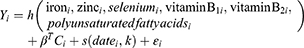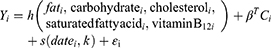Back to Journals » Journal of Inflammation Research » Volume 14
Investigating Associations Between Depressive Symptoms and Anti-/Pro-Inflammatory Nutrients in an Elderly Population in Northern China: A Bayesian Kernel Machine Regression Approach
Authors Li R, Zhan W, Huang X, Zhang L, Sun Y , Zhang Z, Bao W, Ma Y
Received 23 July 2021
Accepted for publication 16 September 2021
Published 9 October 2021 Volume 2021:14 Pages 5201—5213
DOI https://doi.org/10.2147/JIR.S330300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ruiqiang Li,1,* Wenqiang Zhan,2,* Xin Huang,1 Limin Zhang,1 Yan Sun,1 Zechen Zhang,1 Wei Bao,1 Yuxia Ma1
1Department of Nutrition and Food Hygiene, School of Public Health, Hebei Medical University, Hebei Province Key Laboratory of Environment and Human Health, Shijiazhuang, People’s Republic of China; 2School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuxia Ma
Department of Nutrition and Food Hygiene, School of Public Health, Hebei Medical University, Hebei Province Key Laboratory of Environment and Human Health, Shijiazhuang, People’s Republic of China
Email [email protected]
Backgroud: The potential for dietary inflammation has been shown to be associated with a variety of chronic diseases. The relationship between the potential for dietary inflammation and depression in the elderly is unclear.
Objective: This study aimed to exam the relationship between different nutrients and the risk of depression symptoms in the elderly.
Methods: In total, 1865 elderly in northern China were investigated at baseline from 2018 to 2019 and followed up in 2020. We measured the baseline intake of 22 nutrients and used Least Absolute Shrinkage and Selection Operator(LASSO) regression analysis and Bayesian Kernel Machine Regression (BKMR) to explore the association between exposure to a variety of nutrients with different inflammatory potentials and the risk of depressive symptoms.
Results: A total of 447 individuals (24.0%) were diagnosed with depressive symptoms. Through the lasso regression model, it was found that 11 nutrients are significantly related to the risk of depressive symptoms, of which 6 nutrients are pro-inflammatory nutrients (inflammation effect score> 0), and 5 are anti-inflammatory nutrients (inflammation effect score< 0). We incorporated the inflammatory effect scores of 11 nutrients into the BKMR model at the same time, and found that the overall inflammatory effect of 11 nutrients increased with the increase of total inflammatory scores, suggesting that the overall effect was pro-inflammatory. BKMR subgroup analysis shows that whether in the pro-inflammatory nutrient group or the anti-inflammatory nutrient group, multiple nutrients have a significant combined effect on depressive symptoms. By comparing the overall and group effects, we found that the inflammatory effects of the pro-inflammatory diet and the anti-inflammatory diet in the study’s diet are offset by each other (P< 0.005).
Conclusion: We determined the combined effect of multiple nutrients of different inflammatory potential classifications on depressive symptoms in the elderly.
Keywords: depression, anti-inflammatory, pro-inflammatory, elderly, Bayesian kernel machine regression approach
Introduction
Due to rapid economic growth and changes in lifestyles, China is undergoing a rapid epidemiological transition from infectious diseases to non-communicable diseases (NCDs). Mental disorders such as depression are an important but often overlooked non-communicable disease, and they are becoming an increasingly serious cause of disability, suicide and disease burden. It is estimated that depression and depressive symptoms accounted for 14.7% of China’s personal medical expenditure in 2012, almost three times that of obesity and overweight.1 The elderly in rural China have experienced a series of mental disorders, the most common of which is depression.2
In terms of clinical manifestations, there is heterogeneity in depression of different ages. Elderly patients with depression tend to show more activity retardation, cognitive impairment, and a large number of somatic complaints. Among physiological factors, vascular depression is regarded as a type of depression disorder in the elderly and an important cause of depression.3 On the one hand, evidence from neuroimaging suggests that white matter lesions in patients with late-onset depression may be related to the decreased vasogenic connection of the frontal lobe-limbic system and the decreased functional connection of the amygdala to the anterior cingulate gyrus, but the exact cause is not clear.On the other hand, late onset depression is also associated with more cardiovascular disease. So depression in the elderly is a chronic disease that needs urgent attention.4
Depression is a intricate public health problem with the characteristics of large differences in response to treatment.The systemic complexity of depression, or the feedback process between the different drivers of the disorder, leads to the persistence of depression.5 According to reports, systemic chronic low-grade inflammation is related to the progression of Major depressive disorder (MDD) by affecting monoaminergic and glutamatergic neurotransmission.However, whether various pro-inflammatory cytokines are abnormally elevated before the first onset of depression remains unclear.6 Recent studies have found that depression is related to chronic, low-grade inflammation. It is characterized by an increase in circulating pro-inflammatory cytokines, a change in the frequency of white blood cell populations in the blood, the accumulation of immune cells in tissues including the brain, and the activation of these immune cells. Under chronic stress, some evidence suggests that these cytokines promote depression through similar behaviors that disrupt neurotransmitter synthesis and signal transduction.7 Meanwhile, studies have shown that inflammation is closely related to the pathogenesis of depression.8 The role of inflammation can be understood as affecting complex neurobiological, cognitive and emotional processes, rather than directly affecting the widespread depression syndrome.9
Nutrition, as part of the changeable lifestyle factors, can be considered an important way to control inflammation.10 The relationship between diet and inflammation has been fully proven, and the inflammation mediated by it can increase the risk of related chronic diseases.11 Dietary Inflammatory Index (DII) is a instrument to quantify personal dietary inflammation potential The goal is to evaluate the impact of diet-related inflammation on health outcomes.12,13 In short, DII is a weighted index of the degree of influence of diet on known serum inflammatory markers. Each food parameter has its own inflammation score.14 “+” stands for pro-inflammatory, and “-” stands for anti-inflammatory.
Bayesian Kernel Machine Regression is a original statistical approach that settles interactions and nonlinear relationships by flexibly modeling exposure.15,16 Here, we use this method to test the association of 11 nutrients with the risk of depressive symptoms in the elderly. Nutrients are divided into two categories, including 6 pro-inflammatory nutrients (Fe, zinc, selenium, vitamin B1, vitamin B2, polyunsaturated fatty acids) and 5 anti-inflammatory nutrients (fat, carbohydrate, cholesterol, saturated fat, vitamin B12). The objective is to inspect (1) the relationship between different nutrients and the risk of depressive symptoms among the elderly; (2) the overall impact of nutrients; (3) the interaction between different nutrients.
Methods
Study Participants
Participants in the current study were derived from the baseline of the Community Cohort Study of Nervous System Diseases (CCSNSD), an ongoing longitudinal study established by the project in 2018–2019, focusing on potential factors related to the risk of three neurological diseases, including epilepsy in patients >1-year-old and Alzheimer’s disease (AD), Parkinson’s disease (PD) in people ≥55 years old. The project is undertaken by the Institute of Nutrition and Health of the Chinese Center for Disease Control and Prevention, in cooperation with the Center for Disease Control and Prevention. The project applies a multistage random cluster sampling method to extract samples. The protocol of this study was reviewed and approved by the Institutional Review Board of the National Institute for Nutrition and Health (No. 2017020, November 6, 2017). In addition, the written informed consent of each participant was obtained before the investigation.
In allusion to subjects recruited in the CCSNSD cohort, the samples eligible for inclusion were (1) 55 years old and older, (2) resident population living in the sampled community, (3) absence of clinically diagnosed depressive symptoms, (4) be able to perform a normal depressive symptoms assessment, (5) completed data of sociodemographic characteristics, disease history, and food frequency questionnaire (FFQ). We excluded subjects because of (1) no depressive symptoms assessment results, (2) lack of baseline status such as education and physical activities, (3) nutrient deficiency, (4) abnormal energy intake. Finally, a total of 1865 participants were involved in the analysis (Figure 1).
 |
Figure 1 Selection process of subjects. |
Depressive Symptoms
We defined depressive symptoms according to the Geriatric Depression Scale (GDS),This scale is one of the most widely used scales assess the depressive symptoms of the elderly.17 It consists of 30 self-assessment items with yes/no response options. A score of 0–10 indicates no depressive symptoms, a score of 11–20 indicates mild depressive symptoms, and a score of 21–30 indicates severe depressive symptoms.18
Dietary Measurements
A previously validated 116 semi-quantitative FFQ (24) was used to assess diet. For each item, participants were asked to specify how often they consumed food or beverages on average in the previous year. The frequency of consumption response is divided into never, <1 time/month, 1–3 times/month, 1 time/week, 2–4 times/week, 5–6 times/week, 1 time/day, 2–3 times /day, 4–5 times/day, ≥6 times/day. Energy and nutrient intake is calculated by multiplying the frequency of consumption per unit of food by the energy and nutrient content of a specified serving. Evaluate the composition value of energy and nutrients by using the Chinese food content database.
Food Parameter Specific DII Score
In our study, DII was calculated using baseline FFQ data, which captured 22 of the 45 possible foods and nutrients in the original DII. The 22 parameters are carbohydrates, protein, total fat, β-carotene, fiber, cholesterol, saturated fat, monounsaturated fat,polyunsaturated fats, niacin, thiamine, riboflavin, vitamin B12, vitamin B6, Fe, magnesium, Zn, selenium, vitamin A, vitamin C, vitamin E and folic acid.
we use a representative global database to get the standardized intake and convert it to a percentile score; in order to obtain a symmetrical distribution centered on 0, we multiply the percentile score by 2 and subtract 1. Then, we multiply the percentile score by the inflammatory effect score, as defined by “Food parameter specific DII score”. A positive value of the DII score is considered a pro-inflammatory diet, while a negative value is considered an anti-inflammatory diet.19 For our analysis, among 22 food parameters, 16 are pro-inflammatory nutrients and 6 are anti-inflammatory nutrients.
Least Absolute Shrinkage and Selection Operator Regression
LASSO regression is an effective method for high-dimensional predictors, especially when the number of predictors far exceeds the number of observations.20 This method uses the L1 penalty to reduce the coefficient to zero. The penalty parameter λ, also known as the tuning constant, controls the intensity of the penalty. If we reduce λ and relax the penalty, then more predictors can enter the model. In this study, five cross-validation was used to determine the optimal value of λ. Finally, select λ through the 1 standard error (SE) criterion.21
Bayesian Kernel Machine Regression
Kernel machine regression is a popular tool in machine learning, which flexibly simulates the relationship between a large number of variables to a specific result, by mapping or projecting one data sequence to another in a one-to-one manner.22 BKMR can flexibly model exposure to resolve interaction and nonlinear relationships.15,16 The model used in this study is below:
Proinflammatory
Anti-Inflammatory
The function h() is modeled using the Gaussian kernel exposure-response machine function, which allows interaction terms to be included.The coefficient βT is the estimated value of the effect of the Cth covariate for the ith individual. The function s() represents the natural splines for date of visit, using kknots as described above. The residual is represented by εi. Intuitively, the Gaussian kernel assumes that two subjects with similar exposure characteristics will have more similar depressive symptoms risk characteristics, and this similarity is the use of the kernel function.The method handles this complexity by using a kernel exposure–response machine representation for h(). We used noninformative priors for all model parameters.21,23
Once fitted, summary statistics of the model for the estimation of the exposure-response function and the confidence interval (CI) for each nutrient, the overall link between the total nutrient level and the result, and the interaction between each nutrient pair are provided. We ran 11 Bayesian kernel machine regression models, which contained 6 pro-inflammatory nutrients (Fe, Zn, selenium, vitamin B1, vitamin B2, polyunsaturated fatty acids) and 5 anti-inflammatory nutrients (fat, carbohydrate, cholesterol, saturated fat, vitamin B12) as a result of depressive symptoms. All models adjusted for all covariates.
Covariates
The incorporated baseline characteristics include self-reported age (years), BMI (<18.5 kg/m2, 18.50–23.9 kg/m2, 24–28 kg/m2, and ≥28 kg/m2), gender (female or male), education level (illiterate, elementary school, junior high school and above), employment status (yes or no); lifestyle and health-related variables include smoking (yes or No), drinking (yes or no), physical activity (yes or no), daily energy intake (kcal), diabetes (yes or no), high blood pressure (yes or no). The missing values of all variables are less than 5%, so the median and mode of quantitative and qualitative variables are used for inference.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and the number of categorical variables (percentage) is expressed. Continuous variables, where appropriate, were compared by independent samples, unpaired, 2-sided t-test or the Mann–Whitney U-test. Categorical variables were compared by the X2 test or Fisher’s exact test. The glmnet package in R is used for Least Absolute Shrinkage Sum Selection Operator (LASSO) logistic regression, where important nutrients are used to assess the association with the risk of depressive symptoms.
In addition, considering the nonlinearity and interaction of multiple nutrient exposures on depressive symptoms, BKMR analysis was performed to evaluate the combined effects of multiple nutrients based on Gaussian process regression. BKMR uses Bayesian variable selection to facilitate exposure-response functions and improve the inference of highly correlated nutrients. First, analyze the cumulative effect on depressive symptoms by fixing all other nutrients to a specific percentile and increasing the 5th percentile. Then, while keeping other nutrients at the median, use the exposure-response function to explore the association between a single nutrient and depressive symptoms. Finally, the predicted response function of a single nutrient to other nutrients at different quantiles (and the rest of the nutrients are fixed at the median) is studied. By using the Markov Chain Monte Carlo algorithm, the model can run up to 10,000 iterations.
Analyses were performed on R software (version 3.6.0; R Core Team), and the two-sided statistical significance level was set at α = 0.05.
Results
The study included 1865 elderly participants older than 55 years old, of which 447 individuals (24.0%) were diagnosed with depressive symptoms. The demographic characteristics of the participants are shown in Table 1. Compared with non-depressed residents, depressed residents are younger [65(61–70) years old vs 68(63–72) years old], most of them are women, and more people live in rural areas. Table 2 shows the distribution of 22 dietary nutrients. There were significant differences in the levels of carbohydrates, protein, Total fat, Fiber, Saturated fat, Monounsaturated fat, Polyunsaturated fats, Niacin, Thiamine, Riboflavin, Fe, Magnesium, Zinc, Selenium, and Vitamin E between depressed residents and non-depressed residents(P<0.05), while no significant differences were found in other nutrients.
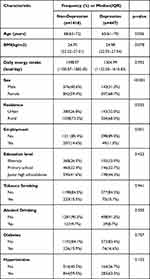 |
Table 1 Baseline Characteristics of the 1865 Subjects [Median(IQR) or Frequency(%)] |
 |
Table 2 Nutrient Content of Study Participants |
The LASSO method is suitable for regression of high-dimensional data because it can be used to extract the most important predictors from the original data set. In this study, the depressive symptoms risk score of each subject was calculated by linear combination of factors weighted by its coefficient. 22 nutrition variables are reduced to 11 potential predictors by the LASSO regression model. Then, a coefficient distribution map is generated. The cross-validation error graph of the LASSO regression model is shown in Figure 2A. The most regularized and reduced model, the cross-validation error is within 1 standard error of the minimum value, including eleven variables. The coefficient paths included in the model have different logarithmic conversion lambda values, as shown in Figure 2B. The model contains eleven independent predictors (Fe, Zn, selenium, vitamin B1, vitamin B2, polyunsaturated fatty acidsfat, carbohydrate, cholesterol, saturated fat, vitamin B12).
Calculate the dietary inflammatory effect scores of 11 nutrients after LASSO regression screening, and divide the nutrients into pro-inflammatory and anti-inflammatory groups according to the positive or negative of the mean value of each nutrient [pro-inflammatory nutrients: Fe, Zn, selenium, vitamin B1, vitamin B2, polyunsaturated fatty acids; anti-inflammatory nutrients: fat, carbohydrate, cholesterol, saturated fat, vitamin B12] (Table 3).
 |
Table 3 Food Parameter Specific DII Score |
We incorporated the inflammatory effect scores of 11 nutrients into the analytical BKMR model at the same time, and found that the overall inflammatory effect of 11 nutrients increased with the increase of total inflammatory scores, suggesting that the overall effect was pro-inflammatory (Supplementary Figure 1A). We also found that Fe, Zn, vitamin B1, vitamin B2 and polyunsaturated fatty acids had significant positive effects. Variations in five nutrients (Fe, Zn, Se, vitamin B1, and vitamin B2) from 25% to 75% significantly increased the risk of depressive symptoms, while three nutrients (cholesterol, SFA, and vitamin B12) were significantly negatively associated with depression risk (Supplementary Figure 1B). Observing the effects of different quantiles and the interaction between nutrients at the same time, the results showed no significant significance (Supplementary Figure 1C and D).
Although the overall effect of nutrients is shown to be pro-inflammatory, it may be meaningless to consider all pro-inflammatory and anti-inflammatory elements in the general population at the same time. Therefore, in this study, we further evaluated the pro-inflammatory nutrients and anti-inflammatory nutrients in groups to prove whether there is a certain offset between the two groups.
We show the visualization of the BKMR model. First, we found the cumulative inflammatory effect of mixed nutrients in the pro-inflammatory nutrient group. We can see that the risk of depressive symptoms increases with the inflammatory effect score. In particular, when all metabolites are at or above their 55th percentile, compared to when all metabolites are at their median value, the overall effect is statistically significant. We observed similar results in the anti-inflammatory nutrient group (Figures 3A and 4A). Then, we tried to understand the single effect of a nutrient by estimating a univariate summary of the change in depressive symptoms risk associated with a change in a single nutrient from 25% to 75%, where all other nutrients are fixed at a certain threshold (25th, 50th or 75th) Percentile). In the pro-inflammatory nutrient group, we found that Fe, Zn, vitamin B1, vitamin B2 and polyunsaturated fatty acids showed significant positive effects. The change in the content of five nutrients from 25% to 75% is associated with a significant increase in the risk of depressive symptoms. Similarly, we show significant positive effects on fats, carbohydrates, saturated fat, and vitamin B12 (Figures 3B and 4B). To study the potential nonlinearity of the exposure-response function, we then estimated the univariate relationship between each nutrient and depressive symptoms risk in the proinflammatory nutrient group, with all the remaining nutrients fixed at the 50th percentile. The graph shows that the levels of inflammatory effects of Fe, Zn, vitamin B1 and vitamin B2, and polyunsaturated fatty acids are increased, and the risk of depressive symptoms is significantly increased (Figures 3C and 4C). Similarly, in the anti-inflammatory nutrient group, increased levels of inflammatory effects of fats, carbohydrates, saturated fat and vitamin B12 were observed, and the risk of depressive symptoms was significantly decreased. There is no evidence that there is an interaction between the nutrients in the pro-inflammatory nutrient group and the anti-inflammatory nutrient group (Figures 3D and 4D).
The results of the overall effect of all nutrients associated with depression estimated that the diet in this study was characterized as a pro-inflammatory or anti-inflammatory diet. Comparing with the pro-inflammatory grouping and anti-inflammatory grouping, it is found that the overall effect result is significantly different from the pro-inflammatory grouping result effect map, and the single nutrient result and the pro-inflammatory grouping can reach the same conclusion. It shows that the diet in this study does have an offsetting effect between the pro-inflammatory diet and the anti-inflammatory diet. At the same time, the dietary inflammation scores of the overall group and the pro-inflammatory group are compared, and the results show that there is a significant difference between the two groups (1.20±1.40 VS 4.06±1.08, P<0.05).
Discussion
In this study, we found that the intake of 11 nutrients are significantly related to the occurrence of depressive symptoms in the elderly through the Lasso regression model. By calculating the inflammatory effect scores of 11 nutrients after each lasso regression screening, and divided the nutrients into pro-inflammatory and anti-inflammatory groups based on the scores[6 pro-inflammatory nutrients((Fe, Zn, selenium, vitamin B1, vitamin B2, polyunsaturated fatty acids) and 5 anti-inflammatory nutrients (fat, carbohydrate, cholesterol, saturated fat, vitamin B12)]. By incorporating the inflammatory effect scores of 11 nutrients into the analytical BKMR model at the same time, and found that the overall inflammatory effect of 11 nutrients increased with the increase of total inflammatory scores, suggesting that the overall effect was pro-inflammatory. BKMR analysis shows that Iron, zinc, vitamin B1, vitamin B2, and polyunsaturated fatty acids are significantly associated with depression in the pro-inflammatory group, while in the anti-inflammatory group, fats, carbohydrates, Saturated fat and vitamin B12 are significantly associated with depressive symptoms. There is no interaction both in the pro-inflammatory and anti-inflammatory group. When the nutrient concentration is higher than the 55th percentile, the mixture of nutrients in the two groups has a significant combined effect on depressive symptoms in the elderly. By comparing the overall and group effects, we found that the inflammatory effects of the pro-inflammatory diet and the anti-inflammatory diet in the study’s diet are offset by each other (P<0.005).
It has been widely recognized that peripheral inflammation can reach the central nervous system and cause neuroinflammation. This neuroinflammation can cause several issues related to the pathophysiology of depression, including: disorders of the hypothalamic-pituitary-adrenal axis; reduced neuroplasticity and neurogenesis;24,25 increased glutamate, which stimulates neurotoxicity; altered monoamine metabolism, reduce serotonin and increase quinine pathway; reduce BDNF; increase oxidative stress, etc., among which inflammatory oxidative stress plays a role that cannot be ignored.26,27
The mechanism by which a pro-inflammatory diet increases the risk of depressive symptoms may be through the activation of the innate immune system through pro-inflammatory nutrients, leading to low-grade inflammation and chronic diseases such as cardiovascular disease (CVD), diabetes, and mental health disorders. At the molecular and cellular level, more and more studies have shown that dietary factors affect neuronal function and synaptic plasticity markers, and these mechanisms are related to the etiology of depression.28–30 For example, in mice, a combination of exercise and an anti-inflammatory diet has been found to increase the expression of genes that have a positive effect on neuronal plasticity and decrease the expression of genes involved in harmful processes including inflammation.31
The two-way relationship between mental disorders (including depression) and inflammation has been established. Although neuropsychiatric diseases (such as depression) can promote inflammation; inflammation can promote neuropsychiatric disorders including depression. It has been repeatedly reported that patients with neuropsychiatric disorders exhibit all the classic features of inflammation.32,33 Pro-inflammatory cytokines regulate cognitive and emotional behaviors by reducing brain monoamine levels, inhibiting neuroendocrine responses, and promoting excitotoxicity. Neuroendocrine regulation, metabolism and changes in diet/microbiota are the main triggers leading to inflammation and susceptibility to depression.An unhealthy diet can also cause changes in the function of the gut and gut microbiota.34 The microbiota-gut-brain axis has been extensively studied. Much remains to be discovered, but it is well known that communication between these two organs can occur through a variety of mechanisms, including neuroanatomical communication (generated through the vagus nerve), intestinal hormones and neurotransmitters, neurotrophic factors, metabolites, bacteria, Neuroendocrine factors and inflammatory mediators. Although some mechanisms still need to be further elucidated, a number of clinical studies and reviews have shown that the gut microbiota of patients with depression has changed, and it is necessary to consider adjusting the microbiota-gut-brain axis to prevent and treat depression.35
B vitamins are water-soluble vitamins necessary to maintain the normal metabolic process of the human body. Human cells cannot synthesize by themselves and need to be ingested through external sources or absorbed and transformed by other substances in the body to obtain them.36,37 B vitamins participate in cell metabolism in different roles, including the production of monoamine oxidase and the synthesis of DNA, RNA, protein and phospholipids during cell repair. In addition, sufficient levels of B vitamins are required to maintain the balance of inflammation in the human body.As part of single-carbon metabolism, B6, folic acid (B9) and B12 metabolize homocysteine (Hcy), which is a non-protein, sulfur-containing amino acid.Hcy is a recognized marker of inflammation, and high levels of Hcy are independent risk factors for cardiovascular disease and neuropsychiatric problems.38,39 A study found that an increase of 5-μmol/l in plasma Hcy may increase the risk of schizophrenia by 70%. Considering the relationship between B6, folic acid and B12 and Hcy metabolism, it can be assumed that supplementation of these vitamins may reduce high levels of Hcy, thereby reducing inflammation and oxidative stress, and may improve symptoms of neurological diseases.40
As essential trace elements, Zn and Fe play a vital role in growth and development. Zn and Fe play an important biological role in regulating cell function and nerve function.There is now some evidence that major depression is accompanied by the activation of the inflammatory response system (IRS).41 According to reports, in patients with depression, the level of pro-inflammatory cytokines increases, the number of activated macrophages, T helper cells increases, the ratio of CD4+/CD8+ cells increases, and the level of activated CD4+ cells increases, with high expression of CD25+, in the acute phase Increased levels of positive proteins, such as C-reactive protein, reduce serum zinc (Zn) and serum albumin (Alb) concentrations. Studies have shown that the decrease of serum zinc level was significantly positively correlated with the decrease of negative proteins (albumin-zinc transporter, transferrin) in the acute phase.42,43 The level of neopterin increased, and the ratio of CD4+/CD8+ was negatively correlated with IL-6. The pro-oxidant-antioxidant imbalance in depressed patients may lead to oxidative damage to red blood cells, making red blood cells more fragile, thereby reducing the lifespan of red blood cells, and possibly leading to suicidal death (erythema) and red blood cell phagocytosis.44 Red blood cell maturation and lifespan disorders can be reflected by changes in red blood cell and Fe status. When senile-damaged red blood cells are cleared, Fe remains in the reticuloendothelial macrophages.45,46 In addition to playing a role in innate immunity, macrophages are also at the center of the Fe cycle of the system, and these functions are interrelated. Iron overload has been shown to induce uncontrolled activation of macrophages, which is considered a key event in the pathogenesis of chronic inflammatory diseases, and can further affect red blood cells and Fe metabolism.47,48
There is ample evidence supporting the role of inflammation in the pathophysiology of mental health disorders, including depression. Meta results showed a significant association between a pro-inflammatory diet and an increased risk of depression diagnosis or symptoms.30 Moreover, adults with depression have a higher inflammatory potential.49 Population-based research data shows that there is a correlation between the quality of habitual diet and systemic inflammation. The quality of diet affects immune function and systemic inflammation levels, which can lead to depression.50 Diet is the main factor that determines the composition of the gut microbiota,51 which can directly and indirectly affect health through inflammation and gut microbiota.52 Changes in the gut microbiome increase the release of microbial lipopolysaccharide (LPS), which activates the intestinal inflammatory response. Intestinal pro-inflammatory cytokines stimulate the afferent vagus nerve, which in turn affects the hypothalamus-pituitary-adrenal (HPA) axis and induces symptoms related to depression. Recent studies have shown that intestinal inflammation can induce neuroinflammation, which in turn stimulates the activation of microglia and the kynurenine pathway, and can activate systemic inflammation-induce depressive symptoms.53
Our research has the following advantages. First of all, our research is the first time that nutrients are divided into anti-inflammatory and pro-inflammatory according to dietary inflammation potential to evaluate the association with depression. Secondly, we use LASSO regression when selecting variables. LASSO regression aims to identify the variables that lead to the model and the corresponding regression coefficients, thereby minimizing the prediction errors of high-dimensional data, and weighing potential deviations when estimating individual parameters. Finally, the BKMR model was used to identify a single potential interaction and to assess the relationship between nutrients and depression risk as a whole.
However, the present study has some limitations. First of all, the nutrients we included did not include all the nutrients contained in the diet, which would bring potential bias to the evaluation of the results. Secondly, due to all of participants recruited into the cohort are from the same province, the true state of the nation’s elderly may not be accurately reflected. Finally, there are few studies on the relationship between the inflammation potential of the nutrients and the risk of depression, and further mechanism studies are needed to verify our results.
Ethics Approval and Consent to Participate
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving participants were approved by the institutional review board of the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention (approval number: 2017-020). Written informed consent was obtained from all participants.
Funding
Community Cohort Study on Specialized Nervous System Diseases (No.2017YFC0907701).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hsieh CR, Qin X. Depression hurts, depression costs: the medical spending attributable to depression and depressive symptoms in China. Health Econ. 2018;27:525–544. doi:10.1002/hec.3604
2. Gao X, Feng T. Public pension, labor force participation, and depressive symptoms across gender among older adults in rural China: a moderated mediation analysis. Int J Environ Res Public Health. 2020;17(9):3193. doi:10.3390/ijerph17093193
3. Empana JP, Boutouyrie P, Lemogne C, et al. Microvascular contribution to late-onset depression: mechanisms, current evidence, association with other brain diseases, and therapeutic perspectives. Biol Psychiatry. 2021;90(4):214–225. doi:10.1016/j.biopsych.2021.04.012
4. O’Neill RA, Maxwell AP, Kee F, et al. Association of reduced retinal arteriolar tortuosity with depression in older participants from the Northern Ireland cohort for the longitudinal study of ageing. BMC Geriatrics. 2021;21(1):62. doi:10.1186/s12877-021-02009-z
5. Wittenborn AK, Rahmandad H, Rick J, et al. Depression as a systemic syndrome: mapping the feedback loops of major depressive disorder. Psychol Med. 2016;46(3):551–562. doi:10.1017/S0033291715002044
6. Tao R, Fu Z, Xiao L. Chronic food antigen-specific IgG-mediated hypersensitivity reaction as a risk factor for adolescent depressive disorder. Genomics Proteomics Bioinformatics. 2019;17:183–189. doi:10.1016/j.gpb.2019.05.002
7. Chan Kenny L, Cathomas F, Russo Scott J. Central and peripheral inflammation link metabolic syndrome and major depressive disorder. Physiology. 2019;34:123–133. doi:10.1152/physiol.00047.2018
8. Slavich George M, Irwin Michael R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi:10.1037/a0035302
9. Dooley Larissa N, Kuhlman Kate R, Robles Theodore F, et al. The role of inflammation in core features of depression: insights from paradigms using exogenously-induced inflammation. Neurosci Biobehav Rev. 2018;94:219–237. doi:10.1016/j.neubiorev.2018.09.006
10. Vicente BM, Dos Santos Quaresma MVL, de Melo CM, Ribeiro SM. The dietary inflammatory index (DII®) and its association with cognition, frailty, and risk of disabilities in older adults: a systematic review. Clin Nutr ESPEN. 2020;40:7–16. doi:10.1016/j.clnesp.2020.10.003
11. Shivappa N, Bonaccio M, Hebert James R, et al. Association of proinflammatory diet with low-grade inflammation: results from the Moli-sani study. Nutrition. 2018;54:182–188. doi:10.1016/j.nut.2018.04.004
12. Abdollahpour I, Jakimovski D, Shivappa N, et al. Dietary inflammatory index and risk of multiple sclerosis: findings from a large population-based incident case-control study. Clin Nutr. 2020;39:3402–3407. doi:10.1016/j.clnu.2020.02.033
13. Suhett LG, Hermsdorff HH, Cota BC, et al. Dietary inflammatory potential, cardiometabolic risk and inflammation in children and adolescents: a systematic review. Crit Rev Food Sci Nutr. 2021;61:407–416. doi:10.1080/10408398.2020.1734911
14. Vidal AC, Oyekunle T, Howard LE, et al. Dietary inflammatory index (DII) and risk of prostate cancer in a case–control study among Black and White US Veteran men. Prostate Cancer Prostatic Dis. 2019;22(4):580–587. doi:10.1038/s41391-019-0143-4
15. Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493–508. doi:10.1093/biostatistics/kxu058
16. Valeri L, Mazumdar Maitreyi M, Bobb Jennifer F, et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect. 2017;125:067015. doi:10.1289/EHP614
17. Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. Int Psychogeriatr. 1996;8:103–112. doi:10.1017/s1041610296002505
18. Debruyne H, Van Buggenhout M, Le Bastard N, et al. Is the geriatric depression scale a reliable screening tool for depressive symptoms in elderly patients with cognitive impairment? Int J Geriatr Psychiatry. 2009;24:556–562. doi:10.1002/gps.2154
19. Shivappa N, Steck Susan E, Hurley Thomas G, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi:10.1017/S1368980013002115
20. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–5528. doi:10.1002/sim.3148
21. Chen D, Liu Z, Liu W, et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun. 2021;12:179. doi:10.1038/s41467-020-20429-0
22. Zhao N, Smargiassi A, Hudson M, et al. Investigating associations between anti-nuclear antibody positivity and combined long-term exposures to NO2, O3, and PM2.5 using a Bayesian kernel machine regression approach. Environ Int. 2020;136:105472. doi:10.1016/j.envint.2020.105472
23. Kupsco A, Kioumourtzoglou MA, Just AC, et al. Prenatal metal concentrations and childhood cardiometabolic risk using bayesian kernel machine regression to assess mixture and interaction effects. Epidemiology. 2019;30:263–273. doi:10.1097/EDE.0000000000000962
24. Pitsillou E, Bresnehan SM, Kagarakis EA, et al. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Mol Biol Rep. 2020;47:753–770. doi:10.1007/s11033-019-05129-3
25. Cernackova A, Durackova Z, Trebaticka J, et al. Neuroinflammation and depressive disorder: the role of the hypothalamus. J Clin Neurosci. 2020;75:5–10. doi:10.1016/j.jocn.2020.03.005
26. Adwitia D, Hankey Giblin Pamela A. Insights into macrophage heterogeneity and cytokine-induced neuroinflammation in major depressive disorder. Pharmaceuticals. 2018;11. doi:10.3390/ph11030064
27. Kim YK, Won E. The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder. Behav Brain Res. 2017;329:6–11. doi:10.1016/j.bbr.2017.04.020
28. Shivappa N, Hebert JR, Neshatbini tehrani A, et al. A pro-inflammatory diet is associated with an increased odds of depression symptoms among iranian female adolescents: a cross-sectional study. Front Psychiatry. 2018;9:400. doi:10.3389/fpsyt.2018.00400
29. Açik M, Çakiroğlu FP. Evaluating the relationship between inflammatory load of a diet and depression in young adults. Ecol Food Nutr. 2019;58(4):366–378. doi:10.1080/03670244.2019.1602043
30. Tolkien K, Bradburn S, Murgatroyd C. An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin Nutr. 2019;38:2045–2052. doi:10.1016/j.clnu.2018.11.007
31. Gaspar RC, Veiga CB, Bessi MP, et al. Unsaturated fatty acids from flaxseed oil and exercise modulate GPR120 but not GPR40 in the liver of obese mice: a new anti-inflammatory approach. J Nutr Biochem. 2019;66:52–62. doi:10.1016/j.jnutbio.2018.12.003
32. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. 2019;1437(1):57–67. doi:10.1111/nyas.13712
33. Appiah-Kusi E, Leyden E, Parmar S, Mondelli V, McGuire P, Bhattacharyya S. Abnormalities in neuroendocrine stress response in psychosis: the role of endocannabinoids. Psychol Med. 2016;46:27–45. doi:10.1017/s0033291715001786
34. Fair DA, Graham AM, Mills B. A role of early life stress on subsequent brain and behavioral development. Wash UJL Poly. 2018;57:89.
35. Huang Q, Liu H, Suzuki K, Ma S, Liu C. Linking what we eat to our mood: a review of diet, dietary antioxidants, and depression. Antioxidants. 2019;8:376. doi:10.3390/antiox8090376
36. Mikkelsen K, Stojanovska L, Apostolopoulos V. The effects of vitamin B in depression. Curr Med Chem. 2016;23(38):4317–4337. doi:10.2174/0929867323666160920110810
37. Mikkelsen K, Stojanovska L, Tangalakis K, et al. Cognitive decline: a vitamin B perspective. Maturitas. 2016;93:108–113. doi:10.1016/j.maturitas.2016.08.001
38. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14(1):6. doi:10.1186/1475-2891-14-6
39. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393–1412. doi:10.1586/ern.09.75
40. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661–670. doi:10.1007/s11011-014-9534-3
41. Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res. 2015;59(5):991–999. doi:10.1002/mnfr.201400761
42. Osuna-Padilla IA, Briceño O, Aguilar-Vargas AV, et al. Zinc and selenium indicators and their relation to immunologic and metabolic parameters in male patients with human immunodeficiency virus. Nutrition. 2020;70:110585. doi:10.1016/j.nut.2019.110585
43. Wong CP, Magnusson KR, Sharpton TJ, et al. Effects of zinc status on age-related T cell dysfunction and chronic inflammation. BioMetals. 2021;34(2):291–301. doi:10.1007/s10534-020-00279-5
44. Guo CH, Liu PJ, Hsia S, et al. Role of certain trace minerals in oxidative stress, inflammation, CD4/CD8 lymphocyte ratios and lung function in asthmatic patients. Ann Clin Biochem. 2011;48(4):344–351. doi:10.1258/acb.2011.010266
45. Adam W, Ewa S. Red blood cells parameters in patients with acute schizophrenia, unipolar depression and bipolar disorder. Psychiatr Danub. 2018;30:323–330. doi:10.24869/psyd.2018.323
46. Oliveira SR, Kallaur AP, Lopes J, et al. Insulin resistance, atherogenicity, and iron metabolism in multiple sclerosis with and without depression: associations with inflammatory and oxidative stress biomarkers and uric acid. Psychiatry Res. 2017;250:113–120. doi:10.1016/j.psychres.2016.12.039
47. Rybka J, Kędziora-Kornatowska KK, Banaś-Leżańska P, et al. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013;63:187–194. doi:10.1016/j.freeradbiomed.2013.05.019
48. Fairweather-Tait Susan J, Wawer AA, Gillings R, et al. Iron status in the elderly. Mech Ageing Dev. 2014;136–137:22–28. doi:10.1016/j.mad.2013.11.005
49. Chen W, Faris Mo’ez Al-Islam E, Bragazzi NL, et al. Diet-related inflammation is associated with major depressive disorder in bahraini adults: results of a case-control study using the dietary inflammatory index. J Inflamm Res. 2021;14:1437–1445. doi:10.2147/JIR.S306315
50. Michael B, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi:10.1186/1741-7015-11-200
51. Kostovcikova K, Coufal S, Galanova N, et al. Diet rich in animal protein promotes pro-inflammatory macrophage response and exacerbates colitis in mice. Front Immunol. 2019;10:919. doi:10.3389/fimmu.2019.00919
52. Zheng J, Hoffman KL, Chen JS, et al. Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr. 2020;124(9):931–942. doi:10.1017/S0007114520001853
53. Simkin DR. Microbiome and mental health, specifically as it relates to adolescents. Curr Psychiatry Rep. 2019;21(9):93. doi:10.1007/s11920-019-1075-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.