Back to Journals » Cancer Management and Research » Volume 14
Italian Real-Life Experience on the Use of Mogamulizumab in Patients with Cutaneous T-Cell Lymphomas
Authors Caruso L, Castellino A, Dessì D, Flenghi L, Giordano A , Ibatici A, Massone C, Pileri A, Proietti I , Pupo L, Quaglino P, Rupoli S, Zinzani PL
Received 1 June 2022
Accepted for publication 30 October 2022
Published 22 November 2022 Volume 2022:14 Pages 3205—3221
DOI https://doi.org/10.2147/CMAR.S377015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Laura Caruso,1 Alessia Castellino,2 Daniela Dessì,3 Leonardo Flenghi,4 Antonio Giordano,5 Adalberto Ibatici,6 Cesare Massone,7 Alessandro Pileri,8 Ilaria Proietti,9 Livio Pupo,10 Pietro Quaglino,11 Serena Rupoli,12 Pier Luigi Zinzani13,14
1Hematology and Bone Marrow Transplantation Unit, Azienda Ospedaliero Universitaria Policlinico G. Rodolico - San Marco Di Catania, Catania, Italy; 2Department of Hematology, Santa Croce E Carle Hospital, Cuneo, Italy; 3Department of Hematology, Businco Hospital Arnas AOB, Cagliari, Italy; 4Hematology and Bone Marrow Transplantation Unit, Santa Maria Della Misericordia Hospital, Perugia, Italy; 5Department of Hematology, Fondazione Policlinico Universitario Agostino Gemelli—IRCCS, Rome, Italy; 6Hematology and Transplant Unit, IRCCS Ospedale Policlinico San Martino, Genova, Italy; 7Dermatology Unit, Ospedali Galliera, Genova, Italy; 8Dermatology Unit, IRCCS S. Orsola-Malpighi Polyclinic, Bologna, Italy. Department of Specialistic, Diagnostic and Experimental Medicine (DIMES), Alma Mater Studiorum University of Bologna, Bologna, Italy; 9Dermatology Unit”Daniele Innocenzi”, Department of Medical-Surgical Sciences and Bio-Technologies, Sapienza University of Rome, Terracina, Italy; 10UOC Lymphoproliferative Diseases, Fondazione PTV Policlinico Tor Vergata, Rome, Italy; 11Dermatologic Clinic, Department of Medical Sciences University of Turin Medical School, Turin, Italy; 12Clinic of Hematology, Ospedali Riuniti Ancona, Ancona, Italy; 13IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”, Bologna, Italy; 14Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale Università di Bologna, Bologna, Italy
Correspondence: Pier Luigi Zinzani, IRCCS University Hospital of Bologna, Seràgnoli Institute of Hematology, and Department of Specialized, Diagnostic and Experimental Medicine, University of Bologna, via Massarenti 9, Bologna, 40138, Italy, Tel +39 051 2144042, Fax +39 051 2144037, Email [email protected]
Abstract: Mycosis fungoides and Sèzary syndrome are the most studied subtypes common cutaneous T-cell lymphomas. The current treatment objective is to improve the clinical manifestations of the disease in the affected areas, to relieve symptoms and to halt disease progression. Patients with early-stage mycosis fungoides are usually managed with skin-directed therapies, whereas patients with resistant or advanced-stage mycosis fungoides or Sèzary syndrome often require systemic drugs. Over the last decade, new drugs have been developed, increasing the breadth of treatment options for cutaneous T-cell lymphomas patients. Mogamulizumab is a first-in-class defucosylated humanized IgG1 κ monoclonal antibody, which exerts its anti-tumour action by selectively binding to C-C chemokine receptor 4 and increasing antibody-dependent cellular cytotoxicity activity against malignant T-cells. Several clinical trials showed that mogamulizumab is able to effectively control the cutaneous T-cell lymphomas in each site (skin, blood, lymph nodes and viscera), improving patients’ symptoms, function and overall quality of life with a manageable safety profile. In this report, we discuss 12 cases of patients with mycosis fungoides or Sèzary syndrome successfully treated with mogamulizumab in real-life clinical practice in Italy.
Keywords: cutaneous T- cell lymphoma, mycosis fungoides, Sèzary syndrome, mogamulizumab
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of generally incurable, conditions with different clinical presentations and outcomes, characterized as extranodal non-Hodgkin lymphomas of malignant, mature T lymphocytes that target and persist in the skin.1–3 Mycosis fungoides (MF) and Sèzary syndrome (SS) are the most studied subtypes of CTCLs.4 Dobos et al performed a systematic review and meta-analysis of 16,953 patients in order to evaluate the epidemiology of CTCLs. The results showed that MF represented 62% of CTCLs, with an important heterogeneity in frequencies between studies and continents, while SS made up 3% of cases worldwide.5
MF is a generally indolent neoplasm characterized by different type and extent of skin lesions (erythematous patches, plaques, and less frequently, tumours); some patients can present with or progress to also have extracutaneous disease.6,7 SS is a more aggressive form of CTCL, characterized by a clinical triad of erythroderma (erythema involving >80% of body surface area), lymphadenopathy and significant blood involvement.6,7 Both diseases can have a huge impact on lifelong morbidity and quality of life (with common symptoms including significant itching, disfiguring skin lesions, and infections), and can also cause sleep disturbance and psychosocial problems.8 The prognosis of advanced-stage MF and SS is poor, with a median overall survival of about 5 years.9
The current treatment objective is to improve the clinical manifestations of the disease in the affected areas (skin, blood, lymph nodes and viscera), to relieve symptoms and to halt disease progression.10 Clinical outcome and scheduled treatments are disease-stage dependent.11 Patients with early-stage MF are generally managed with skin-directed therapies (psoralens plus ultraviolet A – PUVA, topical cytostatic agents, topical steroids, total skin electron beam therapy, local radiotherapy), whereas patients with resistant MF, advanced-stage MF or SS often require systemic drugs (retinoids, methotrexate, interferons, histone deacetylase inhibitors such as vorinostat, cytotoxic drugs, or targeted immunotherapies).11 Over the last decade, new drugs have been developed, increasing the breadth of treatment options for CTCL patients.12,13 The newly available drugs have shown clinical efficacy and a generally manageable safety profile, owing to their action on specific targets. Indeed, targeted therapies are able to bind to specific molecules predominantly expressed on MF/SS cells.13 Brentuximab vedotin, an anti-CD30 monoclonal antibody coupled to the anti-tubulin agent monomethyl auristatin E, induces apoptosis of the neoplastic CD30-positive T cells of MF/SS patients.14
Mogamulizumab is a first-in-class defucosylated humanized IgG1 κ monoclonal antibody approved by FDA and EMA in 2018 for the treatment of adult patients with relapsed or refractory mycosis fungoides or Sézary syndrome after at least one prior systemic therapy.12 Mogamulizumab exerts its anti-tumour action by selectively binding to C–C chemokine receptor 4 (CCR4) and increasing antibody-dependent cellular cytotoxicity (ADCC) activity against malignant T cells.15 Phase I/II trials showed that mogamulizumab is effective for the treatment of CTCLs patients, where overall response rates (ORR) ranged between 30% and 40%.16,17 The best results were observed in SS patients: Duvic et al reported an ORR of 47%, with 12% of global complete remission (skin, nodes and blood); the progression-free survival (PFS) was 11.4 months, with a median response duration of 10.4 months.17
The open-label, international, Phase 3, randomised controlled MAVORIC trial evaluated the clinical efficacy of mogamulizumab (1 mg/kg intravenously on a weekly basis for the first 28-day cycle, then on days 1 and 15 of subsequent cycles until disease progression) versus vorinostat (400 mg daily) in 372 eligible patients with stage IB–IVB histologically confirmed relapsed or refractory MF (n = 204) or SS (n = 168). The primary endpoint of the study was met with investigator-assessed median PFS of 7.7 months for mogamulizumab versus 3.1 months for vorinostat (p < 0.0001). The investigator-assessed ORR was significantly higher for mogamulizumab with respect to vorinostat (28% vs 5%, p < 0.0001); in particular, ORRs in MF and SS patients were 21% and 37% in the mogamulizumab group and 7% and 2% in the vorinostat group.7 Besides, a sub-analysis of the MAVORIC trial showed that mogamulizumab is able to improve MF/SS patients’ symptoms, function and overall quality of life respect to vorinostat; this benefit was more evident in patients with highest symptom burden and functional impairment.18
Other recently published papers confirmed the efficacy of mogamulizumab in CTCLs patients both in real-life and allogeneic stem cell transplantation settings.19,20
In this publication, 12 patients with MF or SS treated with mogamulizumab in Italian reference centers will be discussed. No approval was required for this research by an institutional review board or ethics committee because mogamulizumab was used on label in all cases and all patients gave consent to the publication of their clinical data in anonymous form (including images) for scientific and educational purposes. This retrospective study was notified the institutional board of the coordinating center (IRCCS Azienda Ospedaliero-Universitaria di Bologna, id 1043/2021/OssAOUBo) and was conducted according to the Declaration of Helsinki.
Case Reports
Mogamulizumab Treatment in Refractory Sèzary Syndrome
A 70-year-old woman was referred to the Institute of Hematology in Catania on July 2019 with a diagnosis of SS made in another center. At the beginning of the disease, the main symptoms were systemic erythema and cutaneous exfoliation >80% of the body surface. A skin biopsy showed immunohistochemical lymphocyte markers suggestive for a diagnosis of CTCL; the atypical lymphocytes were CD2, CD3, CD4, CD30, and CD45-positive and CD5, CD7, and CD8 negative. The computerized tomography (CT) scan showed enlarged lymph nodes in the abdomen and thorax, without any evidence of visceral lesions. The cytofluorimetric test confirmed the presence of peripheral atypical lymphocytes with typical features of Sèzary cells. During the first visit, the physical examination revealed an extensive erythema. The selected frontline therapy was a systemic treatment with interferon-α combined with extracorporeal photopheresis. After an initial partial response, the disease progressed: the physician decided to use a second-line approach for refractory advanced-stage disease with bexarotene associated to photopheresis for 3 months, without any improvement. Based on these clinical outcomes, a bexarotene-resistant SS was diagnosed and a third line treatment with gemcitabine monotherapy 1 g/m2 weekly was started. The patient began this treatment in another center and then continued the same therapy in our Center for logistic reasons. After 4 months of therapy and an initial partial response of symptoms and skin disease, another disease progression occurred: the subsequent selected treatment option was brentuximab vedotin, due to the fact that biopsy analysis showed 10% of CD30+ malignant cells. After three infusions of brentuximab vedotin, the skin lesions worsened with generalized itching, diffuse confluent erythema and enlarged deep abdominal lymph nodes; the quality of life of our patient got worse quickly and she threatened suicide. At this point, based on MAVORIC trial results and after new restaging of disease, we decided to treat the patient with mogamulizumab 1 mg/kg/week for the first 4 weeks, and every two weeks after the first month of therapy. The patient quickly achieved a response without any toxicity and the quality of life significantly improved; however, after a long treatment period (28 cycles), she discontinued the therapy due to a new disease progression and started a conventional anthracycline-based chemotherapy. This therapy is still ongoing.
Mogamulizumab as Bridge to Allotransplant in Sèzary Syndrome
A 60-year old female, black race, with a familiar history of diabetes and a silent personal medical history, presented to the center of Hematology in Cuneo with diffuse itching and cutaneous erythroderma on August 2020. At clinical examination, she had no fever, no night sweats, moderate weight loss, bilateral axillary and inguinal lymphadenopathy (about 2 centimeters), no hepato- or splenomegaly. The initial laboratory tests showed remarkable leukocytosis with absolute lymphocytosis (white cells blood count (WBC) 10,300/mm3, neutrophils 21%, lymphocytes 71%), normal hemoglobin and platelets levels and elevated serum lactate dehydrogenase (LDH) level. Abdominal ultrasound (US) scan showed some enlarged liver hilum and mesenteric lymph nodes. The CT scan and a positron emission tomography (PET) scan confirmed the enlarged activated lymph nodes in multiple sites above and below the diaphragm (bilateral cervical, axillary, iliac, inguinal, mesenteric and liver hilum). Flow cytometry on peripheral blood lymphocytes showed an invasion by a CD3+, CD4+, CD7+ T lymphocytes (87% of total lymphocyte count, 6344 cells/µL), with a monotypic expression of variable region Vbeta 12 of T-cell receptor. Multiple site biopsies (cutaneous, right axillary lymph node and bone marrow biopsy biopsy) showed an invasion by CD2+, CD3+, CD5+, CD7+ and CD4+ T lymphocytes. A diagnosis of SS was made and the patient started systemic treatment with steroids. After an initial clinical benefit on itching and cutaneous erythroderma, in December 2020 the patient showed a new worsening of both symptoms, with increasing lymphocytosis and growing adenopathies. On December 23rd 2020, a second line treatment with mogamulizumab at the standard dose of 1 mg/kg on day 1, 8, 15, 22 of cycle 1 and on day 1, 15 for the subsequent courses, was started. At the screening analyses, the patient was found to be anti-HBc Ab positive, with normal transaminase levels, HBsAg and HBV DNA negative. A standard HBV reactivation prophylaxis was started before starting mogamulizumab therapy. Since the second administration of mogamulizumab, a clear clinical benefit on itching and erythroderma was observed, as well as a progressive reduction of peripheral leukocytosis and lymphocytosis (Figure 1). Six cycles of mogamulizumab were performed and well tolerated, without any complication. At the same time, a bone marrow donor was searched: the patient had no siblings, matched unrelated donor (MUD) research was negative, but the patient had an available haploidentical son. The treatment with mogamulizumab was stopped in June 2021, after reaching an almost complete disease remission, and the patient underwent haploidentical allotransplant. In order to reduce the risk of acute Graft Versus Host Disease (aGVHD), a wash out period of 2 months from mogamulizumab infusion and allotransplant was performed. The restaging of disease showed a complete cutaneous response, a complete regression of peripheral blood lymphocytosis with negative peripheral blood flow cytometry and negative bone marrow cytometry and biopsy and a good partial response on lymphadenopathy. The patient underwent allotransplant in a good global partial response, with a Sorror score 1 (for HBV prophylaxis). The transplant was planned on August 2021, but then delayed until September 2021, because of donor positivity for malaria PCR. After antimalarial treatment and the negativization of plasmodium PCR on two consecutive analyses, the peripheral blood stem cells (PBSC) collection was performed. A cytomegalovirus (CMV) reactivation prophylaxis with letermovir from the day of transplant (the patient was CMV positive) and an antifungal prophylaxis with micafungin were administered. A transplant conditioning regimen with thiotepa-cyclophosphamide-fludarabine, followed by total-body irradiation (TBI) 200 rads, was used. On day 0, she received fresh PBSC by haploidentical son donor, containing a total of 9.56 x 106 CD34+ cells/kg. She started GVHD prophylaxis with immunosuppressive therapy with cyclophosphamide (day +3 and +4 from transplant), mycophenolate from day +4 to day +35 and cyclosporine from day +4. The clinical post-transplant course was good, with neutrophil recovery observed on day +15 and platelets recovery on day +21. A standard anti-infective prophylaxis for CMV, HBV, antifungal, antiviral was performed, as well as a weekly monitoring of CMV, EBV, Aspergillosis and malaria plasmodium. The admission period was complicated only by a grade 1 mucositis, without the need of a parenteral feeding support, and by a methicillin-sensitive Staphylococcus aureus bacteremia with fever lasting one day with prompt response to antibiotic treatment. The patient was discharged on day +28. The post-transplant clinical course was uneventful, without any clinical concerns and no GVHD onset. Now she is on day +150 post-transplant, in good health, with a complete disease remission (PFS not reached) and no GVHD signs.
A Case of Sèzary Syndrome Successfully Treated with Mogamulizumab
A 64-years-old female patient, with hypertension, type II diabetes mellitus and hypercholesterolemia, was diagnosed in 2019 with atopic dermatitis and treated with dupilumab until February 2020 for the occurrence of lymphadenopathy. A biopsy performed on April 2020 led to a diagnosis of peripheral T lymphocyte-derived lymphoma (PTCL-NOS); a consultation performed on this biopsy modified the diagnosis as being consistent with SS and associated dermatopathic lymphadenitis. On physical examination, the patient showed erythrodermic and desquamated skin, a left laterocervical lymphadenopathy (maximum diameter 3 cm), a left inguinal and bilateral axillary lymphadenopathy (3 cm), without organomegaly. The bone marrow biopsy did not show a localization of the disease. The immunophenotype analysis on peripheral blood showed a prevalence of CD4+ lymphocytes expressing the following phenotype: CD3+, CD7+, CD26-, CD45RO+, CCR7+ (central memory phenotype), CD27+, CD279-. The total body CT scan showed the presence of supra- and sub-diaphragmatic lymph nodes (cm 1.5–2.5) confirmed by PET. The stage of the SS is IV nodal. The laboratory tests were the following: hemoglobin 11.2 g/dl, WBC 12,460 cells/mm3, platelets 542,000 cells/mm3. On June 2020, a treatment with gemcitabine (days 1–8–15) for 6 cycles was started in the Hematology Unit in Cagliari. After the third cycle (August 2020), the patient had a deep venous thrombosis of the left subclavian vein, complicated by fever, treated with ciprofloxacin and antithrombotic therapy. A neck, axillae and abdomen US showed a stationarity of lymphadenopathy compared to the baseline. At the end of the cycle 6, an immunophenotype test on peripheral blood showed a significant reduction of lymphocytes out of the total number of cells (from 57% to 14%). On January 2021, due to the fact that skin hyperpigmentation with axillary lymphadenopathy was still present, it was decided to consolidate the response with bexarotene, cortisone and photopheresis. This treatment was withdrawn after only 3 days for intolerance and a marked increase of erythroderma with intense itching. She started a treatment with photopheresis for only one session, that was interrupted for a marked increase of erythroderma and lymphadenopathy volume. Following this clinical outcome, the disease was considered refractory to these treatments and on February 2021 therapy with chlorambucil per os (26 mg/day from day 1 to day 15) and dexamethasone for 3 cycles was started, waiting for the availability of mogamulizumab. The therapy with chlorambucil and steroids resulted in a good skin response and an almost complete reduction of lymph nodes size. On 05/05/2021, the patient started mogamulizumab IV 1 mg/Kg (g 1/8/15/22) for the first cycle, then switched to two-weekly administration for two cycles. The patient had a good cutaneous response, with no skin or hematological reactions; a total body CT scan showed a clear reduction in lymph node volume (< 1 cm). Due to the excellent response and the good safety, it was decided to switch the drug to a monthly schedule combined with steroid therapy. In November 2021, the patient was hospitalized in a surgical ward for the rupture of a diverticulum and underwent a surgical resection and the placement of an ostomy: for this reason, the treatment with mogamulizumab was then discontinued for 2 months. On 10/01/2022, the patient did not have a diffuse erythroderma but only patches with small papules in the back, no hematological alterations and no lymphadenopathy. The therapy with mogamulizumab was restarted, with a planned schedule of a dose every two weeks for 4 times and then a monthly infusion. After 4 lines of therapy, mogamulizumab was able to induce a clinical response both at cutaneous (significant improvement of itching and erythroderma) and nodal level, without any haematological toxicity or allergic skin reaction. In this patient, the suspension of the therapy due to a surgical complication at intestinal level did not lead to a relapse of the lymphoma. After 9 months of therapy, the patient’s quality of life clearly improved, together with the maintenance of the response.
Rapid and Persistent Improvement After Mogamulizumab Therapy in a Patient with Mycosis Fungoides
We report the case of an 81-year-old male patient followed by the Hematology and Bone Marrow Transplantation Unit in Perugia. After the appearance of erythematous-desquamative skin patches (body surface area – BSA– <5%), in 2015 the patient underwent a skin biopsy that placed the differential diagnosis between a parapsoriasis with epidermotropism vs MF. Following to the progression of skin lesions (BSA 6%), some of them nodular-like, a new skin biopsy performed on 07/11/2017 at the left leg allowed the diagnosis of MF, treated with nbUVB phototherapy starting from January 2018. On July 2018, a chest-abdomen CT-scan excluded lymph adenomegalies, splenomegaly or organ lesions. After a further worsening of the skin lesions occurred on August 2018, the patient started PUVA therapy; however, from March 2019 a progression of skin lesions to plaques was observed. On 16/04/2019, a lymphocyte typing showed a reduction in CD3+, CD2+, CD4+, CD26-, CD7± (50%), CD8- cell population (2.8%) (151 cells/µL). On 25/06/19, a new skin biopsy on the left forearm showed the presence of a MF with a possible evolution into a tumor phase. On July 2019, a chest-abdomen CT-scan showed no lesions related to MF. Therefore, on 08/07/19, a therapy with gemcitabine (1000 mg/m2 days 1, 8, 15, cycles to be repeated every 28 days) was started; after 6 cycles (December 2019), the patient, after an initial partial response, showed a skin progression with disseminated nodules and plaques (left arm 6 cm erosive nodule plaque, dark red in color; similar smaller centimetric lesions on the right arm, on the middle third of the right thigh of about 4 cm, on the left leg about 3 cm). From 7/12 to 31/12/2019, a locoregional radiotherapy performed on plaque lesions of the left upper arm, right thigh and left leg (30 Gy in 15 fractions on each lesion) improved the irradiated areas without any obvious benefit on the other lesions. On January 2020, the chest-abdomen CT-scan and the lymphocyte immunophenotype from peripheral blood were unchanged from previous ones. On 23/01/20, a treatment with bexarotene 300 mg/day per os was started, with a stable clinical disease until January 2021, when a new skin progression was observed. For this reason, bexarotene was replaced by methotrexate 15 mg/week by subcutaneous route associated with PUVA. On 01/06/21, considering the clinical worsening of the skin and pruritus during the treatment with methotrexate and PUVA, a therapy with mogamulizumab 1 mg/kg IV on days 1, 8, 15 and 22 of the first 28-day cycle, then days 1 and 15 of each subsequent cycle, was started. A rapid improvement of the skin lesions was observed until the complete disappearance of plaques and nodules after 4 cycles with a persistence of brownish macular areas without infiltration (Figure 2). The only reported side effect was constipation. To date (July 2022), the patient is still in complete remission and continues the treatment with mogamulizumab.
 |
Figure 2 Right lesions of the leg before (A) and after (B) four cycles of mogamulizumab. |
A Case of Mycosis Fungoides: Mogamulizumab as Bridge to Transplant
A 63-year-old Caucasian man presented to a dermatologist visit in 2016. He reported a 2-year history of pruritic, erythematous skin eruptions subsequently transformed into hypopigmented plaques. He was treated with steroid injections, with a transient benefit, and topical corticosteroids for possible eczema, without benefits. Over 2 years, an 8-cm tumor appeared on the left wrist, as well as additional tumors on the face and bilateral upper and lower extremities, while the remaining body surface presented a diffuse erythema. On January 2019, a diagnosis of CTCL, namely MF, was established following the biopsy of one of the tumors. Polymerase chain reaction (PCR) analysis was positive for T-cell receptor rearrangement. Flow cytometric analysis and peripheral blood smear analysis did not show abnormal cell populations to indicate a possible systemic involvement. PET and CT scans showed a borderline left axillary and left inguinal lymph node with a standardized uptake value maximum of 4.8 (SUV max) thought to be reactive, but without any evidence of additional disease. The stage of MF was IIIA (T4, Nx, M0, B0). Therefore, given the presence of multiple tumors, the patient started a therapy with IV liposomal doxorubicin 20 mg/m2 every two weeks for about 6 cycles in the Hematology Unit in Rome. The patient obtained a partial response with the complete disappearance of the tumor lesions and residual erythematous patches on the trunk > 10% BSA. Subsequently, the patient started a maintenance therapy with retinoids (bexarotene per os) in incremental doses up to the maximum dose of 300 mg/m2. Bexarotene was continued for about three months with the improvement of the erythroderma, but the dose was reduced until discontinuation due to a severe hypertriglyceridemia. Due to the exacerbation of symptoms (pruritus, patches lesions), an extracorporeal photopheresis (3 sessions per week in the first months following weekly sessions) was started on July 2019, with an improvement on pruritus, even if skin lesions remained almost unchanged. At the same time, the patient showed otalgia with ear secretions, and a diagnosis of catarrhal otitis complicated in mastoiditis was done. Microbiological cultures on ear swab showed the presence of Pseudomonas aeruginosa strains susceptible to carbapenems, successfully treated with meropenem for approximately two weeks. On February 2020, PET/CT scans showed new lymphadenopathy in the bilateral axillary, epitrochlear, inguinal, pelvic, and left external iliac lymph nodes suggestive of disease progression; further skin biopsies showed a CD30 positive cells infiltrate. A therapy with brentuximab vedotin 1.8 mg/Kg every three weeks was started on March 2020. However, the drug was withdrawn after 4 cycles due to a neuropathy and a DRESS syndrome (drug rash with eosinophilia and systemic symptoms). The patient has several hospitalizations for infections of open lesions throughout the year. CT scan total body showed a partial response on the lymph nodes but the erythroderma remained unchanged. Due to this latest failure, an allogeneic stem cell transplantation (HSCT) was proposed to the patient as the only curative possibility, although no family donor was available at that time. On July 2020, a treatment with IV mogamulizumab (1.0 mg/Kg on day 1, 8, 15 and 22 of cycle 1 and on day 1 and 15 of all subsequent cycles) was started, with a significant improvement, both radiological and clinical, for the first five cycles. During this period, the search for a stem cell donor from the international registry was positive. PET/CT scans showed a significant reduction of previously observed lymphadenopathies with absence of a metabolic activity, and residual nonspecific uptake in the lung and intestinal area (complete metabolic remission of the disease). The only skin lesion was a slight erythematous rash alternating with hypopigmented patches mainly affecting the trunk. An allogenic hematopoietic stem cell transplantation from an unrelated HLA identical (10/10) and cytomegaly virus matched (recipient and donor IgG positive) 30-year-old male donor with non-myeloablative conditioning (fludarabine 30 mg/m² body surface area on day −4 to −2 and total body irradiation with 2 Gy on day −1) followed by infusion of peripheral blood stem cells was performed. The patient received a total of 5.4×106 CD34+ cells/kg body weight, 1.4×108 CD3+ cells/kg body weight and 0.6×108 CD16+ cells/kg body weight. In the absence of GvHD, mycophenolate-mofetil was tapered to 500 mg every 14 days from day 32 and discontinued on day 45 while cyclosporine A was tapered from day +56, currently in progress. The restaging on day +30 and +90 after allogenic HSCT showed chimerism of 70% and 93% on sorted CD3+ cells. CT scan on day +90 after alloHSCT confirmed the persistent lymphadenopathy axillary (1.5 cm max) and the complete hematological remission (CR) without any infiltration of MF/SS cells in bone marrow biopsy. At the last follow-up visit, no skin lesions indicative of MF was observed, except an erythema on the face suggestive of GvHD. The patient is in post-transplant follow-up and on immunosuppressive therapy, which is planned to be gradually tapered off. No transplant complications due to the use of mogamulizumab were observed.
Mogamulizumab in Combination with Gemcitabine for a Patient with Sèzary Syndrome and Pancreas Adenocarcinoma
An 81-year-old woman was referred to the Cutaneous Lymphoma Metropolitan Centre (Galliera Hospital and San Martino Policlinic) on November 2020 with generalized exfoliative erythroderma started on July 2020 covering approximately 60% of BSA, onychodistrophy, ectropion, mild palmo-plantar hyperkeratosis (Figure 3) and intense pruritus (VAS pruritus: 7). Skin biopsies showed an epidermotropic infiltrate with Pautrier’s microabscesses, atypical lymphocytes and a patchy lichenoid infiltrate in the papillary dermis (Figure 4). Immunohistochemistry disclosed positivity for CD3, CD4, CD5 and focal positivity for CD30. A complete blood count showed leukocytosis (WBC 25,840 cells/mm3, total lymphocytes 18,100 cells/mm3), hemoglobin 13.3 g/dl, platelets 222.000/mm3. The flow cytometry pattern was typical of a SS with positive CD3, CD4, CD5, CD27 and negative CD2, CD26 T cells. Standard fluorescence in situ-hybridization on peripheral blood showed a deletion of Chr.17p. A total body CT scan showed no lymphatic involvement, but a disomogeneous hypovascular pancreatic mass infiltrating the splenic vein associated with multiple hepatic lesions which was confirmed by magnetic resonance imaging. The hematological findings were consistent with a stage IVA1 SS, whereas the transcutaneous biopsies of abdominal lesions diagnosed an unresectable pancreatic adenocarcinoma with liver metastasis. The patient underwent low-dose total skin electron beam (LD-TSEB) in 22 fractions (22 Gy) with only reduction in severity of pruritus and a mild improvement of peripheral blood lymphocytosis. On March 2021, the patient started IV gemcitabine (1000 mg/m2 on day 1, 8, 15 every 21 days). On June 2021, the Disease Management Team decided to add mogamulizumab to gemcitabine. The induction phase with mogamulizumab (standard IV schedule) was well tolerated (gemcitabine was held during the weekly induction). After the first cycle of mogamulizumab, a prompt clinical response was observed, with >90% clearance of skin disease, improvement of pruritus (VAS 3) and normalization of blood involvement. The patient then received further four cycles of mogamulizumab along with 3 cycles of gemcitabine (for a total of 6 cycles) with no drug-related skin or other adverse effects. Skin and blood responses were maintained (Figure 5). The restaging CT scan performed on November 2021 showed the progression of the pancreatic adenocarcinoma and the liver metastasis. The patient died on January 2022.
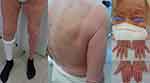 |
Figure 3 Diffuse erythema with scaling covering approximately 60% of BSA, onychodystrophy, ectropion, mild palmo-plantar hyperkeratosis. |
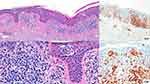 |
Figure 4 Epidermotropic infiltrate with Pautrier’s microabscesses with atypical lymphocytes and a patchy lichenoid infiltrate in the papillary dermis. |
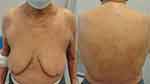 |
Figure 5 Already after the first cycle of mogamulizumab the patient achieved prompt clinical response with >90% clearance of skin disease. |
The combination of mogamulizumab with gemcitabine showed excellent tolerability without significant adverse effects. Gemcitabine represents an effective treatment for SS, but the duration of response is short: as skin features were stable after the first two cycles of gemcitabine, it is plausible that the clinical efficacy the combination regimen was mostly due to mogamulizumab. It is also noteworthy the absence of mogamulizumab-related skin eruption or autoimmune disease: it could be speculated that gemcitabine could have a role as immunosuppressive agent, limiting the occurrence of autoreactivity.
Management of a Case of Mycosis Fungoides in a Patient with Comorbidities
A 72-year-old Caucasian male patient with a granulomatous variant of MF, diagnosed 11 years before in another center, was admitted to the Dermatology Unit of Sapienza University, Polo Pontino, Rome. He had a medical history of hypertension, smoking, Hepatitis B under treatment, a heart attack on 2011, and colon cancer with subsequent adjuvant chemo-radiotherapy on 2012. He showed widespread, erythematous, scaly and indurated plaques, and patches on his trunk (stage Ib, NCCN [TNMB]). Flow cytometry result was negative for SS, without lymphadenopathy or hepatosplenomegaly. He was initially treated with topical steroid ointment only. In 2017, the patient underwent a three-years-long therapy with bexarotene. On January 2017, a one-month treatment with gemcitabine was started but stopped due to infections. A chemotherapy according to CHOP scheme (cyclophosphamide, doxorubicin, vincristine, prednisolone) was able to obtain a complete remission of cutaneous manifestations within five months (from May to September 2017). After a symptom-free period of 7 months, the patient developed a relapse on April (2018). Due to the presence of the CD30 antigen, a therapy with a standard dose of brentuximab vedotin was started. After 17 months of treatment (April 2018 – July 2019) without a full remission, the disease progressed. In the meantime, the patient suffered from symptomatic heart failure (ejection fraction of 49%), which excluded the possibility of photopheresis. Due to a stomatitis, a low-dose of methotrexate therapy was administered and interrupted shortly after. Subsequently, a phototherapy treatment (PUVA) was introduced with a moderate improvement of skin condition, but it was interrupted due to the SARS-coV-2 emergency in March 2020. He was referred to our hospital for further treatment in May 2021. Clinical examination showed erythroderma, scales, and intense itching on his legs, arms, face, and abdomen. CT and PET scans showed no pathological lymphadenopathies, and mogamulizumab was started. Since then, skin lesions or new tumors did not appear for 8 months without severe adverse events. Consistently, PET revealed no increased uptake of lymph nodes. As of October 2021, the patient had received 14 cycles during 6 months and is still in remission.
Long-Term Remission After Mogamulizumab Followed by Allogenic Stem Cell Transplantation in a Patient with Sèzary Syndrome
A 57-year-old woman was admitted to the UOC Lymphoproliferative Diseases in Rome on January 2018 with a 1-month history of itching, erythrodermia and lymphocytosis. A skin biopsy showed CD3 and CD4 positive cells and CD7 and CD26 negative cells. After the histological examination, an epidermotropic T-cell lymphoma was diagnosed. Laboratory test showed leukocytosis with Sèzary cells, and flow cytometry of the peripheral blood a CD4/CD8 ratio >10 and 90% of CD4+ T-cells negative for CD7. CT scan showed supra- and sub-diaphragmatic lymph adenomegalies. These findings led to the diagnosis of SS, stage TNMB IV A1. From February 2018, the patient received a first-line treatment with interferon-alpha-2a 3 MUI twice a week, prednisone 10 mg/die and extracorporeal photopheresis. On September 2018, due to the worsening of the cutaneous disease, she underwent a second-line treatment with gemcitabine 1000 mg/m2 on days 1–8–15 every 28 days associated with extracorporeal photopheresis. In the meantime, we started the research for a matched unrelated donor, since she had not compatible related donor, considering the allogeneic transplantation: an HLA 10/10 possible MUD was found. On March 2020, the patient had a new cutaneous relapse and thus, a third line of treatment with mogamulizumab as bridge-to-transplant was administered. The dose schedule of mogamulizumab was 1 mg/kg once weekly for the first cycle, in a 28-day cycle, and then 1 mg/kg every two weeks, for a total of 11 cycles: at the end of the treatment, a complete response was obtained with a good tolerance. On February 2021, due to an aseptic necrosis of the head of the left femur that required arthroplasty, the patient discontinued the treatment. On September 2021, after 7 months from the discontinuation of mogamulizumab, blood tests, PET/CT and a new skin biopsy confirmed the persistence of the complete response. Therefore, on November 2021, the patient underwent allogeneic hematopoietic stem cells transplantation from matched unrelated donor HLA 10/10. She was admitted in our hospital and she started the conditioning therapy with TBF-RIC (thiotepa-fludarabine-busulfan) and a prophylaxis for GVHD with cyclosporine, methotrexate and thymoglobulin. The transplantation procedure had no complication and no sign of acute GVHD has been reported. The patient discontinued mogamulizumab one year ago, underwent stem cells transplantation and, after 3 months, a complete remission of SS still persists.
Significant Single-Agent Activity of Mogamulizumab in a Patient with Relapsed-Refractory Mycosis Fungoides
A 77-years old woman with a ten-month history of a severe hitching, erythematous, scaly patches involving 40% of BSA was referred in October 2007 to the Haematology Unit in Ancona for confirmatory investigations. Complete blood count showed a mild peripheral blood lymphocytosis (PBL: 3.2 × 109/L), whereas a clonal Vβ20+expansion CD4+CD7-T cells (0.98×109/L) was observed at flow cytometry, without any chromosomal aberrations at karyotype and FISH analysis. According to the ISCL/EORTC classification for MF/SS,21 clinic-pathologic features were consistent with MF stage IIA (T2NXM0B1) and the patient was treated with low-dose interferon-alpha2b (IFN) combined with PUVA, obtaining a clearance of blood tumor lymphocytes and a histological complete remission (CR). After two years, she showed a limited skin disease relapse well-controlled by retreatment with PUVA and oral bexarotene. Unfortunately, on April 2014, the patient had a disease progression due to a generalized scaly erythroderma and rise in blood tumor lymphocytes (1.06 × 109/L) and she was staged as IVA (T4NXM0B2). From April 2014 to September 2019, she received different systemic therapies (prednisone, bexarotene, UVA, pegylated-INF, low-dose methotrexate ± extracorporeal photopheresis) with poor tolerance and not satisfactory clinical outcomes. In June 2021, while undergoing treatment with ECP, the patient has a new disease progression (erythroderma involving 70% of BSA) associated with intolerable itching causing a severe impact on her quality of life. The blood Sèzary flow at that time was 8.90 × 109/L. Conventional cytogenetics showed a clone with a complex karyotype and structural alterations involving several chromosomes, indicating a high degree of genetic disorder. The FISH assay confirmed some alterations highlighted by the karyotype, in particular 9p, 11q and 17p, which correspond to the loss of three important tumor suppressor genes, CDKN2A, ATM and TP53 respectively. Given the recent availability of mogamulizumab (MOGA) and the disease progression after six lines of treatment, the drug was started following the usual protocol schedule. This treatment was effective and well tolerated, except for a drop attack due to orthostatic hypotension and the occurrence of dyspnea caused by hypertensive and valvular heart diseases after the first and fourth infusion of mogamulizumab, respectively.
Our patient had an impressively rapid improvement of symptoms since the second cycle of therapy. At the same time, her blood Sèzary disease cleared quickly and flow cytometry analysis of peripheral lymphocyte subsets accounted for TCR-Vbeta20 clone <0.1% of the circulating leukocytes (B0). After six cycles of mogamulizumab, a sustained blood complete response, a near complete response in skin (Figure 6) and a partial response for histologic criteria of residual disease in the last cutaneous biopsy are maintained. Moreover, the karyotype analysis showed the absence of all genomic aberrations previously identified in peripheral blood with the exception of ATM deletion. The cytogenetic response was achieved already after the first cycle and maintained at the last follow-up (Figure 7).
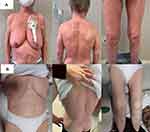 |
Figure 6 Skin involvement before (A) and after (B) therapy with mogamulizumab. |
Overall, this real-life case confirmed the clinical benefit of mogamulizumab regardless the number of specific prior systemic therapies. Additionally, the reliable activity of this drug in the blood compartment was confirmed and the use of cytogenetics to monitor T cell-clones in MF/SS patients, evaluating the potential impact on patient management, is recommended.
Optimization of the Management of a Sèzary Syndrome Patient Treated with Mogamulizumab
A 46-year-old Caucasian man had patchy erythematous skin lesions since 2015 with a diagnosis of dermatitis. On September 2018, due to lymphocytosis observed at the hemochromocytometric test, a skin biopsy and an immunophenotypic study of lymphocyte subpopulations allowed the diagnosis of SS by the Institute of Hematology in Bologna. CT scan showed the presence of lymphadenopathies of peri-centimeter size localized in bilateral laterocervical and occipital sites. The patient reported pruritus and causalgia on the skin patches, without any B symptoms; laboratory tests did not show significant alterations of the main hematochemical parameters. Since October 2018 until May 2020, the patient underwent extracorporeal photopheresis every 15 days in combination with low-dose methylprednisolone, obtaining a partial response on skin lesions and clinical benefit on symptoms. Following a disease progression (erythrodermia), on June 2020 a chemotherapy with single-agent gemcitabine was started: after 2 cycles, the treatment was discontinued for a substantial stability (July 2020). On October 2020, the patient showed erythrodermia with severe pruritus and causalgia. Immunophenotypic study on peripheral blood and skin biopsy confirmed the diagnosis of SS, and a neck-thorax-abdomen CT scan showed the stability of the known adenopathies. Osteomedullar biopsy was negative for disease localization. From December 2020, a therapy with mogamulizumab 1 mg/kg was started. To date, 13 cycles of therapy have been completed. Erythrodermia completely recovered after the four cycles of therapy (modified severity-weighted assessment tool, mSWAT, equal to 0). Starting from the cycle 5, some circumscribed erythematous, slightly desquamating, patches on the back skin, in particular on the right interscapular and intrascapular area, were observed: a dermatological evaluation attributed them to an eczematoid reaction related to mogamulizumab (grade 1, mSWAT 2), treated with topical steroid therapy only. On June 2021, painful adenomegalies in the laterocervical and right retro angulo mandibular areas were observed, in the absence of symptoms referable to an infection and persisting despite a therapy with nonsteroidal anti-inflammatory drugs. A further neck-thorax-abdomen CT scan confirmed the increased size of the adenomegalies at laterocervical, retro clavicular and right retro Angulo mandibular levels, with a maximum diameter of 16–18 mm, together with the presence of adenopathies at the external iliac and bilateral inguinal sites with a maximum size of 26×9 mm; PET scan with 18F-fluorodeoxyglucose showed the presence of hypercaptation at the right retro Angulo mandibular nodal finding. On September 2021, the histological examination of the excisional biopsy of this adenopathy allowed the diagnosis of nodal Rosai-Dorfman disease, considered unrelated to the current treatment. Due to the absence of treatment criteria for Rosai-Dorfman disease and the complete remission of erythrodermia during the treatment with mogamulizumab, the current therapy was continued with a close follow-up of the patient. This case report confirms the efficacy of mogamulizumab in inducing a complete remission in patients with SS, with an excellent control of symptoms and an extremely favorable toxicity profile. A longer follow-up of the patient will allow to verify the duration of this response.
Mogamulizumab-Induced Lichenoid Reaction in a Heavily Pre-Treated Sèzary Syndrome Responder Patient: A Seven-Year Treatment Story
On 2014, a 64-year-old woman with a history of psoriasis showed intensely pruritic, erythematous-desquamative, confluent patches all over the body. Small, palpable lymph nodes were also detectable in the axillary region. A skin biopsy was then performed, confirming an epidermotropic T lymphoproliferative disorder with erythrodermic evolution. Peripheral lymphocytes analysis showed an atypical CD4+ CD7+ CD26- population amounting to 54% of total lymphocytes (1691 cells/mm3). Concomitant CT scan was negative for visceral or lymph node involvement. Therefore, a diagnosis of SS, stage IVA1 (T4N0M0B2) was made by the Dermatology Clinic of Turin. Due to the worsening of the patient’s symptoms, monthly treatment with extracorporeal photopheresis (ECP) was started, subsequently associated with oral bexarotene. An improvement of the skin condition and pruritus, and a reduction of atypical Sèzary cells was obtained (213 cells/mm3). After 15 months of disease control, the ECP was replaced with UVB-nb phototherapy, still combined with bexarotene. Following eighteen cycles of UVB-nb phototherapy, the patient experienced a period of excellent control of her skin-related symptoms but ultimately relapsed on October 2018, when a new cycle of extracorporeal photopheresis was started. On February 2019, due to the persistence of symptoms, the patient was screened for EORTC-1652 protocol. Consequently, atezolizumab IV 1200 mg was administered every three weeks for one year. A stable disease condition was achieved with a persistence of response in the blood (circulating Sèzary cells count 63 cells/mm3). After the end of the treatment, to relieve our patient’s symptoms, ECP was reintroduced monthly until January 2021 with no significant benefit. On June 2021, the patient had a diffuse dark-red erythema extensively all over the body (mSWAT 87.0). On peripheral blood tests, circulating Sèzary cells count was 390 cells/mm3 (B1). Due to the progressive worsening of symptoms, mogamulizumab IV 1.0 mg/kg dose on days 1, 8, 15, and 22 of the first cycle and days 1 and 15 of all subsequent cycles was started. Two months after the start of the therapy, a tenfold decrease in the circulating Sèzary cells count (32 cells/mm3) was already detectable. After 14 infusions, the WBC count was 6.71 x 109/L, and the circulating Sèzary cells count was 14 cells/mm3 with a significant improvement of her skin condition (mSWAT 30.0) and good itch control. After 15 infusions, the patient had an intensely pruritic, confluent lichenoid eruption on lower trunk and upper limbs (Figure 8). Even if distinguishing from mogamulizumab associated rash and the disease progression can be challenging, the previous overall response associated with non-increasing circulating malignant cells, were compatible with a G2 mogamulizumab-associated rash. Therefore, treatment was temporarily discontinued, and an oral steroid regimen was started without performing a skin biopsy. After 15 days, a complete resolution of the rash was obtained, and the treatment was reintroduced with no additional adverse events. To date, our patient remains in great hematologic control (B0) with a substantial benefit in her skin-related symptoms. In this patient, retinoids associated with extracorporeal photopheresis and UVBnb phototherapy obtained the longest period of remission, despite losing their benefit progressively over time. On the other hand, atezolizumab did not significantly impact her skin-related symptoms, although it contributed to the drop of circulating Sèzary cells. Mogamulizumab had an early and drastic impact on circulating malignant cells, which occurred first compared to the skin improvement. The development of a mogamulizumab-associated rash can occur up to 24% of patients, with a median onset of 15 weeks.22 In this patient, the event was a progressively extensive, pruritic, lichenoid rash which required oral steroid therapy and treatment suspension until resolution. It is essential to correctly identify and differentiate a mogamulizumab-associated rash from the disease progression to avoid a premature discontinuation of the treatment, also considering that they seem to occur more frequently in responders.23 For this reason, a cutaneous biopsy should be performed when feasible.
Sebaceous Hyperplasia in a Patient with Sèzary Syndrome Treated with Mogamulizumab
A 46-year-old man with a 4-year-long history of SS referred to the outpatient’s surgery showed eruptive yellowish papules located on his face and forehead (Figure 9). The patient started mogamulizumab 350 days before the consultation in the Dermatology Unit of Bologna after having failed extracorporeal photopheresis and gemcitabine, while the yellowish lesions developed after the summer (+270 from mogamulizumab start). At the time of the dermatologic examination, he had a complete remission for six months, and then a mogamulizumab associated rash appeared 110 days form the drug start (plaques on his scalp and neck), subsiding after one month (+140 days form mogamulizumab beginning) with clobetasol dipropionate cream application. Upon closer inspection, approximately 28 papules, from 3 to 5 mm featuring a yellowish colour and a central depression, were observed. All the lesions developed on the forehead and the cheeks (Figure 9A–C). Dermoscopy showed the presence of well-demarcated yellow globule structures, divided by septa constituted by focused reddish capillaries (Figure 9D). The clinical and dermoscopic features were consistent with the diagnosis of eruptive sebaceous hyperplasia. Based on the Naranjo score, a diagnosis of SH mogamulizumab-related was made and defined as “probable”. Treatment based on daily applications of tretinoin lotion was started to reduce sebaceous hyperplasia growth, while mogamulizumab was continued. Mogamulizumab associated rash have been described recently in MF/SS patients:22–27 they are clinically heterogeneous, and currently, four distinct patterns have been reported: folliculotropic MF-like scalp with alopecia, papules and/or plaques, photo-accentuated dermatitis and morbilliform or erythrodermic dermatitis. It has been hypothesized that mogamulizumab associated rashes may be related to a better clinical outcome in analogy to skin rashes associated with epidermal growth factor receptor inhibitors.28
Sebaceous hyperplasia is a hyperplasia of the sebaceous glands and is commonly observed in sun-damaged skin or in organ transplant recipients who takes cyclosporine A or other immunosuppressive drugs like tacrolimus.29 A possible explanation for the eruptive growth may be related to mogamulizumab action on the tumour microenvironment.24–27 By reducing the presence of immunosuppressive cells within the tumour microenvironment mogamulizumab may have enhanced the anti-tumour Th1 cytokine production, such as interferon-α and tumour necrosis factor, which plays a role in the sebaceous glands by promoting their growth. Unlike mogamulizumab associated rash, mogamulizumab-related sebaceous hyperplasia developed after 270 days, a time-interval twice that of rash development. Due to the presence of an enduring complete remission from the disease both on the dermatologic and hematologic sides, it is unclear whether sebaceous hyperplasia onset may be related to a better clinical response as hypothesized for mogamulizumab associated rash.
Discussion
Mogamulizumab in Italy is indicated for the treatment of adult patients with MF or SS who have received at least one prior systemic therapy. It represents a new innovative therapeutic option, the only one approved, with proven efficacy in patients with SS who have already undergone at least one systemic treatment. Furthermore, it represents a new therapeutic option in MF patients who have already received at least one prior systemic therapy. In fact, other available drugs should only be used in some subpopulations of patients. Given the approval of the product in Italy fairly recently, our first experiences with mogamulizumab have been mainly in complicated patients: heavily pretreated patients and in different stages of the disease. In fact, both MF and SS are characterized by refractoriness to treatments, recurrent disease, often minimal or sometimes absent responses to the administered therapies, requiring the use of different drugs.
These real-life cases confirmed the clinical benefit of mogamulizumab and optimization of management of MF and SS. Our experience showed a significant activity in different schedules: single-agent activity, in combination with other therapies and as bridge to transplant both in MF and in SS. Clinicians using mogamulizumab for MF and SS should be aware of the associated risks, particularly infusion reactions and rash (which rarely may become severe), as well as the increased risk of GVHD, given that allogeneic bone marrow transplantation remains an important curative modality for both advanced CTCLs. However, for the rash, it is essential to correctly identify and differentiate a mogamulizumab-associated rash (MAR) from the disease progression in order to avoid a premature discontinuation of the treatment, also considering that they seem to occur more frequently in responders. In our experience, mogamulizumab-associated rash can be easily managed with steroids.
Furthermore, the SmPC states that the higher risk of transplant complications that has been reported after mogamulizumab treatment is happening within a short-time frame (approximately 50 days) before HSCT, we observed a wash out period of 2 months from mogamulizumab infusion and allotransplant, following up patients closely for early evidence of transplant-related complications. In our experience, the post-transplant patients were in good health, in a complete disease remission and without GVHD signs.
In any case, although clinical experience in Italy is still limited, the real-life experience confirms that mogamulizumab can be used in all stages of disease and in many cases the treatment can be anticipated, such as when a blood involvement is present. In fact, blood tumour burden in patients with MF or SS is recognized as a negative prognostic factor for survival and risk of progression. A post hoc analysis of the MAVORIC trial shows a greater clinical benefit with mogamulizumab vs the comparator in MF and SS patients with B1 and B2 blood involvement.30
The Italian experts suggest that in some cases, it may also prove useful to combine mogamulizumab with other systemic agents and there are ongoing clinical trials involving mogamulizumab in combination with total skin electron beam (TSEB) therapy (NCT04128072) or in combination with extracorporeal photopheresis (ECP; NCT04930653).
Conclusion
Mogamulizumab is a useful novel tool in the management of MF and SS, and considering a favorable risk/benefit ratio, it is likely to be widely used for patients with relapsed disease. This series of clinical cases confirm its efficacy and good safety profile in different clinical situation of the real life. Mogamulizumab offers some unique benefits over alternative therapies: the relatively long duration of remission confirmed in a large trial, high response rates within the blood compartment and consistent efficacy, especially in SS population. It may also prove useful in combination with other systemic agents, both as a direct antineoplastic agent and as immune modulator. Clinicians using mogamulizumab for MF and SS should be aware of the associated risks, particularly infusion reactions and rash (which rarely may become severe), as well as the increased risk of GVHD, given that allogeneic bone marrow transplantation remains an important curative modality for both advanced CTCLs.
Acknowledgments
The authors thank the patients for providing their consent to use medical information. The following collaborators contributed to the paper: E. Angelucci (Genova), D. Assalve (Perugia), R. Bencivenga (Ancona), S. Borriello (Turin), F. Cavallo (Turin), G. Discepoli (Ancona), A. Gozza (Genova), G. Goteri (Ancona), A. Massi (Bologna), N. Mordini (Cuneo), E. Morsia (Ancona), L. Nanni (Bologna), A. Olivieri (Ancona), B. M. Piraccini (Bologna), C. Potenza (Terracina), M. Simonacci (Ancona), S. Sola (Genova), E. Torre (Ancona), C. Zengarini (Bologna).
Funding
This study was supported with an unconditional grant by Kyowa Kirin.
Disclosure
Pier Luigi Zinzani received consultant fees from MSD, Eusapharma and Novartis; speaker fees from Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG Therap, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte and Beigene; Advisory Board fees from Secura-Bio, Celltrion, Gilead, Janssen-Cilag, BMS, Servier, Sandoz, MSD, TG Therap, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapy, Incyte and Beigene. Pietro Quaglino received speaker and advisory board fees from Kyowa Kirin, Takeda, Therakos Cellgene, Helsinn, Recordati, 4 SC. Cesare Massone received speaker and advisory board fees from Kyowa Kirin, Takeda. The other authors report no conflicts of interest in this work.
References
1. Tensen CP, Quint KD, Vermeer MH. Genetic and epigenetic insights into cutaneous T-cell lymphoma. Blood. 2022;139(1):15–33. doi:10.1182/blood.2019004256
2. Park J, Daniels J, Wartewig T, et al. Integrated genomic analyses of cutaneous T-cell lymphomas reveal the molecular bases for disease heterogeneity. Blood. 2021;138(14):1225–1236.
3. Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115(4):798–812.
4. Pileri A, Guglielmo A, Grandi V, et al. The microenvironment’s role in mycosis fungoides and Sézary syndrome: from progression to therapeutic implications. Cells. 2021;10(10):1.
5. Dobos G, Pohrt A, Ram-Wolff C, et al. Epidemiology of cutaneous T-cell lymphomas: a systematic review and meta-analysis of 16,953 patients. Cancers. 2020;12(10):2921.
6. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371(9616):945–957.
7. Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204.
8. Demierre MF, Gan S, Jones J, et al. Significant impact of cutaneous T-cell lymphoma on patients’ quality of life: results of a 2005 national cutaneous lymphoma foundation survey. Cancer. 2006;107:2504–2511.
9. Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766–3767.
10. Shalabi D, Bistline A, Alpdogan O, et al. Immune evasion and current immunotherapy strategies in mycosis fungoides (MF) and Sézary syndrome (SS). Chin Clin Oncol. 2019;8(1):11.
11. Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO clinical practice guidelines. Ann Oncol. 2018;29(Suppl 4):iv30–iv40.
12. Kamijo H, Miyagaki T. Mycosis fungoides and Sézary syndrome: updates and review of current therapy. Curr Treat Opt Oncol. 2021;22(2):10.
13. Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37(1):2–10.
14. Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390:555–566.
15. Ni X, Jorgensen JL, Goswami M, et al. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin Cancer Res. 2015;21(2):274–285.
16. Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;2014:1157–1163.
17. Duvic M, Pinter-Brown LC, Foss FM, et al. phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883–1889.
18. Porcu P, Hudgens S, Horwitz S, et al. Quality of life effect of the anti-CCR4 monoclonal antibody mogamulizumab versus vorinostat in patients with cutaneous T-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(2):97–105.
19. Jouandet M, Nakouri I, Nadin L, et al. Impact of mogamulizumab in real-life advanced cutaneous T-cell lymphomas: a multicentric retrospective cohort study. Cancers. 2022;14(7):1659.
20. Kamada Y, Arima N, Hayashida M, et al. Prediction of the risk for graft versus host disease after allogeneic hematopoietic stem cell transplantation in patients treated with mogamulizumab. Leuk Lymphoma. 2022;63(7):1701–1707.
21. Olsen E, Vonderheid E, Pimpinelli N, et al. ISCL/EORTC. revisions to the staging and classification of mycosis fungoides and Sèzary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–1722.
22. Musiek ACM, Rieger KE, Bagot M, et al. Dermatologic events associated with the anti-CCR4 antibody mogamulizumab: characterization and management. Dermatol Ther. 2022;12(1):29–40.
23. Musiek ACM, Whittaker S, Horowitz SM, et al. Characterization and outcomes in patients with mogamulizumab-associated skin reactions in the MAVORIC trial. Eur J Cancer. 2021;156(Suppl 1):S46.
24. Algarni AS, Ram-Wolff C, Bagot M, De Masson A. Mogamulizumab-induced vitiligo in patients with Sézary syndrome: three cases. Eur J Dermatol. 2021;31(2):213–216.
25. Trager MH, de Clippelé D, Ram-Wolff C, et al. Mogamulizumab-induced mucocutaneous lichenoid reaction: a case report and short review. Acta Derm Venereol. 2020;100(10):adv00158.
26. Wang JY, Hirotsu KE, Neal TM, et al. Histopathologic characterization of mogamulizumab-associated rash. Am J Surg Pathol. 2020;44(12):1666–1676.
27. Hirotsu KE, Neal TM, Khodadoust MS, et al. Clinical characterization of mogamulizumab-associated rash during treatment of mycosis fungoides or Sézary syndrome. JAMA Dermatol. 2021;157(6):700–707.
28. Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4(2):107–119.
29. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54(1):185–212.
30. Cowan RA, Scarisbrick JJ, Zinzani PL, et al. Efficacy and safety of mogamulizumab by patient baseline blood tumour burden: a post hoc analysis of the MAVORIC trial. J Eur Acad Dermatol Venereol. 2021;35(11):2225–2238.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




