Back to Journals » Journal of Pain Research » Volume 12
Is There An Effect On The Development Of Postdural Puncture Headache Of Dural Punction Made With The Spinal Needle In Three Different Orientations During Spinal Anaesthesia Applied To Pregnant Patients?
Authors Bıçak M , Salık F , Akelma H
Received 18 August 2019
Accepted for publication 30 September 2019
Published 22 November 2019 Volume 2019:12 Pages 3167—3174
DOI https://doi.org/10.2147/JPR.S227717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Mustafa Bıçak, Fikret Salık, Hakan Akelma
Health Sciences University, Gazi Yaşargil Training and Research Hospital, Department of Anesthesiology and Reanimation Clinic, Diyarbakir, Turkey
Correspondence: Hakan Akelma
Gazi Yaşargil Training and Research Hospital, Department of Anesthesiology and Reanimation Clinic, Diyarbakır 21010, Turkey
Tel +904122580071
Fax +904122580072
Email [email protected]
Background and objectives: Postdural punction headache (PDPH) is a well-known and common complication of spinal anesthesia. The relationship between spinal needle size, configuration and perforation characteristics of the spinal needle and non-essential leak continues to be controversial.
Methods: This prospective-randomized study included 300 patients aged 18–45 years who underwent cesarean section under spinal anesthesia. Spinal anesthesia was performed using a 26G Quincke spinal needle in the L3-4, or L4-5 range in the sitting position. Spinal anesthesia was performed with spinal needle sharp tip opening in the Group 1 patients, right or left laterally in Group 2 and caudal in Group 3, transducing the dural fibers transversely to the subarachnoid area, and directing the free opening of the needle to the spine. The patients were visited in the clinic where they were hospitalized at the 24th and 48th hours postoperatively, and phoned on the 3rd and 5th days after discharge, being questioned for PDPH.
Results: It was observed that 64% of patients with PDPH developed within the first 24 hrs, 24% between 24 and 48 hrs and 48–72 hrs in 12%. The incidence of PDPH was 14% in Group 1, 8% in Group 2 and 3% in Group 3. This difference between the groups was statistically significant (p: 0.019). The incidence of PDPH was lower in Group 3 than in Group 1 and Group 2.
Conclusion: We suggest that when spinal anesthesia is applied in the obstetric patient group if needle opening faces caudal this method will reduce the frequency of PDPH.
Keywords: spinal anaesthesia, pregnant patients, orientations needle, postdural punction headache
Introduction
Spinal anaesthesia is preferred to general anaesthesia in the obstetric patient group for reasons such as the rapid onset, rapid recovery and few side-effects of the drug.1 However, postdural punction headache (PDPH), which is a major complication of spinal anaesthesia, is encountered more in the obstetric patient group than in the general population, because of the widespread use of neuraxial blocks in these young females.2
August Bier, who was one of the pioneers of spinal anaesthesia applications, suggested from the first experience of spinal anaesthesia in 1899 that postdural headache could originate from the loss of cerebral spinal fluid (CSF).3 Although the mechanism of PDPH is not fully known, it is thought to result from a reduction in CSF pressure because of CSF leakage from the dural punction region to the epidural space.4 As a result of reduced pressure, CSF moves downwards towards the cisterna magna. Headache develops as a consequence of contraction of the meninges of pain-sensitive tissues, especially in the basal dura.5,6 Another mechanism is explained as contractions in the cerebral vessels.
A sudden reduction in CSF pressure causes vasodilation in the cerebral vessels in order to stabilise the intracranial volume. This is similar to the pathophysiology of vascular headaches. The use of vasoconstrictor agents such as theophylline and caffeine in PDPH treatment supports this pathophysiological state.7 Whatever the daily rate of CSF production, when the amount of CSF leaking to the peridural compartment exceeds this, even this small amount is seen to be sufficient to affect the sensitive balance between CSF resorption and production.
PDPH is typically in the form of a frontal, occipital or retro-orbital headache that starts 12–72 hrs after the dural punction and increases when standing and decreases when lying down and resting.8–10
The size of the dural defect, the structure of the longitudinal dural fibres, and the thickness of the dura in the procedure region are directly related to the amount of CSF leakage, which is also associated with the diameter and design of the spinal needle used.11 In several comparative studies, the effects of the diameter and design of the spinal needle on the development of PDPH have been investigated.12,13
There are also studies in the literature that have investigated the relationship between the incidence of PDPH and the entry plane (parallel or transverse to the dural fibres) of the spinal needle.5 In a cadaver study, the dura were examined with electron microscope after dural punction made in different planes, and the dimensions of the dural defects were reported to be different.14,15
From the starting point of cadaver studies, this study was designed, unlike previous clinical studies, with the aim of investigating whether dural punction made with the spinal needle in 3 differently angled orientations had any effect on the development of PDPH.
Materials And Methods
Approval for this prospective study was granted by the Ethics Committee of Health Sciences University Diyarbakir Gazi Yaşargil Training and Research Hospital (decision no: 223, dated: 08.02.2019). All procedures in the study were applied in accordance with the 2008 criteria of the Helsinki Declaration.
The study included 300 patients, aged 18–45 years, with American Society of Anaesthesiologists (ASA) score I-II, who were planned to undergo elective caesarean section operation.
Patients were excluded from the study if they were aged <18 years or >45 years, were morbidly obese (BMI >35), had a history of chronic headache or spinal headache, had a vertebral deformity, had any contra-indication for spinal anaesthesia or did not accept spinal anaesthesia.
Before the surgical procedure, all the patients were evaluated by an experienced anaesthetist in the Anaesthesia Polyclinic. Patients were informed about the procedure to be applied and written informed consent was provided by all study participants. The patients were given information about PDPH characteristic features and it was explained in detail what they should do when a headache developed. All the patients were given a contact telephone number for a researcher.
The patients were admitted for surgery after a fasting period of minimum 8 hrs. No premedication was administered. Routine monitorisation was applied to non-invasive arterial blood pressure, electrocardiogram, and oxygen saturation. An 18–20 G peripheral venous route was opened and throughout the procedure, saline was administered at the rate of 10mL/kg/hour. Before the application of spinal anaesthesia, the patient was moved into a sitting position, then the procedure area was cleaned under sterile conditions and sterile draped. The midline L4-5 intervertebral space was identified. A 26G Quincke spinal needle (Egemen, Turkey) was used for the application of spinal anaesthesia in all cases.
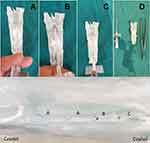
The patients were separated into 3 groups according to the direction of entry of the spinal needle to the longitudinal fibres of the dural sac. The spinal needle reached the subarachnoid area by transversely passing the dural fibres with the sharp tip facing the cephalic direction in Group 1 (Figure 1), by passing parallel to the dural fibres on the left or right laterally, in Group 2 (Figure 2), and by transversely passing the dural fibres with the tip facing caudally in Group 3 (Figure 3). In Figure 4, cross-sectional images of the dura mater are shown, obtained as a result of piercing the dura mater taken from bovine dura in 3 directions with a 16G angiocatheter needle.
To patients in all 3 groups, the needle was entered and passed the skin, subcutaneous tissues, supraspinal ligament, interspinous ligament, ligamentum flavum and dura mater, respectively. When free CSF flow was observed, the needle angle was manoeuvred cephalically in Groups 2 and 3. To all patients, 10–12 mg heavy bupivacaine (Marcaine™ 0.5% Ampoule; Astra Zeneca, Istanbul, Turkey) was administered at the rate of 0.1mL/sec. After the application of spinal anaesthesia, the patients were positioned supine. The sensorial block level was evaluated with a 20G hypodermic needle and the motor block level was evaluated with the Bromage scale.
Bromage scale:
- 0 = no paralysis. The patient can move the foot and knee into full flexion.
- 1 = only the knee and foot can be moved. The leg cannot be raised straight.
- 2 = the knee cannot be bent. Only the foot can be moved.
- 3 = the foot and toes cannot be moved. Classified as total paralysis.
Surgery was started when the motor and sensorial block levels reached T4–T6. Patients applied with spinal anaesthesia and observed to have failure of spinal block were excluded from the study and the same number of new patients were included.
Following the application of spinal anaesthesia, a record was made for each patient of age, body mass index (BMI), number of attempts, number of previous spinal anaesthesia experiences of the patient, and intraoperative complications.
Throughout the operation, non-invasive blood pressure measurements were taken at 3-min intervals. Hypotension was evaluated as systolic blood pressure <95 mmHg or a drop of >30% of the initial value. In these cases, 5–10 mg intravenous ephedrine was applied and intravenous fluid infusion was continued with increases of 20mL/kg. Bradycardia was evaluated as heart rate <45 bpm, and in such cases, 0.015 mg/kg intravenous atropine was administered.
When the operation was completed, the patients were transferred to the Postoperative Anaesthesia Care Unit (PACU), were monitored and when recovery was completed, were transferred to the ward. Postoperative analgesia was administered as 1 g paracetamol at 8 hr intervals. At 24 and 48 hrs postoperatively, the patients were visited on the ward, and on days 3 and 5 after discharge, they were called by telephone and asked about PDPH. Patients with headache were evaluated according to the International Headache Society (IHS) criteria (16). According to the IHS, PDPH diagnosis is made when a headache worsens within 15 mins of standing and improves within 15 mins of lying supine, together with at least one of the following symptoms (17):
- Neck stiffness
- Ringing in the ears
- Over-sensitivity to noise
- Photophobia
- Nausea
- Emergence of the headache in the first 5 days after dural punction
- Recovery of the headache (a) spontaneously within 1 week or (b) within 48 hrs of epidural blood patch.
Patients who described a headache but did not meet the defined criteria were evaluated as non-specific headache and were excluded from the study.
Patients with severe headache related to PDPH were recalled to hospital and treated. The time of onset of headache was recorded, together with the medical treatment applied, interventional treatments applied, and whether or not back pain developed.
Statistical Evaluation
Data obtained in the study were analysed statistically using SPSS for Windows vn 16.0 software. Following power analysis made with Type 1 error 0.5% and 80% power, it was determined that it was necessary to have a minimum of 74 patients in each group. Numerical data were stated as mean±standard deviation values and categorical values as number (n) and percentage (%). In the comparisons of categorical data, the Chi-square test was applied. Conformity of non-categorical data to normal distribution was assessed with the Kolmogorov–Smirnov test. In the comparisons of numerical data, the one-way ANOVA test was applied. A value of p<0.05 was accepted as statistically significant.
Results
The study included 300 patients with ASA I-II who underwent elective caesarean section operation under spinal anaesthesia. The mean age of the patients was 30.4±5.06 years and mean BMI was 28.62±3.26. PDPH developed in 25 (8.3%) patients and back pain in 81 (27%). The demographic data and clinical characteristics of the patients are shown in Table 1.
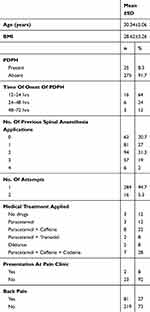 |
Table 1 Demographic Data And Clinical Characteristics Of The Patients |
The onset of PDPH was observed to be within the first 24 hrs in 64%, at 24–48 hrs in 24% and at 48–72 hrs in 12% (Table 1).
When the patients with PDPH were evaluated in respect of medical treatment, no medical treatment was applied to 12%, and the complaints recovered with rest and hydration. Paracetamol was administered to 12%, paracetamol + caffeine to 32%, paracetamol +tramadol to 8%, diclofenac to 8% and paracetamol+caffeine+codeine to 28% (Table 1). Of the patients with PDPH, 8% presented at the pain clinic but epidural blood patch was not applied to any patient (Table 1).
No statistically significant difference was determined between the groups in respect of age, BMI, and number of punction attempts (p:0.98, p:0.34, p:0.63, respectively) (Table 2).
 |
Table 2 Comparisons Of Age, BMI, And Number Of Attempts Between The Groups |
The frequency of PDPH development was determined as 14% in Group 1, 8% in Group 2, and 3% in Group 3. The difference between the groups was determined to be statistically significant (p:0.019). The frequency of PDPH development was determined to be statistically significantly lower in Group 3 than in Groups 1 and 2 (Table 3).
 |
Table 3 Comparisons Of The Groups In Respect Of The Development Of PDPH And Back Pain |
The frequency of back pain was determined as 26% in Group 1, 32% in Group 2, and 23% in Group 3. No statistically significant difference was determined between the groups (p:0.345) (Table 3).
Discussion
There are studies in the literature that have compared dura punction in parallel and transverse planes in respect of PDPH development, but there has been no study conducted on dura punction applied in the transverse plane with the spinal needle angled caudally (reverse transverse). In the current study, reporting reverse transverse punction for the first time, the PDPH incidence in the reverse transverse punction group was found to be statistically significantly lower than that of the other groups (p<0.005).
A series of factors are associated with the risk of PDPH development. The incidence of PDPH can vary according to the population studied, patient position, the plane in which the needle punctures the dura, and the diameter and type of tip of the needle used. Studies related to the relationship of PDPH and the form of puncture of the dura have been conducted since 1954, and this continues to be a matter of debate.17,18
In previous studies of spinal anaesthesia, the PDPH rate has been reported as 9.6% by Kang et al, 22.9% by De Andres et al, and 6.5% by Akdemir et al.19–21 In the current study, the PDPH rate was found to be 8.3%, which was consistent with findings in the literature.
When the relationship between needle diameter and PDPH incidence has been examined in the literature, it has been observed that as the needle diameter decreased so the dural defect decreased and indirectly the amount of leaking CSF.22
In a meta-analysis that compared sitting and lateral decubitus positions in spinal anaesthesia, the PDPH incidence was statistically significantly lower in the lateral decubitus position, and it was concluded that there was no statistically significant difference in respect of the number of entries and successful spinal anaesthesia. Andres Zorilla-Vaca et al explained the lesser development of PDPH in the lateral decubitus position by the difference in CSF pressures in the sitting and lateral decubitus positions.23 While CSF pressure is 40 cm H2O in the sitting position, this pressure falls to 5–20cm H2O in the lateral position.24,25 Hypothetically, this high pressure is related to a larger puncture and longer leakage at a higher pressure. Greater downward movement of the brain and meninges emerges earlier in the sitting position and results in more symptoms. This downward movement does not occur in the lateral decubitus position, so there is a lower risk of PDPH development.26
In studies that have compared parallel or transverse puncture of the dural fibres in obstetric patients in respect of the development of PDPH, the incidence of PDPH has been shown to be lower in patients applied with parallel dura punction.27,28 In a meta-analysis by Richman et al, PDPH incidence in non-obstetric patients was reported as 10.9% in the parallel group and 25.8% in the transverse group. The result in this meta-analysis showed a statistically significant decrease in PDPH when punction was applied with a spinal needle oriented parallel to the long axis of the spine compared to transverse orientation.29 Ready LB et al reported greater CSF leakage in the group where the dura fibres were entered transversely compared to the parallel entry group.30 In the ongoing discussions, the findings of the current study support that the entry direction of the needle during lumbar dural punction and patient position could have significant effects on the incidence of PDPH.
In the current study, overall PDPH incidence was determined as 8.3% (n:25/300). When examined on the group basis, PDPH incidence was 14% in Group 1, 8% in Group 2, and 3% in Group 3. There can be considered to be several mechanisms effective in the lower incidence in Group 3.
The significantly low incidence of PDPH determined in Group 3 (reverse transverse) of the current study compared to the other groups, could be attributed to the development of a dural defect shape that is easier to close with the arachnoid membrane. When within-group evaluations were made, another reason for the statistically significant difference in Group 3 could be that the closure mechanism formed associated with the different puncture form of the dura mater and arachnoid could reduce the amount of CSF leakage. That the PDPH incidence has been found to be lower in spinal anaesthesia made with a parallel approach supports the explanation of a thin cover mechanism.31,32
The low incidence of PDPH in cases where puncture with the spinal needle was made parallel to the dura mater supports the hypothesis that this could be related to separation rather than cutting the dural fibres by the needle in parallel punction, associated with the longitudinal extension of the dural fibres. However, this has been shown not to be the case in the results of light and electron microscope studies in the last 10 years. The concept of longitudinal dura fibre orientation, which was traditionally accepted, has been rejected, and it has been shown that the dural structure is a multi-layered different configuration.33,34 The dura mater is a laminated structure formed of approximately 80 concentric layers including both collagen and elastic fibres parallel to the surface, and it is now known that the fibres in the innermost layers do not show a specific orientation but are oriented in different directions.35,36
Some researchers have stated that the size of the dural defect formed by the spinal needle and the speed of closure of this defect are the most important factors affecting PDPH incidence and have emphasized that prolapse of the arachnoid membrane towards the exterior has an effective role in the closure of the dural defect.28 In a study by Cruickshank et al which evaluated the rate of CSF leakage, there was no significant difference between parallel and transverse groups and the rate of CSF leakage was reported to be related to the needle diameter.37
In our study, we think that CSF leakage rate and amount from the dural defect caused by reverse transverse access create a difference from other plane defects and cause lower incidence of PDPH due to less leakage. To clarify this situation, electron microscopy studies that require advanced technology are needed.
When making the dural punction with a Quincke spinal needle, as used in the current study, the rough parts formed on the edges of the dural defect show a tendency to fold inwards after cutting all the dural and arachnoid layers and the lesion edges moving away from the needle tip. When withdrawing the spinal needle, the inwardly folded lesion in the dura tends to retract because of the visco-elastic property of the dura and thus returns to its previous form.14
In conclusion, caudal orientation of the needle during entry when applying spinal anaesthesia to an obstetric patient group can be considered to be a method that will reduce the incidence of PDPH. However, there is a need for further clinical and cadaver electron and light microscope studies to clarify and confirm these results.
Limitations
Limitations of the study could be said to be that factors reducing PDPH incidence such as postoperative bedrest, and perioperative caffeine and fluid intake were not recorded. In addition, there was no recording of factors that increase the incidence of PDPH, such as psychiatric comorbidity, the time that the spinal needle remains in the intervertebral space, the number of interventions and a history of headaches. In addition, we could not use this method because the electron microscope was not in our hospital. In order to support our study, studies with more patients and supported by electron microscopy are needed.
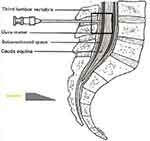 |
Figure 1 Transverse passage of the dural fibres with the tip of the spinal needle facing the cephalic direction. |
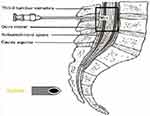 |
Figure 2 Parallel passage of the dural fibres with the tip of the spinal needle facing laterally. |
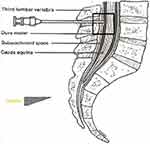 |
Figure 3 Transverse passage of the dural fibres with the tip of the spinal needle facing the caudal direction. |
Patient Consent For Publication
Informed Consent.
Ethics Approval
Health Sciences University Gazi Yaşargil Training and Research Hospital Local Ethics Committee approved this study.
Data Availability Statement
Data are available on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kestin IG. Spinal anaesthesia in obstetrics. Br J Anaesth. 1991;66(5):596–607. doi:10.1093/bja/66.5.596
2. Turnbull DK, Shepherd DB. Post‐dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91(5):718–729. doi:10.1093/bja/aeg231
3. Goerig M, Agarwal K, J. S AE. The versatile August Bier (1861–1949), father of spinal anesthesia. J Clin Anesth. 2000;12(7):561–569. doi:10.1016/S0952-8180(00)00202-6
4. Morewood GH. A rational approach to the cause, prevention and treatment of postdural puncture headache. Cmaj. 1993;149(8):1087–1093.
5. Salik F, Kiliç ET, Akelma H, Güzel A. The effects of the quincke spinal needle bevel insertion on postdural puncture headache and hemodynamics in obstetric patients. Anesth Essays Res. 2018;12(3):705–710.
6. Lütfiye P, Halil Ibrahim Ö, Pınar T. Postdural puncture headache: incidence and predisposing factors in a university hospital. Agri. 2018;31(1):1–8.
7. Grant R, Condon B, Hart I, Teasdale GM. Changes in intracranial CSF volume after lumbar puncture and their relationship to post-LP headache. J Neurol Neurosurg Psychiatry. 1991;54(5):440–442. doi:10.1136/jnnp.54.5.440
8. Aftab S, Nur-Ul-Haq S, Ara A, Hassan JA. Post dural puncture headache: comparison of 26G quincke with 25 G whitacre needle for elective caesarian section. Pak J Surg. 2009;25(4):257–261.
9. Vallejo MC, Mandell GL, Sabo DP, Ramanathan S. Postdural puncture headache: a randomized comparison of five spinal needles in obstetric patients. Anesth Analg. 2000;91(4):916–920. doi:10.1097/00000539-200010000-00027
10. Etezadi F, Yousefshahi F, Khajavi M, Tanha F, Dahmarde A, Najafi A. Post dural puncture headache after cesarean section, a teaching hospital experience. J Family Reprod Health. 2012;17–21.
11. Yang J, Hai Y, Yin P, Li N, Zhou L, Pan A. Clinical guiding significance of abdominal organs projection on the lateral lumbar X-ray for spinal microendoscopy punctures. J Spinal Cord Med. 2018;1–7. doi:10.1080/10790268.2018.1533318
12. Ayub F, Ahmad A, Aslam KZ, Saleem I. Frequency of headache with 25G or 27G quincke needles after spinal anesthesia in patients undergoing elective cesarean section. Anaesth Pain Intensive Care. 2017;21(2):170–173.
13. Zorrilla-Vaca A, Mathur V, Wu CL, Grant MC. The impact of spinal needle selection on postdural puncture headache: a meta-analysis and metaregression of randomized studies. Reg Anesth Pain Med. 2018;43(5):1–7. doi:10.1097/AAP.0000000000000775
14. Reina MA, Puigdellívol-Sánchez A, Gatt SP, De Andrés J, Prats-Galino A, Van Zundert A. Electron microscopy of dural and arachnoid disruptions after subarachnoid block. Reg Anesth Pain Med. 2017;42(6):1–10. doi:10.1097/AAP.0000000000000667
15. Reina MA, Lopez A, Badorrey V, De Andres JA, Martin S. Dura-arachnoid lesions produced by 22 gauge Quincke spinal needles during a lumbar puncture. J Neurol Neurosurg Psychiatry. 2004;75(6):893–897. doi:10.1136/jnnp.2003.017624
16. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, (beta version). Cephalalgia. 2013;33(9):629–808. doi:10.1177/0333102413485658
17. Angel Reina M, Dittmann M, Garcia AL, van Zundert A. New perspectives in the microscopic structure of human dura mater in the dorsolumbar region. Reg Anesth Pain Med. 1997;22(2):161–166. doi:10.1016/S1098-7339(06)80036-2
18. Flaatten H, Thorsen T, Askeland B, et al. Puncture technique and postural postdural puncture headache. A randomised, double‐blind study comparing transverse and parallel puncture. Acta Anaesthesiol Scand. 1998;42(10):1209–1214. doi:10.1111/j.1399-6576.1998.tb05279.x
19. Kang SB, Goodnough DE, Lee YK, et al. Comparison of 26-and 27-G needles for spinal anesthesia for ambulatory surgery patients. Anesthesiology. 1992;76(5):734–738. doi:10.1097/00000542-199205000-00011
20. Reina MA, de Leon-casasola OA, Lopez A, De Andres J, Martin S, Mora M. An in vitro study of dural lesions produced by 25-gauge quincke and whitacre needles evaluated by scanning electron microscopy. Reg Anesth Pain Med. 2000;25(4):393–402. doi:10.1097/00115550-200007000-00013
21. Akdemir MS, Kaydu A, Yanlı Y, Özdemir M, Gökçek E, Karaman H. The postdural puncture headache and back pain: the comparison of 26-gauge atraucan and 26-gauge quincke spinal needles in obstetric patients. Anesth Essays Res. 2017;11(2):458. doi:10.4103/0259-1162.194591
22. Santanen U, Rautoma P, Luurila H, Erkola O, Pere P. Comparison of 27-gauge (0.41-mm) whitacre and quincke spinal needles with respect to post-dural puncture headache and non-dural puncture headache. Acta Anaesthesiol Scand. 2004;48(4):474–479. doi:10.1111/aas.2004.48.issue-4
23. Zorrilla-Vaca A, Healy R, Zorrilla-Vaca C. Finer gauge of cutting but not pencil-point needles correlate with lower incidence of post-dural puncture headache: a meta-regression analysis. J Anesth. 2016;30(5):855–863. doi:10.1007/s00540-016-2221-2
24. Choi PT, Galinski SE, Takeuchi L, Lucas S, Tamayo C, Jadad AR. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Can J Anaesth. 2003;50(5):460–469. doi:10.1007/BF03021057
25. Eriksson AL, Hallén B, Lagerkranser M, Persson E, Sköldefors E. Whitacre or Quincke needles–does it really matter. Acta Anaesthesiol Scand Suppl. 1998;113:17–20. doi:10.1111/j.1399-6576.1998.tb04981.x
26. Hwang JJ, Ho ST, Wang JJ, Liu HS. Post dural puncture headache in cesarean section: comparison of 25-gauge Whitacre with 25- and 26-gauge Quincke needles. Acta Anaesthesiol Sin. 1997;35(1):33–37.
27. Norris MC, Leighton BL, DeSimone CA. Needle bevel direction and headache after inadvertent dural puncture. Anesthesiology. 1989;70(5):729–731. doi:10.1097/00000542-198905000-00002
28. Halpern S, Preston R. Postdural puncture headache and spinal needle design. Metaanalyses. Anesthesiology. 1994;81(6):1376–1383. doi:10.1097/00000542-199412000-00012
29. Richman JM, Joe EM, Cohen SR, et al. Bevel direction and postdural puncture headache: a meta-analysis. Neurologist. 2006;12(4):224–228. doi:10.1097/01.nrl.0000219638.81115.c4
30. Ready LB, Cuplin S, Haschke RH, Nessly M. Spinal needle determinants of rate of transdural fluid leak. Anesth Analg. 1989;69(4):457–460. doi:10.1213/00000539-198910000-00006
31. Mathews DM. Dural tissue trauma and cerebrospinal fluid leak after epidural needle puncture. Surv Anesthesiol. 2004;48(5):255–256. doi:10.1097/01.sa.0000140548.08719.05
32. Vilming ST, Schrader H, Monstad I. Post‐lumbar‐puncture headache: the significance of body posture: a controlled study of 300 patients. Cephalalgia. 1988;8(2):75–78. doi:10.1046/j.1468-2982.1988.0802075.x
33. Angel Reina M, De Leon Casasola O, López A. The origin of the spinal subdural space: ultrastructure findings. Anesth Analg. 2004;94(4):991–995. doi:10.1097/00000539-200204000-00040
34. Fink BR, Walker S. Orientation of fibers in human dorsal lumbar dura mater in relation to lumbar puncture. Anesth Analg. 1989;69(6):768–772. doi:10.1213/00000539-198912000-00014
35. Haller FR, Low FN. The fine structure of the peripheral nerve root sheath in the subarachnoid space in the rat and other laboratory animals. Am J Anat. 1971;131(1):1–19. doi:10.1002/(ISSN)1553-0795
36. Kaar GF, Fraher JP. The sheaths surrounding the attachments of rat lumbar ventral roots to the spinal cord: a light and electron microscopical study. J Anat. 1986;148:137.
37. Cruickshank RH, Hopkinson JM. Fluid flow through dural puncture sites: an in vitro comparison of needle point types. Anaesthesia. 1989;44(5):415–418. doi:10.1111/ana.1989.44.issue-5
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.