criteria? A critical review. Chest. 2001;120:1049–1053. doi:10.1378/chest.120.4.1049
3. Cunha BA. Severe Legionella pneumonia: rapid presumptive clinical diagnosis with Winthrop-University Hospital’s weighted point score system (modified). Heart Lung. 2008;37:311–320. doi:10.1016/j.hrtlng.2007.12.003
4. Muder RR, Yu VL, Fang GD. Community-acquired Legionnaires’ disease. Semin Respir Infect. 1989;4:32–39.
5. Viasus D, Di Yacovo S, Garcia-Vidal C, et al. Community-acquired Legionella pneumophila pneumonia: a single center experience with 214 hospitalized sporadic cases over 15 years. Medicine. 2013;92:51–60. doi:10.1097/MD.0b013e31827f6104
6. Chahin A, Opal SM. Severe pneumonia caused by Legionella pneumophila: differential diagnosis and therapeutic considerations. Infect Dis Clin North Am. 2017;31:111–121. doi:10.1016/j.idc.2016.10.009
7. Diederen BMW. Legionella spp. and Legionnaires’ disease. J Infect. 2008;56(1):1–12. doi:10.1016/j.jinf.2007.09.010
8. Fraser DW, Tsai TR, Orenstein W, et al. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. doi:10.1056/NEJM197712012972201
9. WHO. Legionella and the Prevention of Legionellosis. China: WHO Press; 2007:19–21.
10. Bartram J, Chartier Y, Lee JV, Pond K, Surman-Lee S. Legionella and the prevention of legionellosis. Geneva, Switzerland: World Health Organization Press; 2007. Available from: http://www.who.int/water_sanitation_health/emerging/legionella.pdf.
11. Edelstein PH, Cianciotto NP. Legionella. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases.
12. Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. 2015;28:95–133. doi:10.1128/CMR.00029-14
13. Edelstein PH. Legionella. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology.
14. Cunha BA, Burillo A, Bouza E. Legionnaires′ disease. Lancet. 2016;387:376–385. doi:10.1016/S0140-6736(15)60078-2
15. Yue R, Wu X, Li T, Chang L, Huang X, Pan L. Early detection of Legionella pneumophila and Aspergillus by mNGS in a critically ill patient with legionella pneumonia after extracorporeal membrane oxygenation treatment: case report and literature review. Front Med. 2021;8:686512. doi:10.3389/fmed.2021.686512
16. Li SY, Tong J, Liu Y, Shen W, Hu P. Targeted next generation sequencing is comparable with metagenomic next generation sequencing in adults with pneumonia for pathogenic microorganism detection. J Infect. 2022;2022:
17. Cunha BA. Legionnaires’ disease: clinical diff erentiation from typical and other atypical pneumonias. Infect Dis Clin North Am. 2010;24:73–105. doi:10.1016/j.idc.2009.10.014
18. Woodhead MA, Macfarlane JT. Comparative clinical and laboratory features of legionella with pneumococcal and mycoplasma pneumonias. Br J Dis Chest. 1987;81:133–139. doi:10.1016/0007-0971(87)90130-6
19. Granados A, Podzamczer D, Gudiol F, Manresa F. Pneumonia due to Legionella pneumophila and pneumococcal pneumonia: similarities and diff erences on presentation. Eur Respir J. 1989;2:130–134. doi:10.1183/09031936.93.02020130
20. Roig J, Aguilar X, Ruiz J, et al. Comparative study of Legionella pneumophila and other nosocomial-acquired pneumonias. Chest. 1991;99:344–350. doi:10.1378/chest.99.2.344
21. Gupta SK, Imperiale TF, Sarosi GA. Evaluation of the Winthrop-University Hospital criteria to identify Legionella pneumonia. Chest. 2001;120:1064–1071. doi:10.1378/chest.120.4.1064
22. Levy ML, Le Jeune I, Woodhead MA, Macfarlaned JT, Lim WS; the British Thoracic Society Community Acquired Pneumonia in Adults Guideline Group. Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK. Prim Care Respir J. 2010;19:21–27. doi:10.4104/pcrj.2010.00014
23. Domazetovska A, Jensen SO, Gray M, Radzieta M, Maley M. Culture-free phylogenetic analysis of legionella pneumophila using targeted CRISPR/Cas9 next-generation sequencing. Clin Microbiol. 2022;10(4):e00359–22.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.