Back to Journals » Clinical Epidemiology » Volume 15
Is Age the Most Important Risk Factor in COVID-19 Patients? The Relevance of Comorbidity Burden: A Retrospective Analysis of 10,551 Hospitalizations
Authors Valero-Bover D, Monterde D , Carot-Sans G , Cainzos-Achirica M, Comin-Colet J , Vela E, Clèries M, Folguera J , Abilleira S , Arrufat M , Lejardi Y, Solans Ò, Dedeu T, Coca M, Pérez-Sust P , Pontes C , Piera-Jiménez J
Received 14 February 2023
Accepted for publication 26 May 2023
Published 30 June 2023 Volume 2023:15 Pages 811—825
DOI https://doi.org/10.2147/CLEP.S408510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Damià Valero-Bover,1,2 David Monterde,2,3 Gerard Carot-Sans,1,2 Miguel Cainzos-Achirica,4,5 Josep Comin-Colet,6– 8 Emili Vela,1,2 Montse Clèries,1,2 Júlia Folguera,1,2 Sònia Abilleira,9 Miquel Arrufat,3 Yolanda Lejardi,3 Òscar Solans,2,10 Toni Dedeu,11 Marc Coca,1,2 Pol Pérez-Sust,1 Caridad Pontes,1,2,12 Jordi Piera-Jiménez1,2,13
1Catalan Health Service, Barcelona, Spain; 2Digitalization for the Sustainability of the Healthcare System (DS3) – Institut d’Investigacions Biomèdiques de Bellvitge (IDIBELL), Barcelona, Spain; 3Catalan Institute of Health, Barcelona, Spain; 4Center for Outcomes Research, Houston Methodist, Houston, TX, USA; 5Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Medical Institutions, Baltimore, MD, USA; 6Cardiology Department, Bellvitge University Hospital (IDIBELL), Barcelona, Spain; 7Department of Medicine, University of Barcelona, Hospitalet de Llobregat, Barcelona, Spain; 8CIBER Cardiovascular (CIBERCV), L’Hospitalet de Llobregat, Barcelona, Spain; 9CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain; 10Health Department, eHealth Unit, Barcelona, Spain; 11WHO European Centre for Primary Health Care, Almaty, Kazakhstan; 12Department of Pharmacology, Autonomous University of Barcelona, Barcelona, Spain; 13Faculty of Informatics, Telecommunications and Multimedia, Universitat Oberta de Catalunya, Barcelona, Spain
Correspondence: Jordi Piera-Jiménez, Catalan Health Service, Gran Via de les Corts Catalanes 587, Barcelona, 08007, Spain, Tel +34 93 403 85 85, Email [email protected]
Purpose: To assess the contribution of age and comorbidity to the risk of critical illness in hospitalized COVID-19 patients using increasingly exhaustive tools for measuring comorbidity burden.
Patients and Methods: We assessed the effect of age and comorbidity burden in a retrospective, multicenter cohort of patients hospitalized due to COVID-19 in Catalonia (North-East Spain) between March 1, 2020, and January 31, 2022. Vaccinated individuals and those admitted within the first of the six COVID-19 epidemic waves were excluded from the primary analysis but were included in secondary analyses. The primary outcome was critical illness, defined as the need for invasive mechanical ventilation, transfer to the intensive care unit (ICU), or in-hospital death. Explanatory variables included age, sex, and four summary measures of comorbidity burden on admission extracted from three indices: the Charlson index (17 diagnostic group codes), the Elixhauser index and count (31 diagnostic group codes), and the Queralt DxS index (3145 diagnostic group codes). All models were adjusted by wave and center. The proportion of the effect of age attributable to comorbidity burden was assessed using a causal mediation analysis.
Results: The primary analysis included 10,551 hospitalizations due to COVID-19; of them, 3632 (34.4%) experienced critical illness. The frequency of critical illness increased with age and comorbidity burden on admission, irrespective of the measure used. In multivariate analyses, the effect size of age decreased with the number of diagnoses considered to estimate comorbidity burden. When adjusting for the Queralt DxS index, age showed a minimal contribution to critical illness; according to the causal mediation analysis, comorbidity burden on admission explained the 98.2% (95% CI 84.1– 117.1%) of the observed effect of age on critical illness.
Conclusion: Comorbidity burden (when measured exhaustively) explains better than chronological age the increased risk of critical illness observed in patients hospitalized with COVID-19.
Keywords: COVID-19, comorbidities, comorbidity burden, risk assessment, hospitalized patients, case-mix tool
Introduction
Since the beginning of the coronavirus disease (COVID-19) pandemic, age and the presence of comorbidities have both been pointed out as critical risk factors for developing severe illness.1 Various authors have found that the influence of age on severe outcomes decreased when adjusting for other factors, including but not limited to comorbidities.2–4 Nevertheless, in most models such attenuation of the effect of age was only partial, and age was still acknowledged as the most important risk factor for severe illness.5–7 In this context, age has been used as the main criterion for prioritizing vaccine allocation in many countries and driving many stratify-and-shield campaigns worldwide. However, using age as a risk proxy for prioritization of healthcare interventions risks ageism, with quantifiable adverse consequences in several settings, including COVID-19.8,9
While the effect of age can be easily measured in multivariate models, assessing the contribution of comorbidity burden has several challenges, which may bias the results. Most studies in the COVID-19 setting have assessed the presence or absence of a specific, relatively limited number of chronic conditions.5,6,10–13 However, this approach addresses the effect of certain comorbidities rather than the effect of comorbidity burden as a whole. Alternatively, other authors have used summary measures of comorbidity burden, such number of chronic conditions (eg, stratified into categories from 0 to up to ≥3),6,14,15 or summary indices such as the Charlson or Elixhauser.16–21 These indices might underestimate comorbidity burden due to the limited number of diagnoses considered.22
Taking advantage of the systematic collection and integration of routine care data, we recently developed a set of comprehensive indices for risk assessment in hospitalized patients, which includes an index for measuring comorbidity burden of these patients: the Queralt index for comorbidities (Queralt DxS).23 The index combines and weighs more than 3145 relevant acute and chronic diagnostic codes and provides a numerical index of comorbidity burden on admission. When used as adjustment factor in risk assessment of patients hospitalized with COVID-19, the Queralt DxS showed a remarkable contribution to explaining the risk of critical illness (ie, admission to intensive care unit or death) in individuals hospitalized with COVID-19.24
In this analysis, we investigated how the effect of chronological age on the risk of critical illness changes when comorbidity burden is measured using increasingly exhaustive tools: the Charlson index (17 diagnostic group codes), the Elixhauser index (31 diagnostic group codes), the count of diagnoses included in the Elixhauser index, and the Queralt DxS (3145 diagnostic group codes). We also aimed at evaluating the mediation role of comorbidity burden in the relationship between age and critical COVID-19 illness.
Materials and Methods
Study Design and Setting
This was a retrospective analysis of individuals hospitalized with COVID-19 as the primary diagnosis in the seven public hospitals of the Catalan Institute of Health, the leading healthcare provider in Catalonia (North-East Spain). The Catalan Institute of Health provides universal healthcare to nearly 70% of the Catalan population and accounts for 30% of the total acute hospitalizations in Catalonia.
We screened the database of the Catalan Institute of Health for all individuals admitted with COVID-19 as the primary diagnosis between March 1, 2020 and August 31, 2022. The database was locked on January 25, 2023. Patients derived from other hospitals or transferred to other hospitals on discharge were excluded from the record. For the primary diagnosis, we considered the following diagnosis codes of the international classification of diseases 10th version, clinical modification (ICD-10-CM): B97.29, B97.21, B34.2, J12.81, J12.89, and U07.1. The vaccination campaign in Catalonia started on December 27, 2020. Figure S1 summarizes the prevalence of each variant of concern throughout the investigated period.
All data were handled according to the General Data Protection Regulation 2016/679 on data protection and privacy for all individuals within the European Union and the local regulatory framework regarding data protection. The study protocol was approved by the independent ethics committee of the Bellvitge Biomedical Research Institute (IDIBELL, Spain), which waived obtaining informed consent for the secondary use of data collected during routine care (ref. PR123/22).
Variables and Data Sources
The study outcome was a composite outcome of critical illness, which included the need for invasive mechanical ventilation, transfer to the intensive care unit (ICU), or in-hospital death (any of them) as described elsewhere.13 Information about admission to ICU and death are systematically collected in the electronic health records of Catalan Institute of Health hospitals, whereas the need for invasive mechanical ventilation was determined by the following codes of hospital procedures: 5A09357, 5A09457, 5A09557, 5A1935Z, 5A1945Z, 5A1955Z, 09HN7BZ, 09HN8BZ, 0BH13EZ, 0BH17EZ, 0BH18EZ, 0CHY7BZ.
Primary explanatory variables included age, sex, and measures of the comorbidity burden present on admission. The comorbidities present on admission were retrieved from a common electronic health record shared by all hospitals of the Catalan Institute of Health. We used four summary measures of comorbidity burden: the Charlson index,25 the Elixhauser count (ie, number of diagnoses among the 31 codes considered in the Elixhauser index) and index,26 and the Queralt index for secondary diagnoses present on admission (Queralt DxS).23 The ICD-10 coding for the Charlson and Elixhauser scores was based on work by Quan et al.27 Weights for the Charlson score are based on the original formulation by Charlson et al in 1987,25 while weights for the Elixhauser score were based on work by Moore et al.28 The Queralt DxS is part of a set of three indices for measuring the clinical complexity of hospitalized patients. It provides a numerical value from the weighted sum of secondary diagnoses present on admission from a list of 3145 diagnostic code groups.23 The weights of the version used in this analysis (version 6.3) were estimated from health data collected in hospitalizations reported between 2018 and 2019 in the Catalan Institute of Health and were, therefore, not specific to COVID-19 patients.
Adjusting variables included the wave in which the admission occurred, the hospital, and the vaccination status for COVID-19 (the last used only in the secondary analyses presented in the Supplementary File). The vaccination status was retrieved from the K2 platform database, held by the Catalan Department of Health and used as a source of information for issuing COVID-19 certificates. The K2 database includes information on COVID-19 diagnoses from the primary and hospital care setting (both public and private) and vaccination information. Vaccination categories, used in the secondary analysis only, were defined as follows: partial vaccination (ie, one dose of a 2-dose regimen of an RNA-based vaccine), complete vaccination (ie, either two doses of an RNA vaccine or one dose of a single-dose regimen vaccine), and booster (ie, an additional dose to the complete vaccination regimen).
Analysis
We defined two analysis datasets. The primary analysis dataset, presented in tables and figures in the main text, excluded vaccinated individuals and those admitted during the first wave of the COVID-19 outbreak in Catalonia (from March 1 to June 23, 2020). The reason for excluding these individuals was the high risk of bias. The first outbreak in Spain severely overwhelmed hospital resources and occurred very early in the pandemic, with limited knowledge on the management of COVID-19 in the hospital setting.13,29 These two factors were expected to affect the mortality rate and factors influencing it. Likewise, vaccination significantly limits the progression toward severe illness and may challenge result interpretation. The secondary dataset, presented in the Supplementary File, included the entire retrospective cohort, irrespective of the time of admission and vaccination status. In the secondary analysis, patients were stratified according to the wave in which they were admitted to facilitate results interpretation regarding the severity of the wave, the availability of vaccines, or the prevalence of different variants of concern (Figure S1).
For description purposes, age and the summary measures of comorbidity were categorized. Age was split into the following groups: 0–39, 40–49, 50–59, 60–69, 70–79, and ≥80). The indices of comorbidity were categorized into three risk levels of comorbidity burden that yield homogeneously sized high-risk groups: the Charlson index scores were grouped into low health risk (score 0), moderate (1–2), and high (≥3); the Elixhauser and Queralt DxS indices were grouped into low (below the 50th percentile), moderate (50th – 85th), and high (>85th percentile); the Elixhauser count was grouped into low (0–1), moderate (2–3), and high (≥4). The definition of the cut-off percentiles for the Elixhauser and Queralt DxS indices sought homoscedasticity with age (ie, 15% of the study sample was allocated in the upper age group).
The association between explanatory variables (age, sex, and comorbidity burden) and the study outcome (development of critical illness) was investigated using multiple logistic regression models for each measure of the comorbidity burden: Charlson index, Elixhauser index, Elixhauser count, and Queralt DxS index. Age was introduced as a categorical variable to ease the interpretation of the resulting model, although the same models with age as a continuous variable plus an additional quadratic term were built to confirm the equivalent performance of the model. In addition, while acknowledging potential clustering of patient characteristics by hospital,29 all models were further adjusted by considering the random effects of hospitals in which admission occurred. First, we built separate models for age, sex, and each measure of the comorbidity burden; then, we built multivariate models including age, sex, and one comorbidity measure; finally, we built the same models accounting for interactions between age and the comorbidity measures. The same methodology was applied to secondary analyses in which each wave was analyzed separately, with models adjusted for hospital and vaccination status.
Finally, we used a causal mediation analysis30 to investigate the hypothesis that comorbidity burden, would fully mediate the association between age (exposure factor or treatment variable) and critical illness (outcome). In this analysis, age and the comorbidity indices were used as continuous variables. The control and treatment age groups, required for the mediation analysis, were established based on the 50 and 75 years cutoffs. The average causal mediation effect of comorbidity (mediator), the average direct effect of age, and the total effect were estimated, and the 95% CI obtained using bootstrap from 2000 simulations, considered adequate for this type of analysis. The contribution of the comorbidity-mediating pathway was assessed using the proportion of the average causal mediation effect over the total effect. All analyses were performed using R 4.1.2.31 The causal mediation analysis was conducted using the library mediation by Tingley et al,32 the linear and mixed model adjustments were conducted using the lme4 library,33 and the analyses of the ROC and precision-recall curves were done using the pROC34 and PRROC35 libraries, respectively. The Charlson and Elixhauser indices were computed using the Comorbidity library by Gasparini,36 whereas the Queralt DxS was estimated using the updated version of the index R function (version 6.3; the software can be accessed from https://ics.gencat.cat/ca/assistencia/coneixement-assistencial/sistema-de-Queralt/).
Results
Study Population
Between March 1, 2020 and August 31, 2022, 19,662 individuals were admitted to the hospitals of the Catalan Institute of Health with COVID-19 as the main diagnosis (Figure 1). Of them, 10,551 were non-vaccinated individuals admitted after the first wave (ie, from June 23, 2020 on) and were, therefore, included in the primary analysis: 3632 (34%) with critical illness and 6919 (66%) without critical illness. The second wave contributed the largest number of admissions to this analysis.
Table 1 summarizes the main demographic, epidemiological, and clinical characteristics of the study population of the primary analysis. The characteristics according to COVID-19 wave are listed in Tables S1–S7. The proportion of patients with critical illness increased with age and comorbidity burden, irrespective of the type of measure used. The same trends were observed when age and comorbidity burden were described as continuous variables. The greatest differences in the proportion of critical illness according to comorbidity were observed for the Queralt DxS.
 |
Table 1 Demographic, Clinical, and Epidemiological Characteristics of Individuals Included in the Primary Analysis |
Estimated Risk and Critical Illness
The distribution of the study population across the Queralt DxS risk groups showed a higher proportion of individuals at high and moderate risk among patients who experienced critical illness (Figure 2). The distribution according to the successive waves showed a similar trend (Figures S2–S8).
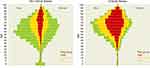 |
Figure 2 Distribution of Queralt DxS-based risk groups, by age and sex, among individuals who did not and did develop critical illness. Primary analysis population (N=10,551). |
Risk Factors for Critical Illness
According to the baseline model, adjusted by age, sex, hospital, and wave, the risk of critical illness increased progressively with age and was higher in men (Figure 3). When adjusting also for summary indices of comorbidity, the effect size of sex remained relatively stable, whereas that of age progressively decreased with the exhaustivity of the comorbidity measure. Moreover, the risk of critical illness increased linearly with age and comorbidity burden, irrespective of the index used. The reduction of the effect size of age was the highest when comorbidity burden was summarized using the Queralt DxS (ie, the comorbidity measure that considers the highest number of possible diagnostic groups). We observed the same trend in all waves, including those occurred after the beginning of the nationwide vaccination campaign, except the first one, in which the effect of age was significant in group ages above 60 years after adjusting for the Queralt DxS (Tables S8–S14).
Performance of Prediction Models for Critical Illness
Table 2 summarizes the BIC, AUROC, and precision-recall estimates for models in three series of models: (1) Models including only sex, age, or a summary measure of the comorbidity burden (adjusted by hospital and wave), (2) Multivariate models including age, sex, and a comorbidity measure (also adjusted by hospital and wave), and (3) The corresponding models accounting for interactions between age and the comorbidity measures. In all model series, the performance increased with the number of diagnoses considered for the comorbidity burden estimate, with models using the Charlson index showing the poorest performance and models using the Queralt DxS the highest. According to the analysis with the entire cohort stratified by waves, models using the Queralt DxS for adjusting for the comorbidity burden had the highest performance in all waves (Tables S15–S21).
 |
Table 2 Performance of the Models for Explaining Critical Illness. The Baseline Model Included Age, Sex, Wave, and the Hospital in Which Admission Occurred |
Mediation Analysis
According to the causal mediation analysis, the proportion of mediation by comorbidity burden over the total effect of age on the risk of critical illness increased with the exhaustivity of the comorbidity measure used (Figure 4). Using the Charlson index (ie, the comorbidity measure with the smallest effect size in our multivariate analysis), the proportion of the effect that was mediated was remarkably lower than the direct effect of age. In contrast, the reverse was true when comorbidity burden was measured using the Queralt DxS. In the latter analysis, the direct effect of age was no longer significant (Figure 4). The proportion of mediation effects was highest for the Queralt DxS index in all waves of the COVID-19 outbreak in Catalonia, with values ranging from 0.68 (95% CI 0.59–0.79) (first wave) to 1.30 (95% CI 0.64–5.29) (seventh wave) (Figure 5 and Tables S22–S28).
Discussion
In this retrospective analysis of 19,662 hospitalizations due to COVID-19, we found that the contribution of comorbidity burden to critical illness increases with the number of diagnoses considered for its measurement. When measured using a comprehensive index such as the Queralt DxS, which considers more than 3000 possible diagnosis groups, the contribution of age to critical illness was remarkably reduced, with age groups 70–79 years and >80 years no longer associated with the odds of developing critical illness and comorbidity burden explaining a significant proportion of the effect of chronological age on this outcome.
The effect of comorbidities on COVID-19 outcomes has been extensively investigated, with some reports showing an age-dependent effect of comorbidities (and other risk factors for severe COVID-19) in hospitalized patients.37–39 However, the vast majority of studies included in systematic reviews addressing the role of comorbidities in these patients measured the effect of individual comorbidities such as hypertension, diabetes or COPD, among others,40–44 or stratifying patients based on the presence of one or multiple comorbidities.45 In line with the general trend approaching the assessment of comorbidities in COVID-19, studies investigating the question of whether age is the most important factor in explaining COVID-19 outcomes have assessed the effect of comorbidities by considering the presence or absence of a pre-defined list of diagnoses. Semenzato et al concluded that age was the most important factor based on the individual hazards of an extensive list of 47 comorbidities.5 However, the authors acknowledged that the sum of the number of comorbidities does not account for the different severity of each of them. Henkens et al followed a similar approach using a shorter list of comorbidities.6 Additionally, the authors investigated the mediation effect of each comorbidity on the effect of age on in-hospital mortality and found that the direct effect of age was ≥95% in all diagnoses. However, when adjusting for the comorbidity burden, they used an unweighted count of diagnoses, stratified as 0, 1–2, and >2 comorbidities. These two approaches (ie, the risk estimate of each diagnosis independently and the unweighted count of the number of diseases) lose sight of the actual disease burden and are likely to underestimate the effect of comorbidity burden as a whole, particularly in patients with relevant diagnoses simultaneously.
Alternatively, we measured the comorbidity burden using a very comprehensive index that considers more than 3000 possible diagnosis groups and weights them according to their impact on health outcomes.23 This measure has shown a high capacity for explaining hospital outcomes in other settings. Our results regarding the explaining capacity of this variable for COVID-19 outcomes were consistent across various waves of the outbreak in our region and in different analysis approaches. This trend was weaker in patients admitted during the first wave, which were excluded from the primary analysis. The extreme demand for ICU beds during the first wave exceeded by far the ICU capacity in our region. In this context, along with the limited information on therapeutic options of hospitalized patients,46,47 the criteria for ICU admission were unclear, and we cannot rule out an age bias in ICU transfers. This limitation was also noted by Henkens et al.6 Furthermore, retrospective analyses of the clinical presentation and hospital outcomes throughout successive waves have highlighted the progressive consolidation of evidence-based practices in the management of COVID-19 patients in the hospital setting,13,29 which may contribute to explaining the differences between waves. These two factors (ie, improvement in clinical knowledge and management of COVID-19 cases and reduction of hospital services burden), likely to contribute to reducing the frequency of critical illness, would align with the drop in AUPRC, which depends on the incidence of the measured outcome. Finally, during the first wave, quick decision-making in a context of overwhelmed systems and a very high number of cases might have resulted in suboptimal documentation of chronic comorbidities.
Besides age and the comorbidity burden, we included sex in our analyses based on its relevance in COVID-19 and health outcomes in general.48 As expected, female sex was associated with lower risk of severe disease. Interestingly, although the inclusion of the comorbidity burden into the model reduced the size effect of sex, females remained as an important factor significantly reducing the risk of severe disease. This observation supports the hypothesis that male sex is independently associated with poorer COVID-19 outcomes, regardless of the presence of other risk factors.49
Our analysis is strengthened by the exhaustive data collection of a large number of diagnoses routinely reported in the hospital records in our region. Although we could not cover all hospitalizations occurred in Catalonia, the percentage of critically ill patients in our cohort was in line with population-based reports, which showed nearly 40% of critically ill cases among hospitalized patients in early waves,29,50 suggesting representativeness of our figures. The exhaustive data collection allowed us to identify and consider in our models most (if not all) concomitant clinical conditions in patients admitted to the hospital because of COVID-19. It is noteworthy, however, that our model can only be applied in healthcare systems with a systematic and exhaustive collection of diagnostic information. On the other hand, the use of administrative databases of routine care data has some limitations that must be considered for result interpretation. First, although we selected only hospitalizations with COVID-19 as a main diagnosis, we cannot rule out that, in some cases, complications of other underlying conditions triggered ICU admission or death. Another important limitation associated with the retrospective design is the constraint of the analysis to the information recorded in the databases. Thus, aside from the diagnoses, other clinical conditions not recorded in these databases may play a role in the observed effect of age. These conditions include―but are not limited to―frailty (ie, physical deterioration, not considered a diagnosis per se), weight loss, mild cognitive decline (ie, not qualifying for dementia), the recent loss of a relative, or subclinical depression. These conditions are likely to impact mortality, regardless of the presence of comorbidities.51,52 Therefore, they should also be considered to understand the effect of chronological age on COVID-19 outcomes completely. Another potential limitation in this regard is the lack of adjustment by baseline drug treatments; although the therapeutic burden is expected to reflect comorbidity burden, the type and number of drugs may differ between patients and impact in a different way on the risk of critical illness.53 Nevertheless, the features captured by Queralt DxS were already able to explain almost the full effect of age on the odds of developing critical COVID-19 disease. Retrospective analyses based on data collected during routine care can be also affected by the quality of the data. Hence, although the tool considers all possible diagnoses, not all clinical conditions are reported and recorded with the same accuracy, and some conditions could be underestimated. Likewise, it is worth mentioning that we covered only deaths occurred in the hospital setting; while this was the case for most people, we cannot rule out a certain number of deaths occurred outside the hospital, particularly during the first wave. Finally, the factors influencing critical illness may change based on the emergence of new variants and progression of population vaccination campaigns; therefore, besides the main analysis, we conducted subanalyses for each wave, thus covering a broad period with different scenarios.
Our findings have various implications. First, in light of the prognostic importance of comorbidity burden as a whole, comprehensive and weighted metrics of this variable may increase the accuracy of risk estimate, as suggested by Semenzano et al.5 This approach would reduce the risk of ageism (and the potential deleterious effects associated) in health planning and healthcare delivery, providing professionals with an autonomous risk score for these patients. It is worth mentioning that, unlike age, which is always available and accessible, not all healthcare organizations systematically collect all diagnoses in a central registry. However, in healthcare environments in which diagnoses are adequately and exhaustively reported in the electronic health records, the Queralt DxS, freely available, might be used to summarize the comorbidity burden in a single index used as an adjustment covariate for autonomous risk scores informing complexity on admission. The same adjustment can be used to develop predictive models using machine learning approaches, in which the summary of multiple comorbidities into a single weighted index may prevent overfitting. Finally, in healthcare systems that integrate primary and hospital care data,54 comorbidity burden could be estimated in advance to anticipate resource prioritization. These models would allow identifying patients at higher risk of critical illness and creating stratification systems for prioritizing and allocating healthcare resources such as COVID-19 vaccines or anticipating the demand of hospital services.
Our findings highlight the importance of considering the comorbidity burden as a whole (rather than individual diagnoses) and in a comprehensive way for assessing the risk of critical illness in COVID-19 patients. This perspective encourages the digitalization of healthcare systems for the systematic collection and integration of healthcare data that provides an accurate view of the clinical complexity of patients. Future steps in this pathway include the external validation of this tool and the inclusion of social care and functional information in these records.
Conclusion
In summary, although age is often regarded as a key prognostic factor in people hospitalized with COVID-19, our study suggests that when measured exhaustively, comorbidity burden explains better than chronological age the higher risk of critical illness. Moving forward, greater attention to comorbidity burden rather than to chronological age may inform more accurate risk stratification, management, and preventive therapy allocation.
Acknowledgments
The authors would like to thank Toni Fuentes for his support in building the dataset used in this analysis. The study did not receive specific funding.
Disclosure
DM declares that he is the developer of the Queralt System, which is freely available. The other authors report no conflicts of interest in this work.
References
1. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:26. doi:10.1136/bmj.m1328
2. Starke KR, Petereit-Haack G, Schubert M, et al. The age-related risk of severe outcomes due to covid-19 infection: a rapid review, meta-analysis, and meta-regression. Int J Environ Res Public Health. 2020;17(16):1–24. doi:10.3390/ijerph17165974
3. Ho FK, Petermann-Rocha F, Gray SR, et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One. 2020;15(11 November):e0241824. doi:10.1371/journal.pone.0241824
4. Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. doi:10.1016/S2214-109X(20)30264-3
5. Semenzato L, Botton J, Drouin J, et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health. 2021;8:100158. doi:10.1016/j.lanepe.2021.100158
6. Henkens MTHM, Raafs AG, Verdonschot JAJ, et al. Age is the main determinant of COVID-19 related in-hospital mortality with minimal impact of pre-existing comorbidities, a retrospective cohort study. BMC Geriatr. 2022;22(1):1–11. doi:10.1186/S12877-021-02673-1
7. Gupta RK, Marks M, Samuels THA, et al. Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: an observational cohort study. Eur Respir J. 2020;56(6):2003498. doi:10.1183/13993003.03498-2020
8. Chang E-S, Kannoth S, Levy S, Wang S-Y, Lee JE, Levy BR. Global reach of ageism on older persons’ health: a systematic review. PLoS One. 2020;15(1):e0220857. doi:10.1371/journal.pone.0220857
9. Ehni H-J, Wahl H-W. Six propositions against ageism in the COVID-19 pandemic. J Aging Soc Policy. 2020;32(4–5):515–525. doi:10.1080/08959420.2020.1770032
10. Gidari A, De Socio GV, Sabbatini S, Francisci D. Predictive value of National Early Warning Score 2 (NEWS2) for intensive care unit admission in patients with SARS-CoV-2 infection. Infect Dis. 2020;52(10):698–704. doi:10.1080/23744235.2020.1784457
11. Huang H, Cai S, Li Y, et al. Prognostic factors for COVID-19 Pneumonia progression to severe symptoms based on earlier clinical features: a retrospective analysis. Front Med. 2020;7. doi:10.3389/FMED.2020.557453
12. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi:10.1016/S0140-6736(20)31189-2
13. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369. doi:10.1136/bmj.m1966
14. Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C mortality score. BMJ. 2020;370:22. doi:10.1136/bmj.m3339
15. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi:10.1001/JAMAINTERNMED.2020.2033
16. Zou X, Li S, Fang M, et al. Acute physiology and chronic health evaluation II score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med. 2020;48(8):E657–E665. doi:10.1097/CCM.0000000000004411
17. Stalter RM, Atluri V, Xia F, et al. Elucidating pathways mediating the relationship between male sex and COVID-19 severity. Clin Epidemiol. 2022;14:115–125. doi:10.2147/clep.s335494
18. Qeadan F, VanSant-Webb E, Tingey B, et al. Racial disparities in COVID-19 outcomes exist despite comparable elixhauser comorbidity indices between Blacks, Hispanics, Native Americans, and Whites. Sci Rep. 2021;11(1). doi:10.1038/S41598-021-88308-2
19. De Caprio D, Gartner J, McCall CJ, et al. Building a COVID-19 vulnerability index. J Med Artif Intell. 2020;3. doi:10.21037/JMAI-20-47
20. Cho SI, Yoon S, Lee HJ. Impact of comorbidity burden on mortality in patients with COVID-19 using the Korean health insurance database. Sci Rep. 2021;11(1). doi:10.1038/S41598-021-85813-2
21. Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310–e2022310. doi:10.1001/JAMANETWORKOPEN.2020.22310
22. Gulbech Ording A, Toft Sørensen H. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol. 2013;5(1):199–203. doi:10.2147/CLEP.S45305
23. Monterde D, Cainzos-Achirica M, Cossio-Gil Y, et al. Performance of comprehensive risk adjustment for the prediction of in-hospital events using administrative healthcare data: the queralt indices. Risk Manag Healthc Policy. 2020;13:271–283. doi:10.2147/RMHP.S228415
24. Monterde D, Carot-Sans G, Cainzos-Achirica M, et al. Performance of three measures of comorbidity in predicting critical covid-19: a retrospective analysis of 4607 hospitalized patients. Risk Manag Healthc Policy. 2021;14:4729–4737. doi:10.2147/RMHP.S326132
25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Clin Epidemiol. 1987;40(5):373–383.
26. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi:10.1097/00005650-199801000-00004
27. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
28. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ elixhauser comorbidity index. Med Care. 2017;55(7):698–705. doi:10.1097/MLR.0000000000000735
29. Roso-Llorach A, Serra-Picamal X, Cos FX, et al. Evolving mortality and clinical outcomes of hospitalized subjects during successive COVID-19 waves in Catalonia, Spain. Glob Epidemiol. 2022;4:100071. doi:10.1016/j.gloepi.2022.100071
30. Imai K, Keele L, Yamamoto T. Identification, inference and sensitivity analysis for causal mediation effects. Statist Sci. 2010;25(1):51–71. doi:10.1214/10-STS321
31. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available from: https://www.r-project.org.
32. Tingley D. Mediation: R package for causal mediation analysis. R package version 4.5.0. Available from: http://cran.r-project.org/package=mediation.
33. Bates D, Maechler M, Bolker BM, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi:10.18637/jss.v067.i01
34. Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12(1):1–8. doi:10.1186/1471-2105-12-77
35. Grau J, Grosse I, Keilwagen J. PRROC: computing and visualizing precision-recall and receiver operating characteristic curves in R. Bioinformatics. 2015;31(15):2595–2597. doi:10.1093/bioinformatics/btv153
36. Gasparini A. Package “comorbidity”; 2020. Available from: https://cran.r-project.org/web/packages/comorbidity/comorbidity.pdf.
37. Goodman KE, Magder LS, Baghdadi JD, et al. Impact of sex and metabolic comorbidities on coronavirus disease 2019 (COVID-19) mortality risk across age groups: 66 646 inpatients across 613 US hospitals. Clin Infect Dis. 2021;73(11):e4113–e4123. doi:10.1093/cid/ciaa1787
38. Bae S, Kim SR, Kim M-N, Shim WJ, Park S-M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107(5):373–380. doi:10.1136/heartjnl-2020-317901
39. Molani S, Hernandez PV, Roper RT, et al. Risk factors for severe COVID-19 differ by age for hospitalized adults. Sci Rep. 2022;12(1):6568. doi:10.1038/s41598-022-10344-3
40. Awortwe C, Cascorbi I. Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions. Pharmacol Res. 2020;161:105250. doi:10.1016/j.phrs.2020.105250
41. Qiu P, Zhou Y, Wang F, et al. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: a systematic review and meta-analysis. Aging Clin Exp Res. 2020;32(9):1869–1878. doi:10.1007/S40520-020-01664-3
42. Chen Z, Peng Y, Wu X, et al. Comorbidities and complications of COVID-19 associated with disease severity, progression, and mortality in China with centralized isolation and hospitalization: a systematic review and meta-analysis. Front Public Health. 2022;10:1.
43. Péterfi A, Mészáros Á, Szarvas Z, et al. Comorbidities and increased mortality of COVID-19 among the elderly: a systematic review. Physiol Int. 2022. doi:10.1556/2060.2022.00206
44. Tian Y, Wu Q, Li H, et al. Distinct symptoms and underlying comorbidities with latitude and longitude in COVID-19: a systematic review and meta-analysis. Can Respir J. 2020;2022. doi:10.1155/2022/6163735
45. Thakur B, Dubey P, Benitez J, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 2021;11(1):1–13. doi:10.1038/s41598-021-88130-w
46. Mannucci E, Silverii GA, Monami M. Saturation of critical care capacity and mortality in patients with the novel coronavirus (COVID-19) in Italy. Trends Anaesth Crit Care. 2020;33:33. doi:10.1016/j.tacc.2020.05.002
47. Hick JL, Hanfling D, Wynia MK, Pavia AT. Duty to plan: health care, crisis standards of care, and novel coronavirus SARS-CoV-2. NAM Perspect. 2020;2020. doi:10.31478/202003b
48. Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2021;64(1):36–47. doi:10.1159/000512592
49. Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116(14):2197–2206. doi:10.1093/cvr/cvaa284
50. Vela E, Carot-Sans G, Clèries M, et al. Development and validation of a population-based risk stratification model for COVID-19 in the general population. Sci Rep. 2022;12:3277. doi:10.1038/s41598-022-07138-y
51. Saragih ID, Advani S, Saragih IS, Suarilah I, Susanto I, Lin CJ. Frailty as a mortality predictor in older adults with COVID-19: a systematic review and meta-analysis of cohort studies. Geriatr Nurs. 2021;42(5):983–992. doi:10.1016/j.gerinurse.2021.06.003
52. Hussien H, Nastasa A, Apetrii M, Nistor I, Petrovic M, Covic A. Different aspects of frailty and COVID-19: points to consider in the current pandemic and future ones. BMC Geriatr. 2021;21(1). doi:10.1186/s12877-021-02316-5
53. Bliek-Bueno K, Mucherino S, Poblador-Plou B, et al. Baseline drug treatments as indicators of increased risk of COVID-19 mortality in Spain and Italy. Int J Environ Res Public Health. 2021;18(22):11786. doi:10.3390/ijerph182211786
54. Gude-Sampedro F, Fernández-Merino C, Ferreiro L, et al. Development and validation of a prognostic model based on comorbidities to predict COVID-19 severity: a population-based study. Int J Epidemiol. 2021;50(1):64–74. doi:10.1093/ije/dyaa209
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




