Back to Journals » Journal of Inflammation Research » Volume 14
Irreversible Electroporation Plus Anti-PD-1 Antibody versus Irreversible Electroporation Alone for Patients with Locally Advanced Pancreatic Cancer
Authors He C , Sun S, Zhang Y, Li S
Received 3 August 2021
Accepted for publication 9 September 2021
Published 21 September 2021 Volume 2021:14 Pages 4795—4807
DOI https://doi.org/10.2147/JIR.S331023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Chaobin He,1,* Shuxin Sun,2,* Yu Zhang,3,* Shengping Li1
1Department of Pancreatobiliary Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, 510060, People’s Republic of China; 2Department of General Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong Province, People’s Republic of China; 3State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, Guangdong, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shengping Li Email [email protected]
Background: Irreversible electroporation (IRE) is shown to not only improve the prognosis of patients with locally advanced pancreatic cancer (LAPC) but also activate the immune system. Considering the immune-activating function of IRE, IRE may enhance the effect of immune checkpoint inhibitors in the treatment of LAPC. We aimed to compare the effect and safety of IRE combined with toripalimab versus IRE alone for LAPC.
Methods: We retrospectively collected data from LAPC patients treated with IRE plus toripalimab (240mg, 7 days after IRE) or IRE alone at Sun Yat‑sen University Cancer Center. Overall and progression-free survival and treatment-related adverse events were evaluated and compared.
Results: From August 2015 to June 2020, a total of 85 patients were collected and analyzed in this study: 70 in the IRE group and 15 in the IRE plus toripalimab group. The IRE plus toripalimab group showed longer OS [44.33 months (95% CI 17.39– 71.27) versus 23.37 months (95% CI 21.20– 25.54), P=0.010] and PFS [27.5 months (95% CI not reached) versus 10.6 months (95% CI 7.79– 13.42), P=0.036], compared with IRE group. There were no treatment-related deaths in all patients of this study. Although pancreatic fistula, biliary fistula, abscess, vomiting and gastroparesis were a little more common in IRE plus toripalimab group, no significant differences in the rates of all adverse events between these two groups were observed.
Conclusion: IRE plus toripalimab had acceptable toxic effects and might improve survival in LAPC compared with IRE alone.
Keywords: locally advanced pancreatic cancer, irreversible electroporation, toripalimab, efficacy, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal gastrointestinal disease with increasing morbidity, which also has a growing impact on cancer-specific mortality worldwide.1 Nearly 40% of all PDAC cases are localized to the pancreas and characterized with the involvement of major vascular structures, leading to unresectable disease without metastases detected by radiological examinations, which are also known as locally advanced pancreatic cancer (LAPC).2 Despite currently available therapies, the prognosis of LAPC remained unsatisfied with 3% as 5-year survival rate and 14.2 months as median survival.3 The optimal treatment of LAPC was still controversial. Based on the development of chemotherapy, more choices for additional treatment, such as conversional surgical resection, were available. However, the rates of downstaging to resection were as low as 4–15%.4 Moreover, more than 30% of deaths were due to the progression of local disease,5 suggesting the importance of local control approaches.
As an important local destructive method, local ablative therapy was used as a new treatment of LAPC. However, the thermal injury to the adjacent organs and vessels limited the use of most thermal ablative methods, including radiofrequency ablation and microwave ablation.6,7 Instead of relying on thermal energy, IRE induces the apoptosis of tumor cells by destroying the cell membrane integrity with short and high-voltage current pulses.8 The feature of free from the heat sink effect makes IRE more appropriate than radiofrequency ablation (RFA) in the treatment of LAPC. Additionally, the immune response caused by IRE may be stronger, because the advantage of preservation of vessels is helpful for the transmission of immune molecules or cells. Thus, apart from inducing apoptosis of tumor cells, IRE can also reconstruct the microenvironment within the tumor and induce the immune response.9–11 Previous studies had shown that IRE could alleviate immune suppression and induce activation of T cells, indicating that IRE may potentiate the antitumor efficacy of immunotherapy in PDAC.9,12
In recent years, game-changing advances have been achieved in the development of therapies of various cancers, including melanoma, lung and liver cancers with immune checkpoint inhibitors (ICIs).13–15 However, little success had been achieved in the use of ICIs in PDAC.16 The relatively low rates of programmed cell death protein 1 (PD-1) expression, low mutational load and infiltration of T cells, and accumulation of regulatory T-cells (Tregs) could all lead to the failed immunotherapy in PDAC.17,18 The immune-suppressive tumor microenvironment (TME) contributed to the poor response to ICIs, indicating the importance of augmenting the ICI-responsiveness with combination therapy. IRE was shown to not only destroy the tumor itself directly, but also activate the immune system. We previously indicated that IRE could induce immunogenic cell death (ICD) and increase the infiltration of effector CD8+ T cells.9 Additionally, IRE could also enhance the process of antigen presentation by promoting M1 macrophage polarization and maturation of dendritic cells.10,19 Based on these findings, a promising approach to improve the responsiveness of ICIs is to utilize the immunogenic properties of IRE to increase the cytotoxic T cells (CTL) to the “cold” TME, switching its immune status to “hot”. The combination of IRE and PD-1 immune checkpoint blockade could significantly promote survival in Kras-induced pancreatic cancer (KPC) mode by selectively promoting the infiltration of CD8+ T cells.20 Based on these promising results, the clinical effect of the combination of IRE and anti-PD-1 therapy in LAPC could be encouraging and should be investigated. However, up to now, no similar studies had focus on this topic. Therefore, we designed this study to evaluate the survival of LAPC patients after IRE combined with anti-PD-1 therapy.
Methods
Patients
This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (SYSUCC). The ethical standards of the 1964 Helsinki Declaration were strictly followed and written informed consent was obtained from patients prior to treatment. Patients diagnosed with LAPC were included in this study. The exclusion criteria included the following: (1) patients who have received surgical resection or RFA; (2) patients with second primary malignant tumor; (3) patients with incomplete follow-up information or loss to follow-up.
Treatment Procedure
Similar to the procedure reported before,21,22 a 4-month induction chemotherapy and adjuvant FOLFIRINOX chemotherapy were adopted for all patients. During the procedure of IRE, an electric field around the tumor was created with two to six probes. As for the initial setting of electroporation, an electric field strength of 1500 V/cm and a planned delivery of 90 pulses at a pulse length of 70–90 ms were used. Toripalimab, an anti-PD-1 immune checkpoint blockade, was administered a week after IRE and was repeated every 3 weeks thereafter until progressive disease (PD) was detected.
Data Collection
Routine measurements of pathological and clinical data, including age, gender, tumor size, grade, and site, white blood cell (WBC) count, platelet (PLT) count, serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glutamyl transpeptidase (GGT), albumin (ALB), total bilirubin (TBIL), indirect bilirubin (IBIL), C-reactive protein (CRP), hepatitis B surface antigen (HBsAg), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) were carried out. Survival endpoints, including overall survival (OS) and progression-free survival (PFS), which were defined as the duration from the date of diagnosis to death and tumor progression, respectively, or last follow-up, were analyzed in this study. All patients were followed until May 30, 2021. After IRE treatment, the first follow-up visit was performed 1 month later, and follow-up then occurred every 2–3 months, until death or dropout. Physical examination, serum CA19-9, CEA analysis and at least one imaging examination (abdominal CT or MRI) were performed for each follow-up. Development of new low-density lesions in the region of the IRE (within 1 cm) or in the liver or lungs was considered evidence of local recurrence or distant metastasis, respectively, even in the absence of symptoms. Suspicious nodules in the peritoneum or the omentum, or the presence of newly identified ascites, were defined as peritoneal recurrence. If the findings were equivocal with recurrence, then imaging and CA19-9 were repeated at the second month for confirmation of tumor recurrence or not.
Blood Sample Collection and Analysis
All blood samples were collected as the procedure reported before,23 which was simply described as duple blood sample before (BT) and on the seventh day after IRE (AT). The mononuclear cells (PBMCs) were isolated and analyzed with CD3, CD3CD4, CD3CD8, CD3CD16CD56, and CD4CD25 antibodies by flow cytometry (FACS caliber, 4 color system, BD Bioscience, CA, US).
Similar to the procedure described in our previous study.23 Quantitative sandwich enzyme immunoassay technique (ELISA kit, R&D system, Minneapolis, MN) was used to measure the concentrations of serum cytokines and humoral immune parameters, including IL-2, IL-6, IL-10, interferon-γ (IFN-γ), tumor-necrosis factor (TNF), C3, C4, IgA, IgM, and IgG in the peripheral blood.
Statistical Analysis
Categorical data were compared with the chi-square test. Survival differences were calculated with Kaplan–Meier method and compared with Log rank test. Cox regression model was used to determine the significant variables of survival in the multivariate analysis. Two tailed P-value was used and those <0.05 were considered statistically significant. R version 3.4.2 software (The R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org) was adopted to perform all statistical analyses.
Results
Patient Characteristics
The flow diagram for data selection is shown in Figure 1. Between August 2015 and June 2020, 85 patients met the criteria for inclusion: 15 patients received combination therapy with anti-PD-1 therapy plus IRE (IRE plus toripalimab group), and 70 patients received IRE monotherapy (IRE group). The last follow-up was completed on 30 May 2021. No significant differences were observed in the baseline clinical and pathological characteristics between these two groups. Female occupied predominantly (55.3%) in the whole cohort. The median age for all patients was 57.84 years (range 19–87). Specifically, the median ages for IRE plus toripalimab group and IRE groups were 56.47 years (range 40–66) and 58.13 years (range 19–87), respectively. Additionally, for patients in both treatment groups, similar distributions of tumor site and tumor size were also observed. The median tumor sizes for patients in the IRE plus toripalimab group and IRE groups were 3.5 cm (range 2.4–5.7) and 3.75 cm (range 2.2–5.5), respectively. There were also no significant differences in the proportions of neoadjuvant chemotherapy and their regimens between these two groups (Table 1).
 |
Table 1 Clinicopathological Characteristics of Patients with LAPC Stratified by Treatment |
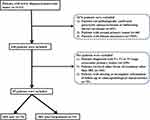 |
Figure 1 Flowchart of the included patients. |
Survival Comparisons Among Three Groups
In this study, disease progression and death were observed in 54 patients and 45 patients, respectively, in the whole cohort. The median OS and median PFS for all patients were 24.63 months [95% confidence interval (CI) 20.91–28.36] and 13.73 months (95% CI 7.91–19.55), respectively. The median OS in the IRE plus toripalimab group was 44.33 months (95% CI 17.39–71.27), compared with 23.37 months (95% CI 21.20–25.54) in the IRE group. The 1-year, 2-year, and 3-year OS rates for IRE plus toripalimab group were 100%, 100%, and 33.3%, respectively, compared with 91.1%, 45.1% and 11.7% for IRE group (P = 0.010, Figure 2A). The median PFS for IRE plus toripalimab and IRE groups were 27.5 months (95% CI not reached) and 10.6 months (95% CI 7.79–13.42 months), respectively. The 1-year and 2-year PFS rates for IRE plus toripalimab group were 88.9% and 52.4%, respectively, compared with 46.8% and 17.6% for IRE group (P = 0.036, Figure 2B).
 |
Figure 2 Overall survival (A) and progression free survival (B) analyses stratified by treatments in LAPC patients. |
Prognostic Factors for Survival
As shown in Table 2, the results of univariate analysis for OS revealed that tumor size, CA125, neoadjuvant chemotherapy regimen, and treatment strategy were associated with OS. Additionally, in the multivariate analysis, it was showed that independent risk factors for OS included tumor size (>4cm versus ≤2cm, HR=39.760, 95% CI 1.908–828.050, P=0.017), CA125 (>45U/mL versus ≤45U/mL, HR=2.373, 95% CI 1.165–4.832, P=0.017), and treatment strategy (IRE + PD-1 versus IRE, HR=0.274, 95% CI 0.076–0.985, P=0.047). In terms of PFS, tumor grade (poor/moderate differentiation versus well differentiation, HR=2.031, 95% CI 1.125–3.669, P=0.019) and treatment strategy (IRE plus toripalimab versus IRE, HR=0.342, 95% CI 0.119–0.978, P=0.045) were identified as significant prognostic factors for PFS (Table 3).
 |
Table 2 Independent Prognostic Factors for OS |
 |
Table 3 Independent Prognostic Factors for PFS |
Variation of Circulating Immune Cells
The isolated mononuclear cells were phenotypically characterized by evaluating the absolute number of B cells (identified as CD19+), T cells (identified as CD3+), CD4+ T cell (helpful T cells, identified as CD3+CD4+), CD8+ T cell (cytotoxic T cell, identified as CD3+CD8+), CD4+ regulatory T cell (CD4+ Treg, identified as CD4+CD25+FoxP3+), CD8+ regulatory T cell (CD8+ Treg, identified as CD8+CD25+FoxP3+), and natural killer cell [natural killer cell (NK cell), identified as CD3−CD16+CD56+] within seven days before (BT) and after (AT) IRE treatment. It was shown that compared with IRE group, the absolute number of CD4+ T cells (P=0.038) and CD8+ T cells (P=0.024) steadily increased, while CD8+ Treg cells (P=0.023) decreased after treatment in IRE plus toripalimab group. The ratio of CD4+ T cells to CD8+ T cells was also decreased (P=0.019) in IRE plus toripalimab group (Figure 3).
Variation of Circulating Cytokines and Humoral Immune Parameters
Apart from the variations of immune cells, there were also huge differences on the serum levels of cytokines and immune parameters after treatment. Compared with levels before treatment, marked increases of IL-4, IL-6, TNF, and IFN-γwere observed after both treatments. Additionally, the increased levels of IL-4, IL-6, TNF, and IFN-γwere much higher in the IRE + PD-1 group than those in the IRE group. The alteration of IL-10 after IRE treatment was not obvious, while it was significant after treatment with IRE combined with toripalimab. Apart from IgM, there were no significant differences in other immunoglobulin, including IgA, IgG, and complements, such as C3, C4, between levels before and after both treatments (Figure 4).
Safety of Treatment
The main treatment-related adverse events are shown in Table 4. No treatment-related deaths were observed in all patients of this study. Loss of appetite, which was observed in 27.06% of all patients, happened most frequently, followed by nausea (25.88%), vomiting (22.35%), diarrhea (20.00%), pain (14.12%), and pancreatic fistula (11.76%). Although pancreatic fistula, biliary fistula, abscess, vomiting and gastroparesis were a little more common in IRE plus toripalimab group, no significant differences were observed in the rates of all adverse events (all P > 0.05). In terms of immune-related adverse events, one patient in the IRE plus toripalimab group developed a grade 2 rash, which was recovered after the treatment with corticosteroids. No immune-related adverse events were observed in IRE group.
 |
Table 4 Comparisons of Complications in Two Treatment Groups |
Discussion
The poor survival for LAPC indicated the need in the development of treatment for these patients. As a special and relatively large group of pancreatic cancer, LAPC showed significantly different biological aggressiveness and histology, compared with metastatic diseases, and also showed limited response to chemotherapy.24,25 Although patients may benefit from the extended surgery, the conversional resection rates varied in a great range from 0% to 43%.22 Additionally, relatively high rates of postoperative complications of extended surgery also prevented the treatment of surgery to some degree.26 Considering the fact that progression of local disease, other than distant metastasis, contributed to most of the mortalities of LAPC patients, local destructive method is worthy of being tried.27 As a non-thermal local destructive method, IRE induces apoptosis of tumor cells through causing irreversible permeabilization in cell membrane.8 Mounting evidences confirmed the safety and effectiveness of IRE in the treatment of LAPC.21,28 Additionally, our previous studies showed that compared with the conventional treatments, including chemotherapy and conversional resection, IRE followed by chemotherapy could further extend the survival time of LAPC patients.22,29 As a local treatment, radiotherapy plays a role in the localized control of LAPC. Chemotherapy followed by chemoradiotherapy is an option in patients with LAPC, demonstrating stability than chemotherapy alone. However, current clinical trials comparing chemotherapy with chemoradiotherapy had reported mixed responses.30,31 Therefore, there was no consensus concerning the survival benefit of radiotherapy in patients with LAPC based on the current evidence and more clinical trials were needed to validate the role of radiotherapy in the treatment of LAPC. IRE is a novel local control treatment which has been recently applied in the treatment of LAPC. IRE was helpful for chemotherapy delivery to tumor by disrupting the dense stroma of pancreatic cancer.20,32 In addition, due to the feature of non-thermal ablation, electric field of extremely high voltage can be applied through the whole tumor without harming nearby important structure in IRE treatment. In contrast, the duodenum and small intestine were easily harmed by high doses of radiation. Conventional radiotherapy was used most frequently in the treatment of LAPC and one of the reasons that studies failed to demonstrate the superiority of radiotherapy was the insufficient dose of radiation.33 Therefore, compared with radiotherapy, IRE is more active in inducing tumor destruction and providing survival benefit for patients with LAPC. All these results indicated that IRE played an important role in the treatment of LAPC. The combination therapy of IRE and other treatment may be a promising method for further improving survival of LAPC patients.
In recent years, along with the improvement in the ICIs, immune therapy became more and more popular in the treatment of several malignant tumors.15,34 However, the immune-suppressive nature limited the efficacy of immune therapy in PDAC.35 Simultaneously, more and more studies indicated that IRE could further be an immune-stimulating method other than induce tumor cell death only.23,36 We previously found that IRE induced local immunomodulation by promoting M1 macrophage polarization and increasing specific T cell infiltration,9,10 indicating that the active immune status stimulated by IRE and the release of tumor antigen might further enhance the efficacy of immune therapy. Additionally, the low expression of PD-1 could contribute to the low response rates of ICIs, which could be partly reversed by the elevated expression of PD-1 in T cells induced by IRE.12 Using the mouse model, it was shown that systemic antitumor immune response triggered by IRE could be enhanced by stimulating the innate immune system with a TLR7 agonist and the adaptive immune system with anti-PD-1 checkpoint blockade simultaneously.37 Therefore, it was reasonable to speculate the hypothesis that IRE could provide sensibilization to ICIs in LAPC. This hypothesis was supported by the finding of Zhao’s study, which reported that IRE enhanced the antitumor efficacy of immunotherapy by reversing resistance to immune checkpoint blockade.20 Notably, the efficacy of this combination therapy was not validated in clinical practice. Subsequently, we firstly built a new treatment regimen with IRE followed by systemic infusion of toripalimab. In the present study, it was shown that compared with IRE monotherapy, IRE combined with toripalimab significantly prolonged the survival of LAPC patients by enhancing immune function and suppressing tumor growth in this study. The OS of LAPC after IRE combined with toripalimab can be elevated to 44.3 months, indicating this treatment has great potential as a promising therapy for LAPC.
Remarkably, it was observed that there was a steady increase of CD4+ T helper cells, CD8+ T cytotoxic cells, while a reverse trend for CD8+ Treg cells was observed after IRE combined with toripalimab. In addition to the increase of T cells, the release of several cytokines, such as IL-4, IL-6, IL-10, TNF, and IFN-γ, were also elevated more significantly after treatment with IRE combined with toripalimab, compared with IRE alone, suggesting a stronger antitumor immune response. The improved expression of TNF and IFN-γ, which were mostly released by CD8+ T cytotoxic cells and induced specific immune killing, indicated that IRE plus anti-PD-1 therapy were more inclined to activate the specific immune system. Additionally, TNF-αnot only played an important role in M1 macrophage polarization, but also contributed to the release of IL-4, IL-6, and IL-10 from M1 macrophage to enhance the antigen-presenting function.38 Similar with other study, higher levels of these immune-activating cytokines contributed to active immune system and maintain longer tumor progression-free status. This might partly contribute to the extended survival in LAPC patients after treatment with IRE combined with toripalimab.
Our study confirmed that the addition of anti-PD-1 therapy could further enhance the efficacy of IRE in the treatment of LAPC. In the present study, neoadjuvant chemotherapy was shown to independently indicate superior survival, indicating neoadjuvant chemotherapy was the basis of the combination therapy. The neoadjuvant chemotherapy, which aimed to eliminate some potential micrometastases, was extremely important for the cancer control. In terms of chemotherapy regimens, it was shown that survival of LAPC patients was not significantly influenced by neoadjuvant chemotherapy regimens. In the present study, only one-third of patients have received chemotherapy with full doses of FOLFIRINOX. This might partly explain the reason for the unobvious survival advantages of full doses of FOLFIRINOX, compared with other chemotherapy regimens. Therefore, it is believed that FOLFIRINOX combined with IRE and anti-PD-1 therapy would further improve the survival of LAPC patients, which will be further validated in the future prospective studies. Additionally, similar with previous study,39 more adverse effects were not observed from this combination therapy, compared with IRE therapy alone, showing the safety of combination method. In this study, toripalimab was administrated on the seventh day after IRE. An appropriate time interval not only provides a time window for immune activation,9 but also is helpful for the recovery from the prior treatment, which may partly contribute to the low rates of adverse effects of this study. In summary, our study indicated that the survival time of LAPC patients was further improved after anti-PD-1 therapy combined with IRE, indicating this combination therapy might be a suitable treatment for LAPC patients.
There were several limitations in this study. First, the retrospective design and the nonrandomized nature made it vulnerable to a variable of potential biases even though the distribution of basic characteristics was balanced between these two groups. The lack of microsatellite instability (MSI) status and the response of neoadjuvant chemotherapy were also limitations. More prospective randomized controlled trials are necessary to verify the results of this study. Second, the numbers of included patients were small. Although no similar studies had been reported before, the efficacy of IRE combined with anti-PD-1 therapy should be validated in more LAPC cohorts. Third, the follow-up time was a little insufficient for OS because the survival endpoint of OS was obtained in only nearly half of all patients. Subsequent results with longer follow-up time were necessary for a more complete overview of this combination therapy.
Conclusion
In conclusion, compared with IRE, IRE combined with toripalimab significantly prolonged survival of LAPC patients. A randomized clinical trial evaluating this combination therapy is therefore warranted.
Acknowledgments
This work was supported by grants from the National Natural Science Funds (No.82102166, No.81972299), Guangdong Basic and Applied Basic Research Foundation (2020A1515110954), and the National Key Research and Development Plan (No.2017YFC0910002).
Disclosure
The authors declare no conflict of interest.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi:10.3322/caac.21654
2. Amin MB, Edge S, Greene F. AJCC Cancer Staging Manual. Chicago, IL: Springer; 2017.
3. Weniger M, Moir J, Damm M, et al. Respect - a multicenter retrospective study on preoperative chemotherapy in locally advanced and borderline resectable pancreatic cancer. Pancreatology. 2020;20:1131–1138. doi:10.1016/j.pan.2020.06.012
4. Loehrer PJ, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi:10.1200/JCO.2011.34.8904
5. Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi:10.1001/jama.2016.4324
6. Girelli R, Frigerio I, Giardino A, et al. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch Surg. 2013;398:63–69. doi:10.1007/s00423-012-1011-z
7. Pezzilli R, Ricci C, Serra C, et al. The problems of radiofrequency ablation as an approach for advanced unresectable ductal pancreatic carcinoma. Cancers (Basel). 2010;2:1419–1431. doi:10.3390/cancers2031419
8. Al Efishat M, Wolfgang CL, Weiss MJ. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ. 2015;350:h521. doi:10.1136/bmj.h521
9. He C, Huang X, Zhang Y, Lin X, Li S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin Transl Med. 2020;10:e39. doi:10.1002/ctm2.39
10. He C, Sun S, Zhang Y, Xie F, Li S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. Oncoimmunology. 2021;10:1897295. doi:10.1080/2162402X.2021.1897295
11. He C, Sun S, Zhang Y, Li S. Identification of circulating biomarkers and construction of a prognostic signature for survival prediction in locally advanced pancreatic cancer after irreversible electroporation. J Inflamm Res. 2021;14:1689–1699. doi:10.2147/JIR.S307884
12. Scheffer HJ, Stam AGM, Geboers B, et al. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology. 2019;8:1652532. doi:10.1080/2162402X.2019.1652532
13. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–3391. doi:10.1172/JCI80011
14. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi:10.1056/NEJMoa1200690
15. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi:10.1016/S1470-2045(21)00252-7
16. Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: barriers and breakthroughs. Ann Gastroenterol Surg. 2018;2:274–281. doi:10.1002/ags3.12176
17. Tsukamoto M, Imai K, Ishimoto T, et al. PD-L1 expression enhancement by infiltrating macrophage-derived tumor necrosis factor-α leads to poor pancreatic cancer prognosis. Cancer Sci. 2019;110:310–320.
18. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30. doi:10.1016/j.ctrv.2019.06.005
19. Yang J, Eresen A, Shangguan J, Ma Q, Yaghmai V, Zhang Z. Irreversible electroporation ablation overcomes tumor-associated immunosuppression to improve the efficacy of DC vaccination in a mice model of pancreatic cancer. Oncoimmunology. 2021;10:1875638. doi:10.1080/2162402X.2021.1875638
20. Zhao J, Wen X, Tian L, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10:899. doi:10.1038/s41467-019-08782-1
21. He C, Wang J, Zhang Y, Lin X, Li S. Irreversible electroporation after induction chemotherapy versus chemotherapy alone for patients with locally advanced pancreatic cancer: a propensity score matching analysis. Pancreatology. 2020;20:477–484. doi:10.1016/j.pan.2020.02.009
22. He C, Sun S, Huang X, Zhang Y, Lin X, Li S. Survival comparison of neoadjuvant chemotherapy followed by irreversible electroporation versus conversional resection for locally advanced pancreatic cancer. Front Oncol. 2020;10:622318. doi:10.3389/fonc.2020.622318
23. He C, Wang J, Sun S, Zhang Y, Li S. Immunomodulatory effect after irreversible electroporation in patients with locally advanced pancreatic cancer. J Oncol. 2019;2019:9346017. doi:10.1155/2019/9346017
24. Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–3043. doi:10.1200/JCO.2010.33.8038
25. Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–1027. doi:10.1001/jamaoncol.2019.0892
26. Beetz O, Sarisin A, Kaltenborn A, Klempnauer J, Winkler M, Grannas G. Multivisceral resection for adenocarcinoma of the pancreatic body and tail-a retrospective single-center analysis. World J Surg Oncol. 2020;18:218. doi:10.1186/s12957-020-01973-x
27. Peixoto RD, Speers C, McGahan CE, Renouf DJ, Schaeffer DF, Kennecke HF. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. 2015;4:1171–1177. doi:10.1002/cam4.459
28. Martin RC, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262:486. doi:10.1097/SLA.0000000000001441
29. He C, Huang X, Zhang Y, Cai Z, Lin X, Li S. Comparison of survival between irreversible electroporation followed by chemotherapy and chemotherapy alone for locally advanced pancreatic cancer. Front Oncol. 2020;10:6. doi:10.3389/fonc.2020.00006
30. Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599.
31. Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi:10.1002/cncr.22735
32. Moir J, White SA, French JJ, Littler P, Manas DM. Systematic review of irreversible electroporation in the treatment of advanced pancreatic cancer. Eur J Surg Oncol. 2014;40:1598–1604. doi:10.1016/j.ejso.2014.08.480
33. Shinoto M, Terashima K, Suefuji H, et al. A single institutional experience of combined carbon-ion radiotherapy and chemotherapy for unresectable locally advanced pancreatic cancer. Radiother Oncol. 2018;129:333–339. doi:10.1016/j.radonc.2018.08.026
34. Yu EY, Petrylak DP, O’Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:872–882. doi:10.1016/S1470-2045(21)00094-2
35. Thind K, Padrnos LJ, Ramanathan RK, Borad MJ. Immunotherapy in pancreatic cancer treatment: a new frontier. Therap Adv Gastroenterol. 2017;10:168–194. doi:10.1177/1756283X16667909
36. Pandit H, Hong YK, Li Y, et al. Evaluating the regulatory immunomodulation effect of Irreversible Electroporation (IRE) in pancreatic adenocarcinoma. Ann Surg Oncol. 2019;26:800–806. doi:10.1245/s10434-018-07144-3
37. Narayanan JSS, Ray P, Hayashi T, et al. Irreversible electroporation combined with checkpoint blockade and TLR7 stimulation induces antitumor immunity in a murine pancreatic cancer model. Cancer Immunol Res. 2019;7:1714–1726. doi:10.1158/2326-6066.CIR-19-0101
38. Li X, Huang Q, Hu X, et al. Evaluating the osteoimmunomodulatory properties of micro-arc oxidized titanium surface at two different biological stages using an optimized in vitro cell culture strategy. Mater Sci Eng C Mater Biol Appl. 2020;110:110722. doi:10.1016/j.msec.2020.110722
39. Lin M, Zhang X, Liang S, et al. Irreversible electroporation plus allogenic Vγ9Vδ2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal Transduct Target Ther. 2020;5:215. doi:10.1038/s41392-020-00260-1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


