Back to Journals » Drug Design, Development and Therapy » Volume 17
Jiawei Danxuan Koukang Alleviates Arecoline Induced Oral Mucosal Lesions: Network Pharmacology and the Combined Ultra-High Performance Liquid Chromatography (UPLC) and Mass Spectrometry (MS)
Authors Zhou L, Tan J, Dai Y, Zhu K, Xiao Y, Wu D, Wang Z, Tan Y, Qin Y
Received 20 April 2023
Accepted for publication 29 August 2023
Published 13 October 2023 Volume 2023:17 Pages 3085—3101
DOI https://doi.org/10.2147/DDDT.S413897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Linghang Zhou, Jin Tan, Yuzhe Dai, Keke Zhu, Yanbo Xiao, Dan Wu, Zongkang Wang, Yisi Tan, Yijie Qin
Department of Stomatology, The First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, 410007, People’s Republic of China
Correspondence: Jin Tan, Department of Stomatology, The First Affiliated Hospital of Hunan University of Chinese Medicine, 95 Shaoshan Middle Road, Changsha, Hunan, People’s Republic of China, Email [email protected]
Purpose: Arecoline is one of the main toxic components of arecoline to cause oral mucosal lesions or canceration, which seriously affects the survival and life quality of patients. This study analyzed the mechanism of Jiawei Danxuan Koukang (JDK) in alleviating arecoline induced oral mucosal lesions, to provide new insights for the treatment of oral submucosal fibrosis (OSF) or cancerosis.
Methods: Metabolomics was applied to analyze the composition of JDK and serum metabolites. The active ingredients of JDK were analyzed by the combined ultra-high performance liquid chromatography and mass spectrometry. The target network of JDK, metabolites and OSF was analyzed by network pharmacology, and molecular docking. Oral mucosal lesions and fibrosis were analyzed by HE and Masson staining. Cell differentiation, proliferation and apoptosis were detected. The expressions of α-SMA, Collagen I, Vimentin, Snail, E-cadherin, AR and NOTCH1 were detected by Western blot.
Results: Arecoline induced the gradual atrophy and thinning of rat oral mucosal, collagen accumulation, the increase expressions of fibrosis-related proteins and Th17/Treg ratio. JDK inhibited arecoline-induced oral mucosal lesions and inflammatory infiltration. Arecoline induced changes of serum metabolites in Aminoacyl-tRNA biosynthesis, Alanine, aspartate and glutamate metabolism and Arginine biosynthesis pathways, which were reversed by M-JDK. Quercetin and AR were the active ingredients and key targets of JDK, metabolites and OSF interaction. Arecoline promoted the expression of AR protein, and the proliferation of oral fibroblasts. Quercetin inhibited the effect of arecoline on oral fibroblasts, but was reversed by AR overexpression. Arecoline induced NOTCH1 expression in CAL27 and SCC-25 cells, and promoted cell proliferation, but was reversed by M-JDK or quercetin.
Conclusion: JDK improved the arecoline-induced OSF and serum metabolite functional pathway. Quercetin targeted AR protein to improve arecoline-induced OSF. JDK and quercetin inhibited arecoline-induced NOTCH1 protein expression in CAL27 and SCC-25 cells to play an anti-oral cancer role.
Keywords: oral submucous fibrosis, Jiawei Danxuan Koukang, arecoline, pharmacology, metabolomics
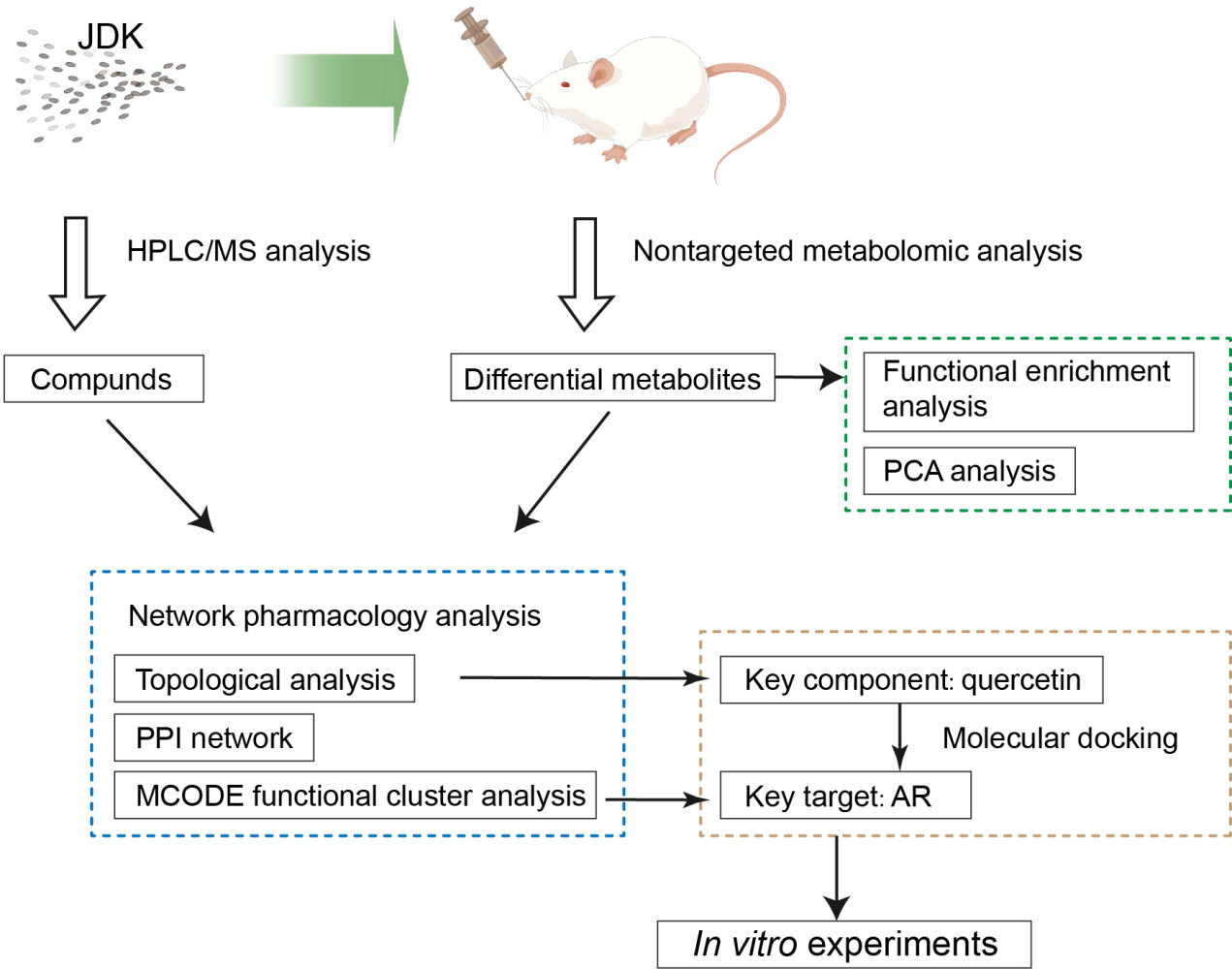
Graphical Abstract:
Introduction
Oral cancer is a major public health problem, is the sixth most common malignancy in the world, with a growing trend affecting young men and women.1 Betel nut is one of the most commonly consumed psychoactive substances in the world, estimated to be consumed by about 10% of the world’s population, and plays a role in regulating the development of several diseases in the mouth.2 Oral leukoplakia and oral submucosal fibrosis (OSF) are two major oral potential malignant diseases caused by chewing areca nut, which may develop into oral cancer if continued consumption of areca nut.3 Arecoline is a major arecoline alkaloid.4 The polymorphism of arecoline’s metabolism-related enzyme encoding gene may predispose organisms to oral cancer.5 Therefore, exploring the pathogenesis of arecoline in OSF or canceration is helpful to develop new therapeutic strategies.
OSF is a collagen deposition disorder that affects oral function and quality of life in patients, and may even turn into malignant tumors.6 At present, there is a lack of effective clinical diagnosis and treatment of OSF, most of which focus on reducing inflammation and improving oral opening to improve patients’ quality of life.7 The expression of Notch was significantly upregulated in OSF tissues and TGF-β1-induced buccal mucosal fibroblasts.8 NOTCH1 was identified as an independent prognostic factor for overall survival of early oral squamous cell carcinoma (OSCC).9 Aberrant activation of Notch signaling was significantly associated with lymph node metastasis (LNM), invasion depth, and local recurrence in OSCC.10,11 NOTCH1 protein expression was also involved in arecoline induction of oral cancer.12 Therefore, we supposed that NOTCH1 may be a potential target for the treatment of arecoline induced oral mucosal lesions or canceration.
OSF was accompanied by significant changes in the immune microenvironment.13 In addition, areca nut can induce the secretion of TNF-α, IL-6 and prostaglandin E2 by oral keratinocytes, which may lead to oral mucosal inflammation.14,15 OSF belongs to the TCM (Traditional Chinese Medicine) category of “imbalance of Yin and Yang, the generation of internal cancer toxins, phlegm and blood stasis”. The treatment principle is to “tonify Qi, activate blood circulation, eliminate phlegm, and detoxify”. The Jiawei Danxuan Koukang (JDK) formula is based on the classic formula Tao Hong Si Wu Tang and Danxuan Koukang (DXKK), with modifications and adjustments. It consists of Chinese herbal medicines such as Salvia miltiorrhiza Bunge, Scrophularia ningpoensis Hemsl, Angelica sinensis (Oliv.) Diels, Carthamus tinctorius Linn, Rehmannia henryi N. E. Brown, Hedyotis diffusa Willd, Astragalus saxorum Simps, Mentha haplocalyx Briq, Platycodon grandiflorus (Jacq.) A. DC, Prunella vulgaris Linn, Artemisia capillaris Thunb and Paeonia lactiflora Pall. In the formula, Salvia miltiorrhiza Bunge, Angelica sinensis (Oliv.) Diels, Carthamus tinctorius Linn, and Paeonia lactiflora Pall promote blood circulation, improve blood vessel function, and relieve blood stasis and pain. Astragalus saxorum Simps strengthens Qi, stabilizes the exterior, and regulates immune function. Rehmannia henryi N. E. Brown, Scrophularia ningpoensis Hemsl, and Artemisia capillaris Thunb clear heat, promote fluid production, nourish Yin, and nourish blood. Hedyotis diffusa Willd and Prunella vulgaris Linn clear heat, detoxify, dissolve stagnation, and disperse masses. Mentha haplocalyx Briq disperses wind-heat, while Platycodon grandiflorus (Jacq.) A. DC eliminates phlegm and drains pus. The JDK formula works together to promote blood circulation, detoxify, dissolve stasis, strengthen the body, and expel pathogenic factors. Previous studies showed that DXKK had an inhibitory effect on the occurrence and development of OSF.16,17 However, the exact mechanism and molecular target of JDK in OSF is not clear. Network pharmacology is a new discipline to elucidate the role of traditional Chinese medicine by constructing a network of “component-target-biomarker” and screening specific targets.18 Therefore, exploring the pharmacological mechanism and molecular target of JDK and OSF may contribute to the development of new treatment methods.
The development of OSF is accompanied by significant changes in metabolites and dysregulation of metabolic events.19 Recent studies have shown that network pharmacology combined with metabolomics (ultra-high performance liquid chromatography and mass spectrometry) or molecular docking can effectively reveal the underlying mechanisms of drug therapy.20,21 For example, researchers found that agarwood intervention can affect the metabolic composition of mice serum, and further combined with network pharmacology to analyze key compounds, targets and biomarkers, clarifying the feasibility of the material basis and mechanism of biomarkers.22 The components of Xihuang Pills can directly act on the target of androtinib, regulate endogenous metabolic disorders in vivo, and thus synergically inhibit tumor growth.23 Similarly, baicalin, the main component of scutellaria baicalensis, may inhibit cholesterol biosynthesis by inhibiting SQLE and LSS, which are important enzymes in the pathway of cholesterol biosynthesis.24 Therefore, the integration of metabolomics and network pharmacology may provide great inspirations for exploring the potential pharmacological activity of JDK and clarifying its possible intervention mechanism.
In this study, the potential molecular targets of JDK were screened by combining network pharmacology and molecular docking. The active ingredients of JDK were analyzed by metabolomics in drug-containing serum. The main regulatory targets of JDK effect were screened by intervening the OSF cell model with drug-containing serum. The potential mechanism of drug components was analyzed at the molecular and cellular levels, so as to clarify the potential effect of JDK on arecoline induced OSF and malignant characterization of oral cancer cells, and to provide reference for the development of new drug therapy.
Materials and Methods
Construction and Grouping of Arecoline Induced Rat Models
The rats were purchased from Hunan Slyke Jingda Experimental Animal Co., LTD. The rats were raised on a 22–24°C, standard diet, and a 12:12 hour light-dark cycle. All the animal care and experimental procedures follow the Guidelines for the Care and Use of Laboratory Animals developed by the Ministry of Science and Technology of China, and the Animal Ethics Committee of Hunan University of Chinese Medicine (No. LLBH-202103100002). This study followed Principles of Declaration of Helsinki. According to previous studies, arecoline was established by oral mucosa injection to induce OSF rat model.25 In short, the rats were injected subbuccal with 0.2 mL arecoline hydrobromide dissolved in 0.9% normal saline every two days (10 mg/mL, B20112, Shanghai Yuanye Bio-Technology Co., Ltd).26 JDK was provided by The First Affiliated Hospital of Hunan University of Chinese Medicine and composed of 10g Salvia miltiorrhiza Bunge, 10g Scrophularia ningpoensis Hemsl, 10g Angelica sinensis (Oliv.) Diels, 5g Carthamus tinctorius Linn, 10g Rehmannia henryi N. E. Brown, 10g Hedyotis diffusa Willd, 10g Astragalus saxorum Simps, 10g Mentha haplocalyx Briq, 10g Platycodon grandiflorus (Jacq.) A. DC, 10g Prunella vulgaris Linn, 10g Artemisia capillaris Thunb and 10g Paeonia lactiflora Pall. JDK (10 mL/kg) was administered by intragastric administration 5 days a week.16,17 JDK was set in order of low dose (L-JDK, 4 g/kg), medium dose (M-JDK, 12 g/kg) and high dose (H-JDK, 24 g/kg) groups. The low, medium, and high dose groups in the rat experiment respectively correspond to 1, 3, and 6 times the clinical equivalent dose in humans. Based on clinical research, lycopene (HY-N0287, MedChemExpress) was used as a positive drug control group.27–29 The dose of lycopene is 8 mg/d according to human clinical use, and the equivalent dose of rats is 0.72 mg/kg according to body surface area.28,30 After 49 days of continuous intervention, the rats were euthanized, and blood (serum) and buccal membrane tissues were collected for follow-up detection and analysis.
Cell Experiment
Rat oral fibroblasts (CP-R230) were purchased from Procell. Cells were cultured in the complete medium (CM-R230, Procell) at 95% air, 5% CO2, 37°C environments. Different concentrations of arecoline (0, 0.05, 0.10, 0.15, 0.20, 0.30 mM) were used to intervene cells in order to screen the suitable concentration of arecoline treatment.31 Subsequently, different concentrations of quercetin (0, 0.05, 0.10, 0.15, 0.20, 0.30 mM) were treated on the basis of arecoline intervention cells. Then, rat oral fibroblasts were randomly divided into control group, Arecoline group, Arecoline + vehicle, Arecoline + quercetin group, Arecoline + quercetin +oe-NC, Arecoline + quercetin +oe-AR group. Cells in groups 2 and 3 were treated with vehicle (Dimethyl sulfoxide) or quercetin for 24 h along with arecoline.32 The cells of the latter two groups were transfected with oe-NC or oe-AR plasmid for 48h, and treated with arecoline and quercetin, respectively.
Human tongue squamous cell carcinoma (CAL27, ZQ0606) and human oral squamous cell carcinoma (SCC-25, ZQ0759) were purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. CAL27 cells were cultured in DMEM medium with 10% fetal bovine serum (FBS). SCC-25 cells were cultured in DMEM/F12 medium, 10% FBS, 400 ng/mL hydrocortisone, 1 mM sodium pyruvate. All cells were incubated at 95% air, 5% CO2, 37°C environments. The two cancer cells were treated with arecoline and quercetin, respectively. The dose and treatment time were the same as above.
Network Pharmacological Analysis
First, compounds of Salvia miltiorrhiza Bunge, Scrophularia ningpoensis Hemsl, Angelica sinensis (Oliv.) Diels, Carthamus tinctorius Linn, Rehmannia henryi N. E. Brown, Hedyotis diffusa Willd, Astragalus saxorum Simps, Mentha haplocalyx Briq, Platycodon grandiflorus (Jacq.) A. DC, Prunella vulgaris Linn, Artemisia capillaris Thunb and Paeonia lactiflora Pall were searched through TCMSP database (https://old.tcmsp-e.com/index.php).33 The screening criteria were OB≥30% and DL≥0.18. SMILES ids of metabolites were obtained based on the pubchem database (https://pubchem.ncbi.nlm.nih.gov/).34 SMILES ids were imported into the Swisstargets database (http://www.swisstargetprediction.ch/)35 to retrieve metabolite targets, which were corrected by the uniprot database (https://www.uniprot.org/).36 With “Oral submucous fibrosi” as key words, human gene search was conducted in GeneCards,37 NCBI,38 OMIM39 and CTD40 databases. The targets of these four databases were summarized and de-weighted, and then analyzed as disease-related genes. The selected drug targets, metabolite targets and disease targets were input into Venny 2.1 to obtain common targets. The common targets of drug diseases were entered into the String database (https://string-db.org/cgi/input.pl) to construct the PPI network.41 The constructed PPI network was imported into Cytoscape3.8.0 to analyze gene clusters and screen core targets by MCODE module.42 A composition-disease-metabolite network map was constructed based on the included components, metabolites and disease targets. Topological analysis screens the key components of drugs. This study has been exempted by the Medical Ethics Committee of The First Affiliated Hospital of Hunan University of Chinese Medicine (No. HN-LL-LW-2023-017).
Identification of JDK Drug Constituents
JDK compound consists of 10g Salvia miltiorrhiza Bunge, 10g Scrophularia ningpoensis Hemsl, 10g Angelica sinensis (Oliv.) Diels, 5g Carthamus tinctorius Linn, 10g Rehmannia henryi N. E. Brown, 10g Hedyotis diffusa Willd, 10g Astragalus saxorum Simps, 10g Mentha haplocalyx Briq, 10g Platycodon grandiflorus (Jacq.) A. DC, 10g Prunella vulgaris Linn, 10g Artemisia capillaris Thunb and 10g Paeonia lactiflora Pall. JDK was identified by AB5600 and high-resolution mass spectrometry. Among them, the flow rate was 0.3 mL/min, the temperature of the sample plate was 4°C, and the temperature of the column was 40°C. Waters HSS T3 column (100×2.1 mm, 1.7µm) column was used with (A) H2O (0.1%FA) and (B) Acetonitrile as mobile phases. The sample size was 3μL. The liquid phase gradient conditions were 1%B for 0.01~1.5min, 99%B for 13~16.5min, and 1%B for 16.6~20min. The Mass condition was tested positive and negative. After detection, the compound spectra are compared with the chemical fingerprint spectra in the library to determine the molecular formula and name of the compound. Subsequently, peak areas are calculated for each compound based on their retention time in the spectra. The final qualitative and quantitative results of the compounds can be found in Supplementary Table 1.
Non-Targeted Metabolomics Analysis
LC-MS/MS analysis was performed using UHPLC system (1290, Agilent Technologies), UPLC BEH amide column (1.7μm 2.1×100mm, Waters), TripleTOF 6600 (Q-TOF, AB Sciex) and QTOF 6550 (Agilent). ProteoWizard was used to convert MS original data files into mzXML format and was processed by R package XCMS (version 3.2). The preprocessing results generated a data matrix, including retention time (RT), mass/charge ratio (m/z) and peak intensity. R package CAMERA is used for peak annotation after XCMS data processing. MetaboAnalyst platform (https://www.metaboanalyst.ca/) for differential metabolite analysis. Kyoto Encyclopedia of Genes and Genomes (https://www.kegg.jp/) was applied to function prediction of metabolites.
Molecular Docking
Molecular docking aims to obtain new drug candidates in a short period of time and at low cost through computational tools.43 Docking aims to closely observe the ligand that binds to the receptor and predict the interactions and energies between the receptor and the ligand. VINA 1.1.2 software was used to study the docking between quercetin and AR protein. Discovery Studio was then used for visual analysis of quercetin and AR proteins.
Hematoxylin-Eosin (HE) Staining
The oral mucosa tissues were fixed, paraffin (Sigma) embedding and sectioned successively by microtome (YD-315, Jinhua Yidi Medical Equipment Co., Ltd). After roasting at 60°C for 12 h, the sections were dewaxed to water. Subsequently, hematoxylin (Abiowell) and eosin (Abiowell) were stained, respectively. Finally, the sections were dehydrated, sealed and examined under a microscope (BA210T, Motic).
Masson Staining
The slices of oral mucosa were roasted at 60°C for 3 h. Sections were dewaxed to water and shaken off the water. An appropriate amount of nuclear dye was added to the sections to stain for 3~5 min. The sections were rinsed completely with running water to remove the dye, and soaked in distilled water. The sections were soaked in ammonia water for 5~10 min to make the nuclei return to blue. Afterwards, the sections were added with Masson staining solution (Abiowell) and stained for 2 min. Then, the sections were separated by color separation solution for about 30s. Finally, the slices were stained with a compound dye, cleaned, dried and sealed for observation.
Cell Counting Kit-8 (CCK8)
Cells in different groups were digested by pancreatic enzyme digestion solution (AWC0232, Abiowell) and inoculated into 96-well plates at a density of 1×104 cells/well. After adherent culture, 10μL/well of CCK8 solution was added to each well (NU679, Dojindo laboratories). The cells were incubated at 37°C with 5% CO2 for 4 h, and analyzed with Bio-Tek assay (MB-530, HEALES).
Enzyme Linked Immunosorbent Assay (ELISA)
Whole blood samples were placed at room temperature for 2 h, and centrifuged at 1000 g at 2~8°C for 15 min. Supernatant was taken for immediate detection. The tissue sample (100 mg) was cut into small pieces and put into a tissue grinder (homogenate tube) with 1 mL 1×PBS to make homogenate. Then, tissue homogenates were subjected to repeated freeze-thaw treatment at −20°C to destroy cell membranes. Tissue homogenates were centrifuged at 5000 g at 2~8°C for 5 min to obtain supernatant for detection. BCA method (AWB0104, Abiowell) was used to quantify the protein in the supernatant of tissue samples, and then used for follow-up detection. Then, TGF-β1 (CB-E04727R, CUSABIO), TNF-α (CB-E11987R, CUSABIO), IL-6 (CB-E04640R, CUSABIO), ROS (rat, E004-1-1, Nanjing Jiancheng Bioengineering Institute), and SOD (A001-3-2, Nanjing Jiancheng Bioengineering Institute) kits were used to detect the levels of TGF-β1, TNF-α and IL-6 in the above samples, respectively.
Flow Cytometry
Cells of different treatments were digested and collected with EDTA-free pancreatic enzyme (AWC0232, Abiowell). The cells were washed with PBS twice, centrifuged at 2000 rpm for 5 min each time to collect 2×105 cells. The cells were suspended with 500 μL binding buffer using the apoptosis kit (KGA1030). Subsequently, the cells were incubated with annexin V-APC and propidium iodide in isolation from light. Finally, apoptosis was detected by flow cytometry.
The fresh tissue was chopped with ophthalmic scissors and added with 5 mL 0.25% collagenase II (AWH0565a, Abiowell), which was digested in a shaking bed at 37°C. A 5 mL complete medium was added to stop digestion and filtered with a 100 μm strainer. The filtrate was centrifuged at 1500 rpm for 5 min to obtain cell precipitation. The 5 mL erythrocyte lysate (AWC0390a, Abiowell) was added for centrifugation to obtain cell precipitation. Cell precipitates were resuspended with 1 mL RPMI containing 2% FBS. For the Th17 detection, cells were added with 1 μL cell stimulation cocktail (00–4975-93, ebioscience) and cultured at 37°C for 4 h. For the Th17 or Treg detection, cells were fixed with 500 μL intracellular fixation buffer (00-5523-00, ebioscience) at room temperature. Cells were cleaned and re-suspended with 1×permeabilization buffer. Cells were incubated with CD4-APC (17-0040-82, ebioscience), CD25-FITC (MA1-35144, ebioscience), IL17A-PE (12-7177-81, ebioscience) or Foxp3-PE (12-5773-82, ebioscience). The cells were washed and resuspended with 0.5% BSA-PBS and detected by flow cytometry (A00-1-1102, Beckman).
Western Blot
Total proteins were extracted from cell or tissue samples using RIPA lysate (AWB0136, Abiowell). BCA method (AWB0104, Abiowell) was applied to detect protein concentration. The obtained protein samples were separated by SDS-polyacrylamide gel electrophoresis (AWT0047, Abiowell). Transfer electrophoresis transferred proteins from a gel to a NC membrane. The membranes were immersed and sealed with 5% skim milk powder (AWB0004, Abiowell), which was prepared with 1×PBST (AWI0130, Abiowell). The membranes were incubated separately with antibodies, including α-SMA (ab5694, 1μg/mL, Abcam, UK), Vimentin (ab92547, 1:2000, Abcam, UK), Collagen I (ab270993, 1:1000, Abcam, UK), SNAIL (#38791: 1000, CST, USA), E-cadherin (20874-1-AP, 1:10000, Proteintech, USA), AR (16036-1-AP, 1:1000, Proteintech, USA), NOTCH1 (10062-2-AP, 1: 2000, Proteintech, USA) and β-actin (66009-1-Ig, 1:5000, Proteintech, USA). Subsequently, the membranes were incubated with HRP goat IgG (SA00001-1/2, 1:5000/6000, Proteintech, USA). Finally, the membrane was incubated with the ECL chemiluminescence solution (AWB0005, Abiowell) and imaged in the imaging system (ChemiScope6100, CLiNX, China).
Data Statistics
The statistical analysis of the data in this study was carried out using Graphpad Prism8.0 statistical software. Measurement data was expressed as mean ± standard deviation. First, the normality and homogeneity of variance were tested. Non-paired t-test was used between groups. One-way ANOVA was used for comparison among multiple groups. Tukey’s conducted post-inspection. P<0.05 indicated that the difference was statistically significant.
Results
JDK Alleviated Arecoline Induced Oral Mucosal Lesions
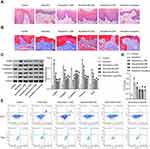
Arecoline induced the gradual atrophy and thinning of rat oral mucosal epithelial cells and increased collagen accumulation (Figure 1A and B). JDK drug treatment could alleviate this phenomenon, among which M-JDK and H-JDK have better effects and similar efficacy to lycopene (Figure 1A and B). In addition, arecoline induced the increase of α-SMA, Collage I, Vimentin and Snail expression, and the decrease of E-cadherin expression (Figure 1C). JDK inhibited the expression of α-SMA, Collage I, Vimentin and Snail proteins and promoted E-cadherin expression, in arecoline group in a dose-dependent manner, and the effects of M-JDK and H-JDK were consistent with those of lycopene (Figure 1C). Flow cytometry showed that arecoline induced the increase of Th17/Treg ratio, but was inhibited by JDK or lycopene (Figure 1D and E). The above results proved that M-JDK could alleviate arecoline induced oral mucosal fibrosis and immune cell infiltration, which was consistent with the efficacy of lycopene, and could be used for subsequent studies.
JDK Improved Serum Metabolism of Arecoline-Induced Rats
Further untargeted metabolomics analysis of arecoline induced serum metabolism in rats showed that serum metabolism changed after arecoline intervention in rats compared with normal rats (Figure 2A). In addition, M-JDK intervention changed the serum metabolism induced by arecoline in rats (Figure 2A). ANOVA and heat map analysis of metabolite abundance showed that compared with control group, cis-4,7,10,13,16,19-Docosahexaenoic acid, Elaidic acid, OLEATE, 6-Octadecenoic acid, Vaccenic acid, Gly-DL-Asp, 2-cis-Eicosenoic acid, Cholic acid, Cholan-24-oic acid, cis-4,10,13,16-Docosatetraenoic acid, CP 47,497 C8-homolog, 4-Hydroxy-2-Oxoglutaric Acid, cis-5,8,11-Eicosatrienoic acid, Uridine, 11,14,17-Eicosatrienoic acid, cis-8,11,14-Eicosatrienoic acid, 5Z,11Z,14Z-Eicosatrienoic acid, 5Z,8Z,14Z-Eicosatrienoic acid, Pentadecanoic acid, PALMITATE, N-Methylnicotinamide and 6-Methylnicotinamide increased in the arecoline group, but decreased with M-JDK intervention (Figure 2B). In addition, compared with the control group, Glutamyl-L-alanine, Ethyl carbamate, SARCOSINE, Alanine, AMINOADIPATE, N-METHYLGLUTAMATE, KYNURENINE, Orsellinic acid, S-Sulfo-L-Cysteine, D-Tagatose, Propionylcarnitine, D-Fructose, 2-Hydroxy-3-methoxychalcone and Deoxycytidine decreased in the arecoline group, but increased after M-JDK intervention (Figure 2B). KEGG function prediction analysis showed that different metabolites were enriched in the Aminoacyl-tRNA biosynthesis (hits of 13/48, P<0.001), Alanine, aspartate and glutamate metabolism (hits of 7/28, P<0.025), and Arginine biosynthesis (hits of 5/14, P<0.032) pathways (Figure 2C). These results indicated that JDK improved serum metabolism induced by arecoline in rats.
AR Was the Target of JDK in Improving Arecoline-Induced Oral Mucosal Lesions in Rats
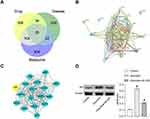
Network pharmacological analysis showed that there were 25 targets of JDK, metabolite and arecoline induced oral mucosal lesions (Figure 3A). The PPI network diagram further showed the interaction network of common targets (Figure 3B). Among them, AR was the key target in the interaction network (Figure 3C). Western blot analysis showed that AR protein was highly expressed in arecoline group, but was inhibited by JDK (Figure 3D). These results indicated that AR was the key target of JDK in improving arecoline-induced oral mucosal lesions in rats.
JDK Medicinal Ingredient Quercetin Targeted AR to Alleviate Arecoline-Induced Proliferation of Oral Fibroblast
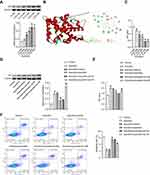
Arecoline at 0~0.20 mM promoted AR expression in fibroblasts in a dose-dependent manner (Figure 4A). However, the effect of 0.30 mM arecoline was similar to that of 0.20 mM arecoline, so 0.20 mM arecoline was selected for subsequent study (Figure 4A). Metabolomics identification and key components screen in network pharmacology of JDK indicated that quercetin and luteolin were key pharmacodynamic components (Supplementary Figure 1, Table 1, Supplementary Table 1 and Supplementary Table 2). Molecular docking further confirmed quercetin bound to AR protein (Figure 4B). 0~0.20 mM quercetin inhibited arecoline induced oral fibroblast activity (Figure 4C). However, 0.30 mM quercetin affected the activity of oral fibroblasts, so 0.20 mM quercetin was selected for subsequent intervention (Figure 4C). In addition, quercetin inhibited arecoline-induced AR protein expression in oral fibroblasts (Figure 4D). At the same time, the inhibition effect of quercetin on arecoline was reversed by AR overexpression (Figure 4D). Quercetin inhibited arecoline-induced decline in oral fibroblast proliferation, but was reversed by overexpression of AR (Figure 4E). Flow cytometry showed arecoline inhibited apoptosis of oral fibroblasts, but was reversed by quercetin (Figure 4F). Meanwhile, overexpression of AR treatment reversed the inhibitory effect of quercetin on arecoline (Figure 4F). These results demonstrated that quercetin of JDK inhibited arecoline-induced fibroblast proliferation by targeting AR.
 |
Table 1 Key Pharmacodynamic Components |
JDK Might Reduce the Risk of Arecoline-Induced Oral Cancer
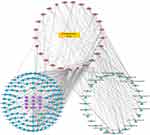
Network pharmacologic analysis showed that there was target interaction between JDK and OSF (Figure 5). In addition, arecoline induced the increase expression of NOTCH1 in oral mucosal tissues of rats, which may be related to the development of oral submucosal fibroma (Figure 6A). However, M-JDK inhibited the expression of NOTCH1 in oral mucosa induced by arecoline (Figure 6A). Considering the antioxidant capacity of lycopene,27 we further evaluated the expression of oxidative stress markers (ROS and SOD) in rats treated with M-JDK. M-JDK can inhibit the increase of ROS level, and the decrease of SOD level induced by arecoline in rat oral mucosa (Figure 6B). In addition, quercetin, a pharmaceutical component of JDK, inhibited arecoline induced NOTCH1 expression in CAL27 and SCC-25 cells (Figure 6C). Arecoline also promoted proliferation and inhibited apoptosis of CAL27 and SC-25 cells (Figure 6D–E). Quercetin inhibited cell proliferation and promoted apoptosis in arecoline-induced CAL27 and SC-25 cells (Figure 6D–E). These results indicated that quercetin of JDK reduced the risk of arecoline-induced oral cancer.
Discussion
OSF is one of the most common precancerous lesions of the oral mucosa, involving any part of the mouth, resulting in tissue scarring, difficulty swallowing, and muscle atrophy.44 Histopathologically, OSF is characterized by epithelial atrophy, chronic inflammation, and hyalinization of the proximal epithelium, leading to submucosal tissue fibrosis.45 α-SMA, Ki67, and CD105 were significantly increased in OSCC in the context of OSF compared to OSF and normal subjects.46 Our study confirmed that M-JDK inhibited arecoline-induced epithelial atrophy, collagen accumulation, the increase of α-SMA, Collage I, Vimentin and Snail proteins, and the decrease of E-cadherin protein in rat oral mucosal tissue, with similar efficacy to that of lycopene. These studies proved that JDK could be used as a potential drug to improve arecoline induced OSF.
Research has shown that several amino acids, including valine, lysine, and phenylalanine, can serve as biological markers for the severity of OSF.47 In addition, the p53 codon 72 polymorphism is predominantly observed as Arginine/Arginine protein in the control group, OSF group, and OSCC group, followed by Proline/Proline and Arginine/Proline proteins.48 These findings suggest that amino acids were involved in protein synthesis-mediated disease progression of OSF. Furthermore, recent studies have confirmed that local photodynamic therapy using 5-aminolevulinic acid was safe and effective for the treatment of oral leukoplakia combined with OSF, but long-term follow-up was required.49 Our study proved that JDK reversed the changes of serum metabolites in Aminoacyl-tRNA biosynthesis, Alanine, aspartate and glutamate metabolism and Arginine biosynthesis pathways induced by arecoline in rats. The above studies confirm that JDK improved disease characteristics in OSF rats by regulating the amino acid transportation-protein synthesis pathway, but further investigation was needed to elucidate the potential molecular targets.
Arecoline affected the production of inflammatory cytokines (IL2, IL-6, TGF-β1) in fibroblasts, which then acted on Th17 and Treg cells to cause fibrosis.50 Th17/Treg ratio is a potential diagnostic indicator of OSF development and malignant transformation, and an independent prognostic factor for OSCC.51 Elevated levels of Th17 cells in peripheral blood and tumor tissue in patients with OSCC reflect T-cell tolerance and a chronic inflammatory state that promotes tumor growth.52 Compared with the control group, OSCC patients had a higher percentage of low-density neutrophils and Th17 cells, as well as a lower percentage of normal-density neutrophils.53 Our study found that JDK inhibited the increase of Th17/Treg ratio in arecoline-induced rat oral mucosa. These studies have demonstrated that JDK could improve chronic inflammation of rat oral mucosa by inhibiting arecoline-induced the increase of Treg/Th17 ratio.
More than 20% AR-positive cytoplasmic staining in neoplastic OSSC epithelium is a significant predictor of the risk of OSCC metastasis.54 OSSC tumor and OSCC cell lines express AR, which promotes cell migration by increasing EGFR phosphorylation.55,56 Triphala restored normal epithelial thickness of oral submucosal fibrosis and inhibited the aging of oral mucosal epithelial cells.57 Quercetin inhibits OSCC cell survival and metastasis through an EMT-mediated pathway, especially Slug.32 Quercetin inhibited cell proliferation, G1 cycle arrest, and apoptosis by activating the p38 signaling pathway in OSCC cells with different p53 states.58 Network pharmacological analysis showed that quercetin and AR were the active ingredients and key targets of JDK, metabolites and oral mucosal fibrosis interaction, and there was interaction between them. Arecoline promoted the expression of AR protein and the proliferation in oral fibroblasts, reflecting the malignant characteristics of OSF histopathology. Quercetin inhibited the effect of arecoline on oral fibroblasts, but was reversed by overexpression of AR. These studies confirmed that quercetin, JDK’s pharmacodynamic ingredient, improved arecoline-induced fibroblast proliferation by targeting AR.
Clinical studies have confirmed the increase of NOTCH1 expression in oral cancer tissues, and the combination of mutated NOTCH1 genes with other clinicopathological parameters may contribute to prognostic stratification of OSCC.59 NOTCH1 regulated TP53 expression from NOTCH1 knockdown oral cancer cells.60 Quercetin (20 μM) and IR (5 Gy) were used in combination to treat colon cancer by targeting colon cancer stem cells and inhibiting Notch-1 signaling.61 Quercetin 3 methyl ether treatment reduced Notch1 expression and induces phosphorylation of phosphoinositol 3 kinase (PI3K) and Akt, thereby inhibiting malignant characterization of human breast cancer cells.62 Our study confirmed that arecoline induced NOTCH1 protein expression and promoted cell proliferation in CAL27 and SCC-25 cells, but was reversed by JDK pharmacodynamic ingredient quercetin. These studies demonstrated that quercetin, a pharmacodynamic component of JDK, inhibited arecoline-induced malignant characterization of oral cancer cells by down-regulating NOTCH1 expression.
In summary, our research confirms the role of JDK in OSF rats and analyzes the improved serum metabolomic profile and function. It is worth further investigating the therapeutic effects of JDK in a clinical study with a large cohort and screening serum metabolic biomarkers for assessing OSF treatment. Furthermore, our study further demonstrates that JDK and its main active component, quercetin, exert anticancer effects by inhibiting arecoline-induced expression of NOTCH1 in oral cancer cells (CAL27 and SCC-25 cells). This is just one molecular target interaction that we have preliminarily confirmed in JDK therapy for OSF. Other potential molecular targets of JDK need to be further explored. This warrants further exploration of the therapeutic value of JDK in improving arecoline-induced OSF and carcinogenesis, potentially opening new avenues for the treatment of OSF and OSCC.
Conclusion
In conclusion, JDK improved arecoline induced oral mucosal lesions and serum metabolite changes in rats. JDK improved arecoline-induced fibroblast fibrosis by targeting AR protein of the pharmaceutical ingredient quercetin. In addition, JDK and its drug ingredient quercetin inhibited arecoline from inducing NOTCH1 expression in CAL27 and SCC-25 cells to play an anti-oral cancer role.
Abbreviations
OSF, oral submucosal fibrosis; OSCC, oral squamous cell carcinoma; LNM, lymph node metastasis; JDK, Jiawei Danxuan Koukang; DXKK, Danxuan Koukang; RT, retention time; m/z, mass/charge ratio; HE, Hematoxylin-eosin; CCK8, Cell Counting Kit-8; ELISA, Enzyme linked immunosorbent assay; UPLC, Ultra-high performance liquid chromatography; MS, Mass spectrometry.
Data Sharing Statement
All original data can be obtained from corresponding authors when necessary.
Ethic Approval
All the animal care and experimental procedures follow the Guidelines for the Care and Use of Laboratory Animals developed by the Ministry of Science and Technology of China, and the Animal Ethics Committee of Hunan University of Chinese Medicine (No. LLBH-202103100002). This study has been exempted by the Medical Ethics Committee of The First Affiliated Hospital of Hunan University of Chinese Medicine (No. HN-LL-LW-2023-017). This study followed Principles of Declaration of Helsinki.
Acknowledgments
Thank you for the technical support provided by FigDraw for the graphical abstract (www.figdraw.com).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81874496).
Disclosure
There is no conflict of interest to declare.
References
1. Warnakulasuriya S, Kerr AR. Oral cancer screening: past, present, and future. J Dent Res. 2021;100(12):1313–1320. doi:10.1177/00220345211014795
2. Ku CW, Day CH, Ou HC, et al. The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats. Open Life Sci. 2021;16:1182–1192. doi:10.1515/biol-2021-0116
3. Warnakulasuriya S, Chen THH. Areca nut and oral cancer: evidence from studies conducted in humans. J Dent Res. 2022;101(10):1139–1146. doi:10.1177/00220345221092751
4. Oliveira NG, Ramos DL, Dinis-Oliveira RJ. Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: an updated review. Arch Toxicol. 2021;95(2):375–393. doi:10.1007/s00204-020-02926-9
5. Das A, Giri S. A review on role of arecoline and its metabolites in the molecular pathogenesis of oral lesions with an insight into current status of its metabolomics. Prague Med Rep. 2020;121:209–235. doi:10.14712/23362936.2020.19
6. Shen YW, Shih YH, Fuh LJ, et al. Oral submucous fibrosis: a review on biomarkers, pathogenic mechanisms, and treatments. Int J Mol Sci. 2020;21(19):7231. doi:10.3390/ijms21197231
7. Fang CY, Chen SH, Huang CC, et al. Fucoidan-mediated inhibition of fibrotic properties in oral submucous fibrosis via the MEG3/miR-181a/Egr1 axis. Pharmaceuticals. 2022;15(7):833. doi:10.3390/ph15070833
8. Wang J, Yang L, Mei J, et al. Knockdown of notch suppresses epithelial-mesenchymal transition and induces angiogenesis in oral submucous fibrosis by regulating TGF-β1. Biochem Genet. 2023. doi:10.1007/s10528-023-10452-3
9. Wang S, Fan H, Xu J, et al. Prognostic implication of NOTCH1 in early stage oral squamous cell cancer with occult metastases. Clin Oral Investig. 2018;22(3):1131–1138. doi:10.1007/s00784-017-2197-9
10. Pandey A, Bhuvanadas S, Joseph JP, et al. Notch signalling: a potential therapeutic pathway in oral squamous cell carcinoma. Endocr Metab Immune Disord Drug Targets. 2021;21(12):2159–2168. doi:10.2174/1871530321666210421125231
11. Yoshida R, Ito T, Hassan WA, et al. Notch1 in oral squamous cell carcinoma. Histol Histopathol. 2017;32:315–323.
12. Nithiyanantham S, Arumugam S, Hsu HT, et al. Arecoline N-oxide initiates oral carcinogenesis and arecoline N-oxide mercapturic acid attenuates the cancer risk. Life Sci. 2021;271:119156. doi:10.1016/j.lfs.2021.119156
13. Wang L, Tang Z. Immunopathogenesis of oral submucous fibrosis by chewing the areca nut. J Leukoc Biol. 2022;111(2):469–476. doi:10.1002/JLB.3MR0521-763RR
14. Tilakaratne WM, Ekanayaka RP, Warnakulasuriya S. Oral submucous fibrosis: a historical perspective and a review on etiology and pathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):178–191. doi:10.1016/j.oooo.2016.04.003
15. Jeng JH, Wang YJ, Chiang BL, et al. Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis. 2003;24:1301–1315. doi:10.1093/carcin/bgg083
16. Li Y, Tan J, Chen A. Effect of Danxuan Koukang on proliferation and PCNA production of human oral mucosal fibroblasts induced by areca nut extract. Chin J Inf Tradit Chin Med. 2006;2006:1.
17. Y-c LI, Tan J, Chen A, et al. Inhibition of Danxuan Koukang on collagen synthesis and TGFβ_1 expression in human oral mucosal fibroblasts induced by areca nut extract. J Tradit Chin Med Univ Hunan. 2007;2007:1.
18. Hao da C, Xiao PG. Network pharmacology: a Rosetta Stone for traditional Chinese medicine. Drug Dev Res. 2014;75(5):299–312. doi:10.1002/ddr.21214
19. Rai V, Bose S, Saha S, et al. Delineating metabolic dysfunction in cellular metabolism of oral submucous fibrosis using 1H nuclear magnetic resonance spectroscopy. Arch Oral Biol. 2019;97:102–108. doi:10.1016/j.archoralbio.2018.10.016
20. Cui X, Du M, Wei K, et al. Study of Xuanhuang Pill in protecting against alcohol liver disease using ultra-performance liquid chromatography/time-of-flight mass spectrometry and network pharmacology. Front Endocrinol. 2023;14:1175985. doi:10.3389/fendo.2023.1175985
21. Yang P, Zhong C, Huang H, et al. Potential pharmacological mechanisms of four active compounds of Macleaya cordata extract against enteritis based on network pharmacology and molecular docking technology. Front Physiol. 2023;14:1175227. doi:10.3389/fphys.2023.1175227
22. Dong M, Du H, Li X, et al. Discovery of biomarkers and potential mechanisms of agarwood incense smoke intervention by untargeted metabolomics and network pharmacology. Drug Des Devel Ther. 2022;16:265–278. doi:10.2147/DDDT.S348028
23. Li C, Wang Z, Chen W, et al. An Integrative Metabolomic and Network Pharmacology Study revealing the regulating properties of xihuang pill that improves anlotinib effects in lung cancer. Front Oncol. 2021;11:697247. doi:10.3389/fonc.2021.697247
24. Ge PY, Qi YY, Qu SY, et al. Potential mechanism of S. baicalensis on lipid metabolism explored via network pharmacology and untargeted lipidomics. Drug Des Devel Ther. 2021;15:1915–1930. doi:10.2147/DDDT.S301679
25. You Y, Huang Y, Wang D, et al. Angiotensin (1–7) inhibits arecoline-induced migration and collagen synthesis in human oral myofibroblasts via inhibiting NLRP3 inflammasome activation. J Cell Physiol. 2019;234(4):4668–4680. doi:10.1002/jcp.27267
26. Chiang MH, Lee KT, Chen CH, et al. Photobiomodulation therapy inhibits oral submucous fibrosis in mice. Oral Dis. 2020;26:1474–1482. doi:10.1111/odi.13409
27. Saran G, Umapathy D, Misra N, et al. A comparative study to evaluate the efficacy of lycopene and curcumin in oral submucous fibrosis patients: a randomized clinical trial. Indian J Dent Res. 2018;29:303–312. doi:10.4103/ijdr.IJDR_551_16
28. Arakeri G, Patil S, Maddur N, et al. Long-term effectiveness of lycopene in the management of oral submucous fibrosis (OSMF): a 3-years follow-up study. J Oral Pathol Med. 2020;49(8):803–808. doi:10.1111/jop.13085
29. Guo J, Xie H, Wu H, et al. Efficacy of lycopene in the treatment of oral submucous fibrosis: a meta-analysis of randomized controlled trials. J Evid Based Dent Pract. 2020;20(4):101471. doi:10.1016/j.jebdp.2020.101471
30. Piyush P, Mahajan A, Singh K, et al. Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: a randomized controlled trial. Oral Dis. 2019;25(1):73–79. doi:10.1111/odi.12947
31. Gu L, Xie C, Peng Q, et al. Arecoline suppresses epithelial cell viability through the Akt/mTOR signaling pathway via upregulation of PHLPP2. Toxicology. 2019;419:32–39. doi:10.1016/j.tox.2019.03.006
32. Kim SR, Lee EY, Kim DJ, et al. Quercetin inhibits cell survival and metastatic ability via the EMT-mediated pathway in oral squamous cell carcinoma. Molecules. 2020:25. doi:10.3390/molecules26010025
33. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminformatics. 2014;6(1):13. doi:10.1186/1758-2946-6-13
34. Kim S, Chen J, Cheng T, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49(D1):D1388–D1395. doi:10.1093/nar/gkaa971
35. Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–W364. doi:10.1093/nar/gkz382
36. UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(D1):D506–D515. doi:10.1093/nar/gky1049
37. Safran M, Dalah I, Alexander J, et al. GeneCards version 3: the human gene integrator. Database. 2010;2010(1):baq020. doi:10.1093/database/baq020
38. Agarwala R, Barrett T, Beck J. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2018;46(D1):D8–D13. doi:10.1093/nar/gkx1095
39. Amberger JS, Bocchini CA, Schiettecatte F, et al. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43(D1):D789–D798. doi:10.1093/nar/gku1205
40. Davis AP, Grondin CJ, Johnson RJ, et al. The comparative toxicogenomics database: update 2019. Nucleic Acids Res. 2019;47(D1):D948–D954. doi:10.1093/nar/gky868
41. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi:10.1093/nar/gky1131
42. Sun C, Yuan Q, Wu D, et al. Identification of core genes and outcome in gastric cancer using bioinformatics analysis. Oncotarget. 2017;8(41):70271–70280. doi:10.18632/oncotarget.20082
43. Liu J, Liu J, Tong X, et al. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of huai hua san against ulcerative colitis. Drug Des Devel Ther. 2021;15:3255–3276. doi:10.2147/DDDT.S319786
44. Gupta S, Jawanda MK. Oral submucous fibrosis: an overview of a challenging entity. Indian J Dermatol Venereol Leprol. 2021;87:768–777. doi:10.25259/IJDVL_371_20
45. Khan I, Pant I, Narra S, et al. Epithelial atrophy in oral submucous fibrosis is mediated by copper (II) and arecoline of areca nut. J Cell Mol Med. 2015;19(10):2397–2412. doi:10.1111/jcmm.12622
46. Kavitha L, Ranganathan K, Shyam S, et al. Immunohistochemical biomarkers in oral submucous fibrosis: a scoping review. J Oral Pathol Med. 2022;51(7):594–602. doi:10.1111/jop.13280
47. Goel R, G S, Chandrasekhar T, et al. Amino Acid profile in oral submucous fibrosis: a high performance liquid chromatography (HPLC) study. J Clin Diagn Res. 2014;8(12):Zc44–Zc48. doi:10.7860/JCDR/2014/10201.5290
48. Hallikeri K, Burde K, Anehosur V, et al. p53 polymorphism and association of human papillomavirus in oral submucous fibrosis and oral squamous cell carcinoma: a case-control study. J Oral Maxillofac Pathol. 2019;23(1):97–103. doi:10.4103/jomfp.JOMFP_180_18
49. Wan W, Gao X, Song S, et al. 5-aminolevulinic acid photodynamic therapy for extensive oral leukoplakia with concomitant oral submucous fibrosis: a case report. Photodiagnosis Photodyn Ther. 2023;41:103203. doi:10.1016/j.pdpdt.2022.103203
50. Wang L, Gu L, Tang Z. Cytokines secreted by arecoline activate fibroblasts that affect the balance of TH17 and Treg. J Oral Pathol Med. 2020;49(2):156–163. doi:10.1111/jop.12965
51. Liu S, Liu Z, Shan Z, et al. Skewed Th17/Treg balance during progression and malignant transformation of oral submucous fibrosis. Oral Dis. 2022;28(8):2119–2130. doi:10.1111/odi.13853
52. Dar AA, Patil RS, Pradhan TN, et al. Myeloid-derived suppressor cells impede T cell functionality and promote Th17 differentiation in oral squamous cell carcinoma. Cancer Immunol Immunother. 2020;69(6):1071–1086. doi:10.1007/s00262-020-02523-w
53. Garley M, Jabłońska E, Dziemiańczyk-Pakieła D, et al. LDGs versus NDGs in patients with oral squamous cell carcinoma (OSCC). Cytokine. 2021;137:155311. doi:10.1016/j.cyto.2020.155311
54. Tomasovic-Loncaric C, Fucic A, Andabak A, et al. Androgen receptor as a biomarker of oral squamous cell carcinoma progression risk. Anticancer Res. 2019;39(8):4285–4289. doi:10.21873/anticanres.13593
55. Liu X, Qing S, Che K, et al. Androgen receptor promotes oral squamous cell carcinoma cell migration by increasing EGFR phosphorylation. Onco Targets Ther. 2019;12:4245–4252. doi:10.2147/OTT.S200718
56. Batelja-Vuletic L, Tomasovic-Loncaric C, Ceppi M, et al. Comparison of androgen receptor, VEGF, HIF-1, Ki67 and MMP9 expression between non-metastatic and metastatic stages in stromal and tumor cells of oral squamous cell carcinoma. Life. 2021:11. doi:10.3390/life12010011
57. Patil S, Sarode SC, Ashi H, et al. Triphala extract negates arecoline-induced senescence in oral mucosal epithelial cells in vitro. Saudi J Biol Sci. 2021;28(4):2223–2228. doi:10.1016/j.sjbs.2021.01.011
58. Son HK, Kim D. Quercetin induces cell cycle arrest and apoptosis in YD10B and YD38 oral squamous cell carcinoma cells. Asian Pac J Cancer Prev. 2023;24:283–289. doi:10.31557/APJCP.2023.24.1.283
59. Wu-Chou YH, Hsieh CH, Liao CT, et al. NOTCH1 mutations as prognostic marker in oral squamous cell carcinoma. Pathol Res Pract. 2021;223:153474. doi:10.1016/j.prp.2021.153474
60. Kuo TM, Nithiyanantham S, Lee CP, et al. Arecoline N-oxide regulates oral squamous cell carcinoma development through NOTCH1 and FAT1 expressions. J Cell Physiol. 2019;234:13984–13993. doi:10.1002/jcp.28084
61. Li Y, Wang Z, Jin J, et al. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting notch-1 pathway. Biochem Biophys Res Commun. 2020;523(4):947–953. doi:10.1016/j.bbrc.2020.01.048
62. Cao L, Yang Y, Ye Z, et al. Quercetin-3-methyl ether suppresses human breast cancer stem cell formation by inhibiting the notch1 and PI3K/Akt signaling pathways. Int J Mol Med. 2018;42:1625–1636. doi:10.3892/ijmm.2018.3741
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.