Back to Journals » Journal of Inflammation Research » Volume 17
Intestinal Mucosal Immune Barrier: A Powerful Firewall Against Severe Acute Pancreatitis-Associated Acute Lung Injury via the Gut-Lung Axis
Authors Li F, Wang Z, Cao Y, Pei B, Luo X, Liu J, Ge P, Luo Y , Ma S, Chen H
Received 8 November 2023
Accepted for publication 20 March 2024
Published 10 April 2024 Volume 2024:17 Pages 2173—2193
DOI https://doi.org/10.2147/JIR.S448819
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Adam D Bachstetter
Fan Li,1– 3,* Zhengjian Wang,4,* Yinan Cao,1– 3 Boliang Pei,1– 3 Xinyu Luo,1– 3 Jin Liu,1– 3 Peng Ge,1– 3 Yalan Luo,1– 3 Shurong Ma,1,3 Hailong Chen1– 3
1Department of General Surgery, the First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, 116011, People’s Republic of China; 2Institute (College) of Integrative Medicine, Dalian Medical University, Dalian, Liaoning, 116011, People’s Republic of China; 3Laboratory of Integrative Medicine, the First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, 116011, People’s Republic of China; 4Department of Hepatobiliary Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shurong Ma; Hailong Chen, Department of General Surgery, the First Affiliated Hospital of Dalian Medical University, Zhongshan Road 222, Dalian, Liaoning, 116011, People’s Republic of China, Tel +86-411-83635963, Fax +86-411-83622844, Email [email protected]; [email protected]
Abstract: The pathogenesis of severe acute pancreatitis-associated acute lung injury (SAP-ALI), which is the leading cause of mortality among hospitalized patients in the intensive care unit, remains incompletely elucidated. The intestinal mucosal immune barrier is a crucial component of the intestinal epithelial barrier, and its aberrant activation contributes to the induction of sustained pro-inflammatory immune responses, paradoxical intercellular communication, and bacterial translocation. In this review, we firstly provide a comprehensive overview of the composition of the intestinal mucosal immune barrier and its pivotal roles in the pathogenesis of SAP-ALI. Secondly, the mechanisms of its crosstalk with gut microbiota, which is called gut-lung axis, and its effect on SAP-ALI were summarized. Finally, a number of drugs that could enhance the intestinal mucosal immune barrier and exhibit potential anti-SAP-ALI activities were presented, including probiotics, glutamine, enteral nutrition, and traditional Chinese medicine (TCM). The aim is to offer a theoretical framework based on the perspective of the intestinal mucosal immune barrier to protect against SAP-ALI.
Keywords: severe acute pancreatitis-associated acute lung injury, intestinal mucosal immune barrier, gut microbiota, gut-lung axis, migration of immune cells
Introduction
Severe acute pancreatitis (SAP) is one of the most common abdominal critical emergencies in clinical practice. According to the 2012 Revision of the Atlanta Classification Standard (RAC) by the International AP Consensus and the 2014 Guidelines for diagnosis and treatment of the acute pancreatitis guide by the Chinese medical association branch, SAP is defined as acute inflammation of pancreas, and its main manifestations include pancreatic edema, necrosis, hemorrhage, infection, peripancreatic fluid collection, wall necrosis (WON), and extrapancreatic organ dysfunction or failure.1,2 The main cause is the destruction of pancreatic acinar cells and bile duct cells, leading to the activation of trypsin and lipase in pancreatic tissue.3,4 And it is often accompanied by organ failure lasting for more than 48 hours. The pathological process is characterized by an aggressive nature and the potential involvement of one or multiple organs, resulting in a mortality rate of up to 35%5,6. The incidence of SAP has increased in recent years, with an estimated number of 110 to 140 cases per 100,000 population.4,7–9 Acute lung jury is one of the most serious and earliest complications secondary to SAP, which usually manifests as a decrease in alveolar surface-active substances, an elevation in oxygen-free radicals and permeability of alveolar epithelial cells, accumulation of inflammatory proteins, and injury to the endothelial cells of the pulmonary microvasculature. This accounts for 70% of the mortality rate among patients with SAP.10–12 The occurrence of SAP-ALI is typically accompanied by the release of inflammatory mediators in a cascading manner, disruption of the intestinal barrier function, imbalance of immune systems, as well as alterations in intestinal bacteria and their pathogen-associated molecular patterns (PAMPs).13–16 SAP-ALI as a typical complication of SAP, is the main cause of death in SAP patients, and its mortality rate is more than 30%.17–23 The current focus of SAP-ALI research is to find ways to reduce the inflammatory response and the expression of inflammatory factors.24,25 Endotoxemia, systemic inflammatory response syndrome and multiple-organ failure are common complications in the development of SAP.26 In SAP, the levels of proinflammatory cytokines TNF-α, IL-6, and IL-1β are elevated, and neutrophils and pancreatic enzymes are key indicators of inflammatory response.27 Pro-inflammatory cytokines enter the intestine through microcirculation, damage the mucosa and destroy the intestinal barrier, which is an important factor affecting the course of SAP-ALI.28,29 However, the exact mechanisms of action and regulatory targets remain to be fully elucidated.30–32
The intestinal mucosal immune barrier is the primary site for the initiation and activation of the immune response, exerting influence on the integrity of the intestinal barrier and attenuation of the systemic inflammatory response. It also plays a crucial role in maintaining intestinal immune homeostasis, and regulating the long-range migration of immune cells along the gut-lung axis.33,34 In addition, crosstalks between the gut microbiota or its derived small molecules and intestinal immune cells are important in the maintenance of a favorable immune microenvironment and facilitating bi-directional communications among intestinal cells, ultimately influencing the integrity of the intestinal barrier.35–37 Current studies have shown that the intestinal mucosal immune barrier actively contributes to the protection against SAP-ALI, and any disruption to this barrier can expedite disease progression and impact the prognosis of patients.38,39
Currently, there is a lack of reviews regarding the role of the intestinal mucosal immune barrier in the pathogenesis of SAP-ALI. In this paper, we provide a detailed account of the composition and potential mechanisms of the intestinal mucosal immune barrier in SAP-ALI. Additionally, we investigate the relationships between the intestinal mucosal immune barrier and gut microbiota via the gut-lung axis to enhance our understanding of bacterial translocation and immune cell migration in SAP. Finally, we summarize several drugs that could strengthen the intestinal mucosal immune barrier, which is prospective to provide new sights to be utilized in SAP-ALI patients in the future.
Composition of the Intestinal Mucosal Immune Barrier
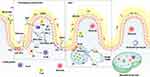
The intestinal mucosal immune barrier is mainly composed of intestinal epithelial cells (IECs), gut-associated lymphoid tissue (GALT), immune cells (macrophages, antigen-presenting cells, monocytes, neutrophils, etc.), the secretion of plasma cells produce secretory IgA (SIgA), and immune active substances (antibodies, cytokines, lysozyme, defensive element).40,41 The intestinal mucosal immune barrier plays an important role in defending against bacterial, viral or toxin invasions, as well as regulation in anti-allergic reactions, and suppression of sustained pro-inflammatory immune responses.42 The intestinal mucosal immune barrier, an important component of the host immune system, plays a crucial role in resisting pathogen translocation, stabilizing the immune microenvironment, attenuating systemic inflammatory responses and regulating the bidirectional communications between the immune cells.
Intestinal Epithelial Cells (IECs)
IECs are the initial cells that contact with the gut microbiome and actively contribute to the immune response of the gastrointestinal tract. It is mainly composed of the intestinal stem cells (ISCs) located in Lieberkühn crypts (marked by Lgr5, leucine-rich repeat-containing G protein-coupled receptor 5) and its differentiated cell lineage including intestinal absorptive epithelial cells (IAECs), goblet cells (GCs), microfold cells (M cells), Paneth cells (PCs) and enteroendocrine cells (EEs).43–45 The intestinal form of the natural physical barrier is composed of the IECs cortex, which separates immune cells in the lamina propria from the lumen and facilitates two-way communication between them and the symbiotic anaerobic bacteria for immune tolerance.46 IECs stimulated by microbiota and IFN-γ promote the differentiation of CD4+ CD8αα+ IELs by expressing MHC class II (MHC II) and programmed death ligand-1 (PD-L1).47 In addition, IECs receive signals via herpes virus entry mediator (HVEM) on their surface and stimulate the synthesis of basement membrane proteins, and they interact with adhesion molecules expressed on the surface of T cells, called integrins, to perform immune patrolling and surveillance functions.48 Endoplasmic reticulum stress in IECs promotes the expression of Duoxa2/Duox2, leading to the increase in reactive oxygen species (ROS). Enhanced ROS signals could activate purine metabolic pathways, which in turn lead to an increased release of xanthine from IECs, ultimately inducing the differentiation of Th17 cells, and it may be an important source of the inflammatory storm triggered during SAP.49,50
Leucine-Rich Repeat-Containing G Protein-Coupled Receptor 5 Positive Intestinal Stem Cells (Lgr5+ ISCs)
IECs process a rapid capacity for renewal and repairment (3–5 days renewal).51 As the initial source of various types of intestinal cells, ISCs provide the neoplastic force to keep the intestines always fresh and alive.52 Lgr5 acts as an important molecule for tracking ISCs in vivo as an ISC-specific marker.53 Human Lgr5 ISCs are predominantly p27-positive cells that occupy ecological niches at the base of crypts, thereby preventing the dedifferentiation of differentiated cells.54 ISCs are located at the basal part of the intestinal raised villous crypts and are arranged in interspersed rows with PCs. Transit amplifying cells (TACs), generated by ISCs, gradually migrate from the bottom of the crypts to the top of the crypts and differentiate into a variety of intestinal cells.55 TACs firstly give rise to the secretory and absorptive progenitors, which subsequently differentiate into mature GCs, EEs, and PCs, and absorptive progenitors differentiate into IAECs.56,57 The differentiated mature cells eventually migrate to the tip of the villi and undergo gradual apoptosis, subsequently being replaced by newly migrated cells from below.58 ISCs are capable of plasticity, and in the event of acute injury resulting in the loss of Lgr5+ ISCs, the intestine triggers a regenerative response to restore self-renewal capacity to compensate for the lost number in cell numbers.59 The behavior of ISCs is influenced by the basolateral microenvironment, which consists of gut bacteria, signaling pathway-associated ligands, soluble cytokines, chemokines and growth factors.59–62 Lymphatic endothelial cells (LECs) and fibroblasts (RSPO3+ GREM1+ fibroblasts, RGFs) in the ISCs microenvironment are the main cellular sources of the key ISC microenvironmental factor RSPO3, which has a major impact on the behavior of ISCs under intestinal homeostasis and the regeneration of intestinal epithelial performs a supportive role for ISCs.63 Current studies have shown that the reduction of Lgr5+ ISCs in response to intestinal injury is accompanied by the increased secretion of stem cell factors and the enhanced ISCs functions, which is associated with the re-entry of PCs into the cell cycle, the loss of the secretory phenotype, and the acquisition of stem cell properties, which contribute to the regeneration of tissues and the response to the inflammation.64,65 The interaction between ISCs and the immune cells represents a promising therapeutic target in the context of SAP-ALI.66
Goblet Cells (GCs)
GCs, a special type of epithelial cell dispersed among IAECs, perform innate immune functions by the secretion of mucus and the production of mucin, chemokines and cytokines. The numbers gradually increase from the duodenum to the end of the ileum.67–69 The formation of goblet cell-associated antigen passages (GAPs) enables GCs to prevent bacterial invasion into the intestinal mucosa and facilitate the transmission of post-feeding protein and antigen signals to the dendritic cells, thereby promoting the development of tolerance.70–72 Alterations in the microenvironment of the mucus system that are caused by dysfunctional GCs or the lack in specific types of GCs can accelerate the process of the inflammatory responses and increase the fixed resistance against pathogenic microorganisms.73,74 In addition, mucus secretion by GCs and mucus layer thickness could be regulated by autophagy and endoplasmic reticulum stress could be attenuated in a bacterial- and Nod2-dependent manner.75,76 As an important factor of intestinal mucus barrier damage in SAP-ALI, its causal relationships with the gut microbiota, the feedback mechanisms in immune responses, and the targets for GCs’ differentiation in SAP are still the current focus of basic research.77–79
Paneth Cells (PCs)
PCs are a type of specialized secretory cells that differentiate at the base of the crypt, and their maintenance requires the secretory regulatory protein Atoh1, a downstream target of Wnt/b-catenin signaling for innate immune functions.80 Upon signals from bacteria in the intestinal mucus layer alter, PCs secret antimicrobial substances such as antimicrobial peptides (AMPs), defensins, and lysozymes into the mucus layer to protect the intestinal barrier.81,82 PCs have a unique ecological niche, which facilitates their interactions with ISCs and protects ISCs from changes in the intestinal microenvironment.83 In addition, the PI3K/AKT/mTOR signaling axis mediated by IL-22 (activated upon SAP) is involved in the differentiation of PCs and influences the expression of intracellular host defense genes (REG1A, REG1B, DMBT1).84–86 Despite the similar number and distribution of LGR5+ ISCs in the small and large intestinal crypts, the number of effective stem cells (capable of forming long-lasting cell clones) in the small intestinal crypts was two times higher than that in the large intestine due to the motility of retrograde cells that is dependent on Wnt signaling.87 In addition, loss of the functions and mutation of PTPN2, a key regulator to maintain the vitality and functions of PCs, could accelerate the expansion of inflammatory response.65,88 In summary, the intestinal tract is not as active as it should be in SAP. The weakness of the intestinal mucus layer, the reduced secretion of AMPs, and the increased abundance of pathogenic bacteria in SAP are closely related to the impaired function of PCs.40,89
Microfold Cells (M Cells)
M cells are key cells in intestinal immunity, characterized by short, few, irregular villi and special micro folds, and are known as the zone where antigens can cross in the host intestinal mucosa.90 Although M cells are not involved in the digestion and absorption, they could selectively capture or transfer antigens to the lamina propria of the mucosa, allowing antigens to be acquired by DCs and then recognized by immune cells to elicit specific immune responses.91,92 As the sentinel cells, the abundance of M cells can be modulated by gastrointestinal injury-sensing neurons and exert resistant effects against pathogenic bacteria through releasing calcitonin gene-related peptide (CGRP).93 The transcription factor ONECUT2 is activated in response to pancreatic alveolar cell damage and acts as a downstream of RANK/RANKL, which supports the differentiation of IEC and inhibits M-cell lineage specialization, thereby indirectly regulating functions of B cells and production of SIgA.94,95 NADPH oxidase-1 (NOX1), which is involved in the production of ROS, is significantly up-regulated during SAP, and the differentiation and plastination of M-cells are inhibited, which in turn affects the course of the adaptive immune response.96,97 In addition, present on M cells is capable of recognizing and internalizing β-glucan-rich probiotics, thereby eliciting an immune response in the intestinal mucosa, emerging as a feasible strategy for the modulation of immunity by regulation in gut microbiota.98
Enteroendocrine Cells (EECs)
EECs are distributed throughout the intestinal epithelium, accounting for merely 1% of total intestinal cells. They possess a high sensitivity to chemical cues such as luminal food stimuli and pH fluctuations, thereby exerting regulatory effects on intestinal functions, systemic metabolism, and food intake through hormone secretion.99,100 As the largest endocrine system in the human body, the gut microbes and their metabolites (SCFAs, secondary bile acids, LPS, etc.) are effective stimulators of EECs, which could produce hormonal signals via G-protein coupled receptors, nutrient transporter proteins and ion channels.101 It has been shown that bacteria in gut could influence the protein modification of O-GlcNAc by increasing the synthesis of SCFAs and regulating the differentiation of intestinal endocrine L-cells to enhance the energy metabolism of IECs, which in turn indirectly enhances the intestinal barrier.102 The necrotic apoptosis of IECs may be closely related to the function of EEs in SAP.103 Apart from this, tryptophan derivatives produced by intestinal bacteria could activate Trpa1 channels on EEs, leading to a rapid activation of the intestinal vagus nerve, resulting in an increase in intestinal peristalsis and a faster rate of substance absorption in SAP.104,105 Enterochromaffin cells, as a type of rare excitatory EECs, are capable of recognizing stimulations and acting on the intestinal innervating afferent nerves to release 5-hydroxytryptamine, thereby transmitting nociceptive signals to the spinal cord, which is involved in the immunomodulation effects in the intestine.106 Although EEs indirectly influence intestinal mucosal immunity, they are essential for the energetic input of IECs and their communication with immune cells.
Gut-Associated Lymphoid Tissue (GALT)
GALT mainly consists of Peyer’s patch (PP), intestinal intraepithelial lymphocytes (IELs), lamina propria lymphocytes (LPLs), lymphoid follicles and mesenteric lymph nodes (MLNs).107 The distribution of immune cells and GALT shows a regional pattern along the intestinal mucosal layers and intestinal canal walls.108
Peyer’s Patch (PP)
PP consists of hundreds of lymphoid follicles surrounded by follicle-associated epithelium containing M cells and is predominantly found in the distal ileum.109 The PP is an important lymphoid organ for bacterial colonization and activation of T cell-dependent IgA signals.110,111 Bacteria clustered on the surface of the PP are predominantly Serratia and Lactobacillus spp, while, those clustered inside the PP are mainly Alkalobacter spp and Pallobacterium spp.112 An important function of PP is the uptake and monitoring of intestinal antigens (including bacteria), and activation of immune cells within PP may be involved in the limitation of bacterial translocation.113 Entry of bacteria into the PP triggers sustained pro-inflammatory immunity, and disruption of the intestinal flora within the PP could cause a severe inflammatory response, resulting in damage to intestinal mucosa on the surface of PP and induction of excessive bacterial invasion.114 Fibroblasts in PP are considered the ecological niche-supporting cells in epithelial crypts, the coordinators of lymphoid tissue and mediators of stromal–immune interactions.115 Innate lymphoid cells (ILCs) in PP could induce the expression of the antimicrobial proteins (RegIIIβ and RegIIIγ in IECs) and maintain homeostasis in gut microbiota by regulating IL-22 and IFN-γ signals.116 PP-derived mesenchymal stromal cells (MSCs) perform enhanced immunomodulatory capacities and inhibit the abnormal proliferation of T-cells as well as the production of inflammatory factors.117 Robo4 is also a regulator of the vascular endothelial barrier during pancreatic inflammation, which could induce the renewal and regeneration of B-cells in PPs,118,119 while, subsequent transcriptomics data are needed in terms of SAP-ALI.
Intraepithelial Lymphocytes (IELs)
IELs, a phenotypically heterogeneous population of T cells within the intestinal epithelium, dominate the defense of the mucosal immune system and are considered the frontline of immune surveillance by fending off pathogens and maintaining the epithelial integrity.120,121 The IELs can be classified into two primary cell subpopulations, the TCRγδ+ T cells and TCRαβ+ T cells.122 IELs are surrounded by IECs with a ratio of approximately 10:1 (IECs: IELs) in the ileum.123 TCR+ IELs express either TCRγδ+ or TCRαβ+, whereas the former is closely associated with the crosstalk with IECs and leads to more effective immune surveillance and higher expression of antimicrobial-associated genes.123,124 IELs are present at the outermost layer of the intestinal epithelial barrier and sense alterations in the intestinal microenvironment (eg, commensal anaerobes, PAMPs, toxins, or other small-molecules).125 Activation of glucagon-like peptide-1 (GLP-1) in intestinal IELs is closely related to the composition of the gut microbiota and the inflammatory state on the intestinal epithelial surface.126 The aryl-hydrocarbon receptor (AhR) is required for the maintenance of IELs and inhibition of AhR prevents lipid peroxidative stress and iron death in IELs.127 It is reported that activation of AhR can reduce the AP-induced inflammation by inducing the expression of IL-22, suggesting that activation of IELs by AhR mediated may be a new direction to protect against SAP-ALI.128
Lamina Proprial Lymphocytes (LPLs)
LPLs, diffusely distributed in the lamina propria of the mucosa, include CD4+ T cells, SIgA+ B cells, NK cells, macrophages, mast cells, dendritic cells, and innate lymphoid cells (ILCs).129,130 T cells could secrete inflammatory cytokines such as IL-10 and TGF-β, and modulate the secretion of SIgA by B cells.131 The immune cells with a large amount in the lamina propria exert innate and adaptive immune functions through the transformation of information.132 The γδ T cells in LPLs are capable of secreting IL-17 and perform similar functions with Th17 effector cells and ILCs.133
The activation of ILCs, which include ILC1, ILC2, and ILC3 as well as NK cells, is independent of antigen-specific recognition and exhibits plasticity as they lack the expression of specific/pan-specific antigen receptors.134,135 ILCs are important in the anti-infected activities in mucosa and the maintenance of homeostasis in tissues.136 The presence of ILC1 cells has been observed in both the small intestine and colon, where they actively secrete cytokines such as IFN-γ and TNF-α. These cells play a crucial role in maintaining the integrity of the intestinal mucosa by exerting potent anti-infective effects in response to microbial dysbiosis.137 The activity of ILC2 is mediated by IL-4, IL-5, IL-9 and IL-13. This response is predominantly driven by IL-33, thereby promoting the hyperplasia of GCs, production of mucus, infiltration of eosinophils, and the fibrotic processes within tissues.136 ILC3 maintains the intestinal barrier integrity as a patrol in the intestinal tract, and induces the expression of IL-22. In addition, altered migration patterns were observed in ILC3.138 Despite the reduced number of NK cells in the early stage, the hyperreactive state of them is considered an important part of SAP, representing a systemic response of inflammation.139 In short, ILCs exert complex and diverse roles in the polarization, homeostasis and apoptosis of macrophages, the network of which is a dominant factor in immune homeostasis.140,141
Secretory IgA (SIgA)
SIgA serves as a primary effector of the immune system, being synthesized by plasma cells in the lamina propria and subsequently released onto the mucosal surface. SIgA is considered the first line of defense against the adhesion and colonization of pathogenic bacteria and the pioneer of mucosal immune protection.142,143 SIgA has multiple functions, including forming antigen-antibody complexes to encapsulate bacteria, stimulating the secretion of intestinal mucus, preventing bacterial adherence, and neutralizing toxins.144–146 SIgA in gastrointestinal mucus directly affects the resistance to pathogens and the local immunity against infections, and a significant reduction in SIgA has been found in SAP.147,148 While, B-1 cell-specific protein (MZB1) is essential for maintaining the secretion of SIgA during acute enteritis.149 The retrograde transport of SIgA-pathogen complexes into the GALT appears to be particularly crucial for initiating an adaptive immune response, which may necessitate the involvement of NOD2 as well as the Dectin-1 receptor.150,151 The remote modulation mechanisms and effectors of SIgA-bacterial complex after a damage in the intestinal mucosal immune barrier under SAP merits further investigations.
Other Immune-Related Active Substances
Other active substances secreted in the intestinal epithelium include defensins, Mucin2 (MUC2), and complements, which are involved in the immune responses during SAP-ALI. It has been reported that they are engaged in the clearance of pathogens or inflammatory mediators, and the activation of humoral and cellular immunity, etc.152 Defensins are mainly divided into two types, namely α-defensins and β-defensins.153 α-defensins exhibit antimicrobial activities even after being degraded by intestinal proteases into fragments. β-defensins perform a strong structural plasticity, which can prevent the direct contact of the pathogenic microorganisms with IECs.154 The expression of defensin is associated with the composition of SCFAs levels, which may involve mTOR and STAT3 pathways.155 MUC2 provides the nutrients and shapes the composition and functions of the intestinal microbes and could maintain the intestinal immune homeostasis by actively regulating the production of AMPs, altering the activity of antigen-presenting cells, enhancing the clearance of bacteria as well as the immune cell communications.156 The complement system comprises a collection of biologically active proteins that are found on the surface of the intestinal epithelium, capable of modulating immune responses and serves as a crucial component in maintaining the intestinal mucosa.157,158 It is expected that MUC2 and complements to be effective therapeutic targets for SAP-ALI (for details see Figure 1).
The Role of Intestinal Mucosal Immune Barrier in SAP
The integrity of the intestinal mucosal barrier and intestinal immune function are closely related to the development of SAP.159 Studies have shown that the intestinal mucosal immune barrier helps to prevent inflammation and pathogen invasion. As the integrity of the intestinal mucosal immune barrier is damaged, the release of endotoxin and D-lactic acid could aggravate the severity of SAP patients.160,161 And the complex process of inflammation and infection caused by intestinal mucosal barrier dysfunction in early SAP patients involves immunosuppression, the increase of regulatory T cells, and the release of related inflammatory cytokines.162 In ALI, the activity and function of innate immune cells (such as monocytes, macrophages and DC) mediated by T cell and B cell differentiation are regulated by SCFAs. Maintaining the balance of the immune system and alleviating the damage of the intestinal barrier and BT are important aspects of alleviating SAP-ALI.163,164 B10, a component of regulatory B cells, improves the severity of SAP.165 In addition, bacterial translocation caused by intestinal flora disorder accelerates the development process of SAP.166 The secretion of SIgA and lymphocytes in the gut during SAP can reduce the concentration of intestinal endotoxin and bacteria, thereby preventing bacterial translocation.167 Studies have shown that oral administration of L-arginine can improve the intestinal mucosal immune functions by increasing the number of CD3+ and CD4+T lymphocytes and the concentration of SIgA in the lamina propria of the intestinal mucosa of SAP rats.167 Notably, macrophages, as the major population of immune cells that migrate into the pancreas, appear to be particularly important in their function in controlling the immune responses.168 Important roles of intestinal mucosal immunity include the recognition of harmful and infectious antigens, effective responses to destructive stimuli, the down-regulatory effects of immune responses to innocuous antigens, and the development of tolerance on which intestinal homeostasis depends. In summary, a deeper understanding of the intestinal mucosal immune barrier may provide new ideas for the clinical treatment of SAP.
Gut Microbiota and Intestinal Mucosal Immune Barrier
Relationships Between Gut Microbiota and Intestinal Mucosal Immunity
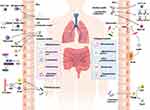
It is now generally accepted that gut microbiota co-evolves with the intestinal mucosal immune system and the former is thought to be a driving factor, which could train the immune system to defend against the pathogenic bacteria.169 The commensal bacteria in gut and their derived metabolites could modulate the intestinal immune microenvironment and the development or maturation of a variety of immune cells, resulting in the enhancement of intestinal mucosal immunity.170,171 The disruption of the microbial community in the early stages of life has been found to alter the development of PP within the GALT and the formation of germinal centers, while fewer IELs, Tregs and SIgA were presented in germ-free mice.172,173 The stimulation of LPLs by Lactobacillus can induce the secretion of IL-22 and activate the STAT3 signaling pathway, thereby promoting the regeneration of ISCs and facilitating the renewal and repairment of IECs.174 Additionally, the gut microbiota exerts an influence on the levels of conjugated linoleic acid within the mouse intestine, thereby augmenting the population of intraepithelial lymphocytes through a mechanism associated with the activation of transcription factors HNF4γ and ThPOK.125 SCFAs, the bioactive molecules by microbes, have been reported to assist in the maintenance of the intestinal barrier and modulation of innate immune cells, and these findings demonstrate their potential in attenuating the systemic inflammatory responses in SAP-ALI.103 About ninety percent of SIgA in gut is produced by microbes, the diversity of which can be sensed by M cells as well as the microbe-associated molecular patterns (MAMPs).175,176 Studies have shown the gut microbiota may influence the mucus by regulating the expression of glycosyltransferases177,178 and the increased permeability in intestinal barrier may be strongly associated with the increased level of serum lipopolysaccharide.179–181 Currently, the investigations in how the gut microbiota enhances bidirectional communications with the intestinal mucosal immune barrier in SAP-ALI remains a prominent area, and exosomes derived from gut bacteria are anticipated to emerge as a novel focal point.
Gut Microbiota and B-Lymphocytes
B-lymphocytes are secondary antigen-presenting cells, which could enhance the immune effect by the release of cytokines, and the stimulation of immunoglobulins. While, B cells have been proven to be activated by the secretion of intestinal epithelial cells by stimulation of bacteria. It is reported that IgA secreted by B-lymphocytes plays a key role in the intestinal mucosal immune system, and Segmented Filamentous Bacteria (SFB) harbored in the flora could effectively stimulate the secretion and synthesis of IgA, thus facilitating the generation of immune responses.
Gut Microbiota and T-Lymphocytes
The differentiation of T cells can be regulated by gut microbiota via the stimulation of dendritic cells and macrophages, and the production of helper T lymphocytes. Regulatory T cells are important components of the immune system in the intestinal mucosa, which could promote the secretion of antimicrobial peptides and clear pathogens. Gut microbes perform a promoting effect on the differentiation and proliferation of regulatory T cells and their metabolites are capable of influencing the autoimmune regulatory responses.182 For example, Clostridium perfringens and Bacteroides could stimulate the proliferation and development of Treg. While, Bifidobacterium can promote the generation of Th17 in gut. Short-chain fatty acids, a type of metabolite derived from gut microbiota, act as the inhibitor of histone deacetylase and could induce the production of regulatory T cells by means of the G-protein-coupled receptor.182 Another group of metabolites, which are tryptophan metabolites, have been proven to perform the activities in inhibiting inflammatory responses via aryl hydrocarbon receptors in T cells or astrocytes.
Crosstalk Between Gut Microbiota and Intestinal Immunity
Gut microbiota maintains the integrity of the gut by regulating the permeability of the barrier and their normal functions, among which the Firmicutes and the Bacteroidetes are capable of absorbing the nutrients and promoting their motility while maintaining the structural integrity of the gut. Antimicrobial peptides secreted by pancreatic alveolar cells and Paneth cells are effective in maintaining intestinal integrity, as well as ensuring the intestinal barrier functions and maintaining intestinal immune integrity. While, the intestinal mucosal immune barrier is the bridge for bacterial translocation during SAP. In SAP, the pro-inflammatory and anti-inflammatory factors exhibit opposite effects with each other, such as increased expression of cytokines lost balances and the expression of several factors such as TNF- α, IL-1β, IL-6, IL-17A, CXCL1 and IL-18 increased.183,184 A summary of the interactions between gut microbiota and intestinal mucosal immunity is shown in Figure 2.
Changes in Gut Microbiota During Sap
As an important part of the intestinal biological barrier, gut microbiota is involved in the development of SAP-ALI. This is also evidenced by the fact that the relatively sterile state of the ileum in the intestine significantly reduces the degree of pancreatic damage during AP.185 Studies based on 16SrRNA gene sequencing have found that the abundance and diversity of intestinal flora are significantly varied in SAP. Specifically, the abundance of pathogenic bacteria such as Escherichia, Shigella, Enterobacteriaceae, Enterococcus and Staphylococcus is significantly increased, and the abundance and diversity of intestinal beneficial bacteria Bifidobacterium and Akkermansia are significantly reduced compared with the control group.50,186–188 At the phylum level, the increase in the relative abundance of Bacteroidetes and Proteobacteria and the decrease in the relative abundance of Firmicutes and Actinobacteria are important characteristics of gut microbiota in SAP.189 At the genus level, abundance of Phascolarctobacterium significantly increased, and Candidatus_Saccharimonas, Prevotellaceae, Lachnospiraceae, and Ruminiclostridium were significantly reduced.89 The data above suggested the potential role of gut microbiota in mediating SAP, as well as a prospective target for the treatment.
The Relationships Between Gut Microbiota and Sap
Several publications have reported the important regulatory role of gut microbiota in the occurrence and development of acute pancreatitis via the “gut-pancreas axis”.190 The damage in the intestinal barrier and the increase in intestinal permeability caused by inflammation are considered important factors that affect the pathology of SAP.191 Bacterial translocation may aggravate SAP-ALI by the increase of oxidative stress levels and intestinal flora carrying pathogen-associated molecular patterns (PAMPs) can transfer from the lamina propria to the lungs once the intestinal barrier is damaged. This process also enhances the secretion of inflammatory molecules including IL-6 and TNF-α.192,193 In addition, there is a positive correlation between the abundance of Enterobacter, Enterococcus and the expression of inflammatory factors (IL-6 and TNF-α), suggesting the role of pathogenic bacteria in accelerating the inflammatory responses under SAP-ALI.188 The relative abundance of Bacteroides and Prevotella, which are important SCFAs-producing bacteria, decreased during SAP, resulting in the reduction of propionate, butyrate and valerate.187 Butyrate can alleviate cerulein-induced AP and intestinal injury by inhibiting the interactions between HDAC and AP1, ATAT1 and the expression of NLRP3 inflammasome.194 In addition, it has been shown that Lactobacillus can reduce the occurrence of pancreatic necrosis and pancreatic abscess, while Akkermansia is essential to form intestinal tight junctions and protect the intestinal barrier.195,196 Further research is required to investigate the impact of gut microbiota on SAP-ALI through the regulation of intestinal mucosal barrier function, specifically focusing on intestinal permeability and antimicrobial peptides.197
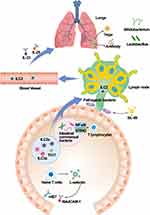
Migration of Immune Cells via Gut-Lung Axis
The effector of gut microbiota on mucosal immunity is not limited to the gastrointestinal tract, but may also perform an impact on the distal mucosal sites, including the lungs. The pathways between the gut and lung tissues and the bidirectional regulations with each other through microbiological or immune systems are known as the “gut-lung axis”.198 The stimulated immune cells within the intestinal mucosa could spread and migrate to other mucosal sites, which is called the “common mucosal immune response”.199 Previous studies have shown that immune cells from the gut, ILC2s, ILC3s, and Th17 cells, could be translocated from the gut to the lungs and participate the inflammation.200 Adhesion cascade reaction of immune cells is the key component of organismal immune cell migration.201 The α4β7 expressed on the surface of immune cells is a resting state in the non-inflammatory state and would not bind to the adhesion molecules on endothelial cells. While in the inflammatory state, L-selectin expressed by immune cells (mainly naive T cells) would bind to MAdCAM-1, which is highly expressed in the endothelial vein on GALT, allowing lymphocytes to slowly move along the vessel wall and expose the binding site for α4β7, ultimately allowing immune cells to adhere to the endothelial cells and the cell cytoskeleton was altered.202 Recent studies have found that BL-99 as prophylaxis could inhibit the growth of potential pathogens (Burkholderia cepacia, Shigella and Clostridium perfringens) and upregulate the expression of receptors associated with SCFAs, resulting in the decrease of inflammatory mediators and inflammatory immune cells (such as monocytes and macrophages) inflex into the lungs.203 Studies have shown that SCFAs can improve inflammatory responses through the gut-lung axis during SAP, specifically, SCFAs regulate intestinal barrier function and reduce SAP-ALI through the AMPK/NF-κB/NLRP3 mediated pathways.187,204 Succinic acid promotes alveolar macrophage polarization through its receptor SUCNR1 and downstream PI3K/AKT/HIF-1α pathway, which also provides further evidence for the gut-lung axis in SAP.205 In addition, SCFAs can affect the expression of free fatty acid receptors FFAR2 and FFAR3 in lungs.206 The significance of the gut-lung axis in SAP-ALI cannot be disregarded, as evidenced by all these findings, and further explorations are still necessary to provide theoretical support for the clinical treatment.
Apart from this, immune cell migration has been reported to be accelerated by gut microbiota and their metabolites via the activation of signaling molecules (eg, IL-33, IL-25).207,208 The symbiotic intestinal immune system acts as an intermediate by regulating immunol signal pathways (such as NF-κB, STING), and modulating the activity of T lymphocyte subsets. This enhances the communication between immune cells and epithelial cells in SAP-ALI. Details are shown in Figure 3. However, further molecular biology research is still required.209
Drugs and the Mechanisms
As mentioned previously, the intestinal mucosal immune barrier exhibits a protective effect in the development of SAP-ALI. Enhancing the functions of the intestinal mucosal immune barrier and inhibiting the translocation of bacteria or inflammatory factors via the gut-lung axis have been considered a promising direction in the treatment of SAP-ALI. Currently, available drugs that enhance the intestinal mucosal immune barrier include probiotics, glutamine, enteral nutrition, and traditional Chinese medicine.
Probiotics
Probiotics are a group of microbes that could help to maintain the balance of intestinal microecology and intestinal mucosal immunity, which could be colonized in the intestinal tract for a long time.210 Current studies have demonstrated that a variety of intestinal probiotics, including Bifidobacterium, Lactobacillus, and Clostridium butyricum, exert protective effects in SAP-ALI, and mechanisms encompass multi-targeted modulation effects on the immune barrier of the intestinal mucosa.40,211–213 Bifidobacteria, mainly colonized in the intestinal mucus layer, could release specific metabolites and control the activity of GCs by increasing the expression of MUC2 and preventing the overgrowth of Enterobacteriaceae. At the same time, Bifidobacteria could improve the efficiency of PD-1, PD-L1 and influence the immune defense of the host.214 Specific commensal Bifidobacterium longum can significantly upregulate the number of immune cells (CD4+ T cells, IgA plasma cells) and modulate the intestinal immune functions by Th1/Th2 and Th17/Treg.215 In addition, AO44, a new strain of Bifidobacterium longum that enrich with extracellular vehicles has shown anti-inflammatory properties and it can induce the release of IL-10 by the splenocytes and DC-CD4+ T cell co-cultures.216 Lactobacillus rhamnosus Probio-M9 strengthens the link between intestinal immune cells and commensal bacteria, which also stabilizes genetic variation within the gut microbiota.217 Lactobacillus Royale can maintain the intestinal barrier integrity by activation of LPLs, STAT signaling pathway and regeneration of ISCs.174 Lactobacillus acidophilus in combination with Bacillus subtilis modulates the intrinsic immunity in the intestine by upregulating the number of CD4+ T cells and SIgA+ cells, as well as the expression of GPR43 and GPR41, aryl hydrocarbon receptors, hypoxia-inducible factor 1 alpha and SIgA.218 The abundance of pathogenic bacteria triggers Clostridium butyricum to enhance Treg responses through tolerogenic antigen presentation signals, while simultaneously suppressing Th1 and Th1.219 The beneficial effects of probiotics on SAP have been demonstrated in previous studies, and it has been reported that probiotics can alleviate the pathological state of pancreatic tissue and reduce the infection rate in SAP rats by reducing the number of potentially harmful bacteria in the duodenum and preventing the migration of intestinal bacteria to other organs such as the pancreas.220 While more studies are needed to determine whether it can be used as a potential target to attenuate the severity of SAP.221–223
Glutamine
Glutamine (Gln) is a common amino acid in the human body and is thought to modulate systemic inflammatory responses while also playing a crucial role in various essential metabolic processes,224,225 and changes in levels have been reported to indicate the severity of diseases.226,227 Serving as a substrate for energy metabolism in IECs, Gln stimulates the differentiation and proliferation of ISCs, and maintains the morphological integrity of the mucosa in the small intestine.228 In addition, Gln is the energy source for numerous immune cells (such as macrophages, lymphocytes, fibroblasts,and monocytes) in the GALT and a precursor for cell proliferation, ensuring the activated status of immune cells and the resistance to the bacterial invasion, thus enhancing the non-specific immunol functions.226,229 Fasting under SAP might lead to the weakness of the mucus layer on the intestinal surface, increased intestinal permeability, and the translocation of bacteria and endotoxins, possibly attributing to entheogenic sepsis or multi-organ failure. While, this condition has been alleviated by the supplement of Gln.228 At present, it is still unknown whether glutamine use affects the abundance of intestinal flora during SAP and future studies can be carried out on whether it affects intestinal mucosal immune functions in SAP-ALI patients.226
Enteral Nutrition
Enteral nutrition (EN) is a safe route to provide nutritional support and stimulate the gastrointestinal motility of patients with SAP.230 The maintenance of cellular functions and integrity can be achieved through localized nutrient delivery, resulting in reduced incidence of stress mucosal disease and subsequent palliation of the condition.231 Previous studies have demonstrated that EN can promote the production of transmembrane-binding proteins, protect the intestinal epithelium from tight junctions, and enhance the intestinal mucosal barrier.232 EN can innate the immune responses by stimulating the intestine and facilitating the migration of α4β7 and L-selectin towards PPs.233 In addition, enteral nutrition has been shown to effectively preserve the population of goblet cells (GCs) and sustain the expression levels of MUC2 and lysozyme.234 The administration of short-peptide-based enteral nutrition (SPEN) can ameliorate intestinal mucosal microcirculatory impairment by mitigating mucosal inflammation, preserving the integrity of the intestinal mucosal barrier and reversing the systemic immunosuppression.235 Arginine supplementation reduces the damage of intestinal mucosa, and stimulates the proliferation of immune cells within PP, and increases the secretion of intestinal IgA.236 However, it is crucial to consider the timing of administration, as there may not be a significant improvement in symptoms when administered within 24 hours compared to that between 24h and 48h,237 which needs further studies.
Traditional Chinese Medicine
The performance of traditional Chinese medicine in the management of SAP should not be underestimated, as it plays a pivotal role in attenuating inflammatory factors and improving the prognosis. Qing-Yi Decoction (QYD), composed of Rhubarb, Radix bupleuri, Radix Aucklandiae, Paeoniae Radix Alba, Natrii Sulfas, Rhizome Corydalis, Gardenia jasminoides and Scutellaria baicalensis Georgi. The formula is commonly employed in the treatment of SAP,238,239 previous studies have shown that QYD could protect the intestinal barrier by inhibiting the release of inflammatory factors, the production of endotoxin, and the expression of NF-κB to block the activation of neutrophil cells.240 QYD could also improve gastrointestinal functions, reduce the infection rates, improve the prognosis and alleviate the lung injury in SAP patients,241,242 mechanisms of which may be related with the inhibition of secretory phospholipase A2 in the intestine and lungs.243 Recent studies have shown that the abundance of SCFAs produced bacteria increases after the treatment of QYD in SAP, and the produced SCFAs could regulate the AMPK/NF-κB/NLRP3 pathway via gut-lung axis, resulting in the reduction of systemic inflammatory responses and restoration of barrier functions.187 The protective effect of QYD against IECs injury in SAP may be related to the CaN/NFAT pathway.244 In addition, the TLR4/NF-κB signaling pathway might also be inhibited by QYD, which in turn prevents the destruction of intestinal mucosa.245 In addition, Cheng Qi Tang appears to be an effective treatment that could reduce the mortality and improve the organ dysfunction symptom scores and abdominal discomfort in SAP patients.5 To sum up, the investigations in the pathogenesis of SAP-ALI are still at the beginning stage, and in-depth studies are needed in the future.
Summary and Prospections
The growing emphasis on the gut-lung axis highlights the role of the intestinal mucosal immune barrier as a crucial “firewall” in SAP-ALI, especially the migration of immune cells and the translocation of bacteria and their PAMPs. The disruption of the intestinal mucosal immune barrier in SAP-ALI may lead to a reconfiguration of the intestinal microenvironment and an alteration in the composition of the gut microbiota, thereby impeding efforts to regain control over it. This review aims to provide a comprehensive overview of the involvement of various intestinal mucosal immune barrier compositions in the development of SAP-ALI through the gut-lung axis, intestinal flora, and immune-active substances. Future studies may focus on the movement of LPLs along the gut-lung axis, which particularly holds the potential to alter immune cell polarization in lung tissue as well as injury-related molecular patterns. In conclusion, to better understand the pathogenesis of SAP-ALI and improve the prognosis of patients, more studies are needed on the intestinal mucosal immune barrier in SAP-ALI.
Abbreviations
AhR, aryl hydrocarbon receptor; ALI, acute lung injury; AMPs, antimicrobial peptide; CGRP, calcitonin gene-related peptide; EEs, enteroendocrine cells; EN, enteral nutrition; GALT, gut-associated lymphoid tissue; GAPs, goblet cell-associated antigen passages; GCs, goblet cells; Gln, glutamine; GLP-1, glucagon-like peptide 1; HVEM, herpes virus entry mediator; IAECs, intestinal absorptive epithelial cells; IECs, intestinal epithelial cells; IELs, intestinal intraepithelial lymphocytes; ILCs, innate lymphoid cells; ISCs, intestinal stem cells; LECs, intestinal epithelial cells; Lgr5, leucine-rich repeat-containing G protein-coupled receptor 5; LPLs, lamina propria lymphocytes; M cells, microfold cells; MAMPs, microbe-associated molecular patterns; MLNs, mesenteric lymph nodes; MODS, multiple-organ dysfunction syndrome; MSCs, mesenchymal stromal cells; MZB1, B-1 cell-specific protein; NOX1, NADPH oxidase-1; PCs, Paneth cells; PD-L1, programmed death ligand-1; PP, Peyer patch; QYD, Qing-Yi Decoction; RGFs, RSPO3+GREM1+ fibroblasts; ROS, reactive oxygen species; SAP, severe acute pancreatitis; SAP-ALI, severe acute pancreatitis acute lung injury; SCF, stem cell factor; SIgA, secretory IgA; SIRS, systemic inflammatory response syndrome; SPEN, short-peptide-based enteral nutrition; TACs, transit amplifying cells.
Acknowledgments
Fan Li and Zhengjian Wang are co-first authors for this study. The authors would like to thank the reviewers and the authors of all the references.
Funding
This study was supported by National Key R&D Program of China (No. 2019YFE0119300) and National Natural Science Foundation of China (No. 82274311, 82074158, and 82304943).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Banks PA, Bollen TL, Dervenis C., et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
2. Li J, Chen J, Tang W. The consensus of integrative diagnosis and treatment of acute pancreatitis-2017. J Evid Based Med. 2019;12(1):76–88. doi:10.1111/jebm.12342
3. Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–496. doi:10.1038/s41575-019-0158-2
4. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–184. doi:10.1038/s41575-018-0087-5
5. Lin J, Han C, Dai N, Bi S, Du D, Xia Q. Effectiveness of Chengqi-series decoctions in treating severe acute pancreatitis: a Systematic review and meta-analysis. Phytomedicine. 2023;113:154727. doi:10.1016/j.phymed.2023.154727
6. Wang Z, Liu J, Wang Y, et al. Identification of Key Biomarkers Associated with Immunogenic Cell Death and Their Regulatory Mechanisms in Severe Acute Pancreatitis Based on WGCNA and Machine Learning. Int J Mol Sci. 2023;24(3):3033. doi:10.3390/ijms24033033
7. Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: a Review. JAMA. 2021;325(4):382–390. doi:10.1001/jama.2020.20317
8. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. doi:10.1016/S0140-6736(14)60649-8
9. Iannuzzi JP, King JA, Leong JH, et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: a Systematic Review and Meta-Analysis. Gastroenterology. 2022;162(1):122–134. doi:10.1053/j.gastro.2021.09.043
10. Hu Q, Yao J, Wu X, et al. Emodin attenuates severe acute pancreatitis-associated acute lung injury by suppressing pancreatic exosome-mediated alveolar macrophage activation. Acta Pharm Sin B. 2022;12(10):3986–4003. doi:10.1016/j.apsb.2021.10.008
11. Owusu L, Xu C, Chen H, et al. Gamma-enolase predicts lung damage in severe acute pancreatitis-induced acute lung injury. J Mol Histol. 2018;49(4):347–356. doi:10.1007/s10735-018-9774-3
12. Zhu X, Duan F, Zhang Y, et al. Acadesine alleviates acute pancreatitis-related lung injury by mediating the barrier protective function of pulmonary microvascular endothelial cells. Int Immunopharmacol. 2022;111:109165. doi:10.1016/j.intimp.2022.109165
13. Bampidis V, Azimonti G, Bastos M, et al. Safety and efficacy of a feed additive consisting of l-methionine produced by the combined activities of Corynebacterium glutamicum KCCM 80245 and Escherichia coli KCCM 80246 for all animal species (CJ Europe GmbH). EFSA J. 2022;20(4):e07247. doi:10.2903/j.efsa.2022.7247
14. Kong L, Deng J, Zhou X, et al. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis. 2021;12(10):928. doi:10.1038/s41419-021-04227-0
15. Larwood DJ. Nikkomycin Z-Ready to Meet the Promise? J Fungi (Basel). 2020;6(4):261. doi:10.3390/jof6040261
16. Zhou Y, Xia H, Zhao L, et al. SB203580 attenuates acute lung injury and inflammation in rats with acute pancreatitis in pregnancy. Inflammopharmacology. 2019;27(1):99–107. doi:10.1007/s10787-018-0522-9
17. Dombernowsky T, Kristensen MØ, Rysgaard S, Gluud LL, Novovic S. Risk factors for and impact of respiratory failure on mortality in the early phase of acute pancreatitis. Pancreatology. 2016;16(5):756–760. doi:10.1016/j.pan.2016.06.664
18. Elder ASF, Saccone GTP, Dixon D-L. Lung injury in acute pancreatitis: mechanisms underlying augmented secondary injury. Pancreatology. 2012;12(1):49–56. doi:10.1016/j.pan.2011.12.012
19. Fanelli V, Ranieri VM. Mechanisms and clinical consequences of acute lung injury. Ann Am Thorac Soc. 2015;12 Suppl 1:S3-S8. doi:10.1513/AnnalsATS.201407-340MG
20. Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi:10.1056/NEJMoa050333
21. Mansfield PB. An apparatus for elective fibrillatory cardiac arrest in experimental and clinical cardiopulmonary bypass operations. J Thorac Cardiovasc Surg. 1962;43:402–405.
22. Pastor CM, Matthay MA, Frossard J-L. Pancreatitis-associated acute lung injury: new insights. Chest. 2003;124(6):2341–2351. doi:10.1378/chest.124.6.2341
23. Jia M, Xu X, Zhou S, et al. Prediction of acute lung injury in severe acute pancreatitis by routine clinical data. Eur J Gastroenterol Hepatol. 2023;35(1):36–44. doi:10.1097/MEG.0000000000002458
24. De Campos T, Deree J, Coimbra R. From acute pancreatitis to end-organ injury: mechanisms of acute lung injury. Surg Infect (Larchmt). 2007;8(1):107–120. doi:10.1089/sur.2006.011
25. Akbarshahi H, Rosendahl AH, Westergren-Thorsson G, Andersson R. Acute lung injury in acute pancreatitis--awaiting the big leap. Respir Med. 2012;106(9):1199–1210. doi:10.1016/j.rmed.2012.06.003
26. Lou D, Shi K, H-P L, et al. Quantitative metabolic analysis of plasma extracellular vesicles for the diagnosis of severe acute pancreatitis. J Nanobiotechnology. 2022;20(1):52. doi:10.1186/s12951-022-01239-6
27. Montravers P, Chollet-Martin S, Marmuse JP, Gougerot-Pocidalo MA, Desmonts JM. Lymphatic release of cytokines during acute lung injury complicating severe pancreatitis. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1527–1533. doi:10.1164/ajrccm.152.5.7582288
28. Teodoro T, Odisho T, Sidorova E, Volchuk A. Pancreatic β-cells depend on basal expression of active ATF6α-p50 for cell survival even under nonstress conditions. Am J Physiol Cell Physiol. 2012;302(7):C992–1003. doi:10.1152/ajpcell.00160.2011
29. Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35(2):758–763. doi:10.1007/s10753-011-9371-z
30. Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20. doi:10.1016/j.jaci.2009.05.038
31. Zhang D, Li L, Li J, et al. Colchicine improves severe acute pancreatitis-induced acute lung injury by suppressing inflammation, apoptosis and oxidative stress in rats. Biomed Pharmacother. 2022;153:113461. doi:10.1016/j.biopha.2022.113461
32. Zhang -X-X, Wang H-Y, Yang X-F, et al. Alleviation of acute pancreatitis-associated lung injury by inhibiting the p38 mitogen-activated protein kinase pathway in pulmonary microvascular endothelial cells. World J Gastroenterol. 2021;27(18):2141–2159. doi:10.3748/wjg.v27.i18.2141
33. Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–685. doi:10.1038/nri3738
34. Perez-Lopez A, Behnsen J, Nuccio S-P, Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol. 2016;16(3):135–148. doi:10.1038/nri.2015.17
35. Li Y, Liu N, Ge Y, Yang Y, Ren F, Wu Z. Tryptophan and the innate intestinal immunity: crosstalk between metabolites, host innate immune cells, and microbiota. Eur J Immunol. 2022;52(6):856–868. doi:10.1002/eji.202149401
36. Gasaly N, de Vos P, Hermoso MA. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: a Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front Immunol. 2021;12:658354. doi:10.3389/fimmu.2021.658354
37. Tan Y-Q, Wang Y-N, Feng H-Y, et al. Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling. Free Radic Biol Med. 2022;184:30–41. doi:10.1016/j.freeradbiomed.2022.03.025
38. Zhuang Q, Huang L, Zeng Y, et al. Dynamic Monitoring of Immunoinflammatory Response Identifies Immunoswitching Characteristics of Severe Acute Pancreatitis in Rats. Front Immunol. 2022;13:876168. doi:10.3389/fimmu.2022.876168
39. Stojanovic B, Jovanovic IP, Stojanovic MD, et al. The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis. Cells. 2023;12(11):1495. doi:10.3390/cells12111495
40. Wang Z, Li F, Liu J, et al. Intestinal Microbiota - An Unmissable Bridge to Severe Acute Pancreatitis-Associated Acute Lung Injury. Front Immunol. 2022;13:913178. doi:10.3389/fimmu.2022.913178
41. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi:10.1038/nri2653
42. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–153. doi:10.1038/nri3608
43. Oda M, Hatano Y, Sato T. Intestinal epithelial organoids: regeneration and maintenance of the intestinal epithelium. Curr Opin Genet Dev. 2022;76:101977. doi:10.1016/j.gde.2022.101977
44. Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res. 2010;156(3):180–187. doi:10.1016/j.trsl.2010.06.003
45. Cohen-Kedar S, Shaham Barda E, Rabinowitz KM, et al. Human intestinal epithelial cells can internalize luminal fungi via LC3-associated phagocytosis. Front Immunol. 2023;14:1142492. doi:10.3389/fimmu.2023.1142492
46. Overcast GR, Meibers HE, Eshleman EM, et al. IEC-intrinsic IL-1R signaling holds dual roles in regulating intestinal homeostasis and inflammation. J Exp Med. 2023;220(6):e20212523. doi:10.1084/jem.20212523
47. Moon S, Park Y, Hyeon S, et al. Niche-specific MHC II and PD-L1 regulate CD4+CD8αα+ intraepithelial lymphocyte differentiation. J Exp Med. 2021;218(4):e20201665. doi:10.1084/jem.20201665
48. Seo G-Y, Takahashi D, Wang Q, et al. Epithelial HVEM maintains intraepithelial T cell survival and contributes to host protection. Sci Immunol. 2022;7(73):eabm6931. doi:10.1126/sciimmunol.abm6931
49. Duan J, Matute JD, Unger LW, et al. Endoplasmic reticulum stress in the intestinal epithelium initiates purine metabolite synthesis and promotes Th17 cell differentiation in the gut. Immunity. 2023;56(5):1115–1131.e9. doi:10.1016/j.immuni.2023.02.018
50. Glaubitz J, Wilden A, Frost F, et al. Activated regulatory T-cells promote duodenal bacterial translocation into necrotic areas in severe acute pancreatitis. Gut. 2023;72(7):1355–1369. doi:10.1136/gutjnl-2022-327448
51. Alcaino C. Mechanosensitive release of 5-HT from specialized intestinal epithelial cells. Nat Rev Gastroenterol Hepatol. 2023;20(1):4. doi:10.1038/s41575-022-00712-9
52. Tan SH, Phuah P, Tan LT, et al. A constant pool of Lgr5+ intestinal stem cells is required for intestinal homeostasis. Cell Rep. 2021;34(4):108633. doi:10.1016/j.celrep.2020.108633
53. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi:10.1038/nature06196
54. Ishikawa K, Sugimoto S, Oda M, et al. Identification of Quiescent LGR5+ Stem Cells in the Human Colon. Gastroenterology. 2022;163(5):1391–1406.e24. doi:10.1053/j.gastro.2022.07.081
55. Sanman LE, Chen IW, Bieber JM, et al. Transit-Amplifying Cells Coordinate Changes in Intestinal Epithelial Cell-Type Composition. Dev Cell. 2021;56(3):356–365.e9. doi:10.1016/j.devcel.2020.12.020
56. Cancedda R, Mastrogiacomo M. Transit Amplifying Cells (TACs): a still not fully understood cell population. Front Bioeng Biotechnol. 2023;11:1189225. doi:10.3389/fbioe.2023.1189225
57. Jing J, Feng J, Li J, et al. Reciprocal interaction between mesenchymal stem cells and transit amplifying cells regulates tissue homeostasis. Elife. 2021;10:e59459. doi:10.7554/eLife.59459
58. Roostaee A, Benoit YD, Boudjadi S, Beaulieu J-F. Epigenetics in Intestinal Epithelial Cell Renewal. J Cell Physiol. 2016;231(11):2361–2367. doi:10.1002/jcp.25401
59. Kurokawa K, Hayakawa Y, Koike K. Plasticity of Intestinal Epithelium: stem Cell Niches and Regulatory Signals. Int J Mol Sci. 2020;22(1):357. doi:10.3390/ijms22010357
60. Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19–34. doi:10.1038/s41575-018-0081-y
61. Zhu P, Lu T, Wu J, et al. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons. Cell Res. 2022;32(6):555–569. doi:10.1038/s41422-022-00645-7
62. Kim S, Shin Y-C, Kim T-Y, et al. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes. 2021;13(1):1–20. doi:10.1080/19490976.2021.1892441
63. Goto N, Goto S, Imada S, Hosseini S, Deshpande V, Yilmaz ÖH. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell Stem Cell. 2022;29(8):1246–1261.e6. doi:10.1016/j.stem.2022.06.013
64. Schmitt M, Schewe M, Sacchetti A, et al. Paneth Cells Respond to Inflammation and Contribute to Tissue Regeneration by Acquiring Stem-like Features through SCF/c-Kit Signaling. Cell Rep. 2018;24(9):2312–2328.e7. doi:10.1016/j.celrep.2018.07.085
65. Canale V, Spalinger MR, Alvarez R, et al. PTPN2 Is a Critical Regulator of Ileal Paneth Cell Viability and Function in Mice. Cell Mol Gastroenterol Hepatol. 2023;16(1):39–62. doi:10.1016/j.jcmgh.2023.03.009
66. Hou Q, Huang J, Ayansola H, Masatoshi H, Zhang B. Intestinal Stem Cells and Immune Cell Relationships: potential Therapeutic Targets for Inflammatory Bowel Diseases. Front Immunol. 2020;11:623691. doi:10.3389/fimmu.2020.623691
67. Knoop KA, Newberry RD. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018;11(6):1551–1557. doi:10.1038/s41385-018-0039-y
68. Liu Y, Yu X, Zhao J, Zhang H, Zhai Q, Chen W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int J Biol Macromol. 2020;164:884–891. doi:10.1016/j.ijbiomac.2020.07.191
69. Johansson ME, Hansson GC. Goblet cells need some stress. J Clin Invest. 2022;132(17):e162030. doi:10.1172/JCI162030
70. Newberry RD, Hogan SP. Intestinal epithelial cells in tolerance and allergy to dietary antigens. J Allergy Clin Immunol. 2021;147(1):45–48. doi:10.1016/j.jaci.2020.10.030
71. Kulkarni DH, Gustafsson JK, Knoop KA, et al. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 2020;13(2):271–282. doi:10.1038/s41385-019-0240-7
72. Gustafsson JK, Davis JE, Rappai T, et al. Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis. Elife. 2021;10:e67292. doi:10.7554/eLife.67292
73. Gustafsson JK, Johansson MEV. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. 2022;19(12):785–803. doi:10.1038/s41575-022-00675-x
74. Nyström EEL, Martinez-Abad B, Arike L, et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science. 2021;372(6539):eabb1590. doi:10.1126/science.abb1590
75. Naama M, Telpaz S, Awad A, et al. Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host Microbe. 2023;31(3):433–446.e4. doi:10.1016/j.chom.2023.01.006
76. Hindson J. Mucus secretion from colonic goblet cells is regulated by autophagy and ER stress. Nat Rev Gastroenterol Hepatol. 2023;20(4):202. doi:10.1038/s41575-023-00761-8
77. Allaire JM, Morampudi V, Crowley SM, et al. Frontline defenders: goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am J Physiol Gastrointest Liver Physiol. 2018;314(3):G360–G377. doi:10.1152/ajpgi.00181.2017
78. Huang Z, Wu H, Fan J, et al. Colonic mucin-2 attenuates acute necrotizing pancreatitis in rats by modulating intestinal homeostasis. FASEB J. 2023;37(7):e22994. doi:10.1096/fj.202201998R
79. Yao Y, Kim G, Shafer S, et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell. 2022;185(7):1172–1188.e28. doi:10.1016/j.cell.2022.02.013
80. Cray P, Sheahan BJ, Dekaney CM. Secretory Sorcery: Paneth Cell Control of Intestinal Repair and Homeostasis. Cell Mol Gastroenterol Hepatol. 2021;12(4):1239–1250. doi:10.1016/j.jcmgh.2021.06.006
81. Fu Y, Mei Q, Yin N, et al. Paneth Cells Protect against Acute Pancreatitis via Modulating Gut Microbiota Dysbiosis. mSystems. 2022;7(3):e0150721. doi:10.1128/msystems.01507-21
82. Yu S, Balasubramanian I, Laubitz D, et al. Paneth Cell-Derived Lysozyme Defines the Composition of Mucolytic Microbiota and the Inflammatory Tone of the Intestine. Immunity. 2020;53(2):398–416.e8. doi:10.1016/j.immuni.2020.07.010
83. Lin X, Gaudino SJ, Jang KK, et al. IL-17RA-signaling in Lgr5+ intestinal stem cells induces expression of transcription factor ATOH1 to promote secretory cell lineage commitment. Immunity. 2022;55(2):237–253.e8. doi:10.1016/j.immuni.2021.12.016
84. He G-W, Lin L, DeMartino J, et al. Optimized human intestinal organoid model reveals interleukin-22-dependency of paneth cell formation. Cell Stem Cell. 2022;29(9):1333–1345.e6. doi:10.1016/j.stem.2022.08.002
85. Luo C, Huang Q, Yuan X, et al. Abdominal paracentesis drainage attenuates severe acute pancreatitis by enhancing cell apoptosis via PI3K/AKT signaling pathway. Apoptosis. 2020;25(3–4):290–303. doi:10.1007/s10495-020-01597-2
86. Sun Z, Li L, Qu J, Li H, Chen H. Proteomic analysis of therapeutic effects of Qingyi pellet on rodent severe acute pancreatitis-associated lung injury. Biomed Pharmacother. 2019;118:109300. doi:10.1016/j.biopha.2019.109300
87. Azkanaz M, Corominas-Murtra B, Ellenbroek SIJ, et al. Retrograde movements determine effective stem cell numbers in the intestine. Nature. 2022;607(7919):548–554. doi:10.1038/s41586-022-04962-0
88. Theiss AL. Ptpn2: a Critical Regulator of Paneth Cell Homeostasis. Cell Mol Gastroenterol Hepatol. 2023;16(1):163–164. doi:10.1016/j.jcmgh.2023.03.010
89. Chen J, Huang C, Wang J, et al. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats. PLoS One. 2017;12(4):e0176583. doi:10.1371/journal.pone.0176583
90. Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6(4):666–677. doi:10.1038/mi.2013.30
91. Dillon A, Lo DD. M Cells: intelligent Engineering of Mucosal Immune Surveillance. Front Immunol. 2019;10:1499. doi:10.3389/fimmu.2019.01499
92. Torow N, Li R, Hitch TCA, et al. M cell maturation and cDC activation determine the onset of adaptive immune priming in the neonatal Peyer’s patch. Immunity. 2023;56(6):1220–1238.e7. doi:10.1016/j.immuni.2023.04.002
93. Lai NY, Musser MA, Pinho-Ribeiro FA, et al. Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell. 2020;180(1):33–49.e22. doi:10.1016/j.cell.2019.11.014
94. Schlesinger Y, Yosefov-Levi O, Kolodkin-Gal D, et al. Single-cell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity. Nat Commun. 2020;11(1):4516. doi:10.1038/s41467-020-18207-z
95. Luna Velez MV, Neikes HK, Snabel RR, et al. ONECUT2 regulates RANKL-dependent enterocyte and microfold cell differentiation in the small intestine; a multi-omics study. Nucleic Acids Res. 2023;51(3):1277–1296. doi:10.1093/nar/gkac1236
96. Shen A, Kim H-J, G-S O, et al. Pharmacological stimulation of NQO1 decreases NADPH levels and ameliorates acute pancreatitis in mice. Cell Death Dis. 2018;10(1):5. doi:10.1038/s41419-018-1252-z
97. Hsu N-Y, Nayar S, Gettler K, et al. NOX1 is essential for TNFα-induced intestinal epithelial ROS secretion and inhibits M cell signatures. Gut. 2023;72(4):654–662. doi:10.1136/gutjnl-2021-326305
98. Lin S, Mukherjee S, Li J, Hou W, Pan C, Liu J. Mucosal immunity-mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer’s patches. Sci Adv. 2021;7(20):eabf0677. doi:10.1126/sciadv.abf0677
99. Guo X, Yin C, Yang F, et al. The Cellular Diversity and Transcription Factor Code of Drosophila Enteroendocrine Cells. Cell Rep. 2019;29(12):4172–4185.e5. doi:10.1016/j.celrep.2019.11.048
100. Beumer J, Puschhof J, Bauzá-Martinez J, et al. High-Resolution mRNA and Secretome Atlas of Human Enteroendocrine Cells. Cell. 2020;181(6):1291–1306.e19. doi:10.1016/j.cell.2020.04.036
101. Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15(4):226–237. doi:10.1038/s41574-019-0168-8
102. Zhao M, Ren K, Xiong X, et al. Protein O-GlcNAc Modification Links Dietary and Gut Microbial Cues to the Differentiation of Enteroendocrine L Cells. Cell Rep. 2020;32(6):108013. doi:10.1016/j.celrep.2020.108013
103. Wang Z, Liu J, Li F, et al. The gut-lung axis in severe acute Pancreatitis-associated lung injury: the protection by the gut microbiota through short-chain fatty acids. Pharmacol Res. 2022;182:106321. doi:10.1016/j.phrs.2022.106321
104. Ye L, Bae M, Cassilly CD, et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29(2):179–196.e9. doi:10.1016/j.chom.2020.11.011
105. Jakkampudi A, Sarkar P, Unnisa M, et al. Kynurenine pathway alteration in acute pancreatitis and its role as a biomarker of infected necrosis. Pancreatology. 2023;23(6):589–600. doi:10.1016/j.pan.2023.07.003
106. Bayrer JR, Castro J, Venkataraman A, et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature. 2023;616(7955):137–142. doi:10.1038/s41586-023-05829-8
107. Chen S, He R, He B, Xu L, Zhang S. Potential Roles of Exosomal lncRNAs in the Intestinal Mucosal Immune Barrier. J Immunol Res. 2021;2021:7183136. doi:10.1155/2021/7183136
108. Bouffi C, Wikenheiser-Brokamp KA, Chaturvedi P, et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat Biotechnol. 2023;41(6):824–831. doi:10.1038/s41587-022-01558-x
109. Park JI, Cho SW, Kang JH, Park T-E. Intestinal Peyer’s Patches: structure, Function, and In Vitro Modeling. Tissue Eng Regen Med. 2023;20(3):341–353. doi:10.1007/s13770-023-00543-y
110. Gribonika I, Strömberg A, Lebrero-Fernandez C, et al. Peyer’s patch TH17 cells are dispensable for gut IgA responses to oral immunization. Sci Immunol. 2022;7(73):eabc5500. doi:10.1126/sciimmunol.abc5500
111. Hirota K, Turner J-E, Villa M, et al. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14(4):372–379. doi:10.1038/ni.2552
112. Obata T, Goto Y, Kunisawa J, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A. 2010;107(16):7419–7424. doi:10.1073/pnas.1001061107
113. Chen H, Zhang Y, Ye AY, et al. BCR selection and affinity maturation in Peyer’s patch germinal centres. Nature. 2020;582(7812):421–425. doi:10.1038/s41586-020-2262-4
114. Sonnenberg GF, Monticelli LA, Alenghat T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–1325. doi:10.1126/science.1222551
115. Fawkner-Corbett D, Antanaviciute A, Parikh K, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell. 2021;184(3):810–826.e23. doi:10.1016/j.cell.2020.12.016
116. Hashiguchi M, Kashiwakura Y, Kojima H, Kobayashi A, Kanno Y, Kobata T. Peyer’s patch innate lymphoid cells regulate commensal bacteria expansion. Immunol Lett. 2015;165(1):1–9. doi:10.1016/j.imlet.2015.03.002
117. Chen J, Huang J, Shi J, et al. Nestin+ Peyer’s patch resident MSCs enhance healing of inflammatory bowel disease through IL-22-mediated intestinal epithelial repair. Cell Prolif. 2023;56(2):e13363. doi:10.1111/cpr.13363
118. Fair-Mäkelä R, Ugur M, Iftakhar-E-Khuda I, et al. Robo4 contributes to the turnover of Peyer’s patch B cells. Mucosal Immunol. 2020;13(2):245–256. doi:10.1038/s41385-019-0230-9
119. Troullinaki M, Chen L-S, Witt A, et al. Robo4-mediated pancreatic endothelial integrity decreases inflammation and islet destruction in autoimmune diabetes. FASEB J. 2020;34(2):3336–3346. doi:10.1096/fj.201900125RR
120. Olivares-Villagómez D, Van Kaer L. Intestinal Intraepithelial Lymphocytes: sentinels of the Mucosal Barrier. Trends Immunol. 2018;39(4):264–275. doi:10.1016/j.it.2017.11.003
121. Hoytema van Konijnenburg DP, Mucida D. Intraepithelial lymphocytes. Curr Biol. 2017;27(15):R737–R739. doi:10.1016/j.cub.2017.05.073
122. Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11(7):445–456. doi:10.1038/nri3007
123. Han J, Liu N, Jin W, et al. TGF-β controls development of TCRγδ+CD8αα+ intestinal intraepithelial lymphocytes. Cell Discov. 2023;9(1):52. doi:10.1038/s41421-023-00542-2
124. Ma H, Qiu Y, Yang H. Intestinal intraepithelial lymphocytes: maintainers of intestinal immune tolerance and regulators of intestinal immunity. J Leukoc Biol. 2021;109(2):339–347. doi:10.1002/JLB.3RU0220-111
125. Song X, Zhang H, Zhang Y, et al. Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature. 2023;619(7971):837–843. doi:10.1038/s41586-023-06265-4
126. Wong CK, Yusta B, Koehler JA, et al. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 2022;34(10):1514–1531.e7. doi:10.1016/j.cmet.2022.08.003
127. Panda SK, Peng V, Sudan R, et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity. 2023;56(4):797–812.e4. doi:10.1016/j.immuni.2023.01.023
128. Xue J, Nguyen DTC, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143(6):1670–1680. doi:10.1053/j.gastro.2012.08.051
129. Isho B, Florescu A, Wang AA, Gommerman JL. Fantastic IgA plasma cells and where to find them. Immunol Rev. 2021;303(1):119–137. doi:10.1111/imr.12980
130. Sandler RS, Hansen JJ, Peery AF, Woosley JT, Galanko JA, Keku TO. Intraepithelial and Lamina Propria Lymphocytes Do Not Correlate With Symptoms or Exposures in Microscopic Colitis. Clin Transl Gastroenterol. 2022;13(3):e00467. doi:10.14309/ctg.0000000000000467
131. Bemark M, Angeletti D. Know your enemy or find your friend?-Induction of IgA at mucosal surfaces. Immunol Rev. 2021;303(1):83–102. doi:10.1111/imr.13014
132. Lin YH, Duong HG, Limary AE, et al. Small intestine and colon tissue-resident memory CD8+ T cells exhibit molecular heterogeneity and differential dependence on Eomes. Immunity. 2023;56(1):207–223.e8. doi:10.1016/j.immuni.2022.12.007
133. Rampoldi F, Prinz I. Three Layers of Intestinal γδ T Cells Talk Different Languages With the Microbiota. Front Immunol. 2022;13:849954. doi:10.3389/fimmu.2022.849954
134. Vivier E, Artis D, Colonna M, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174(5):1054–1066. doi:10.1016/j.cell.2018.07.017
135. Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020;20(9):552–565. doi:10.1038/s41577-020-0282-9
136. Zhao M, Shao F, Yu D, et al. Maturation and specialization of group 2 innate lymphoid cells through the lung-gut axis. Nat Commun. 2022;13(1):7600. doi:10.1038/s41467-022-35347-6
137. Panda SK, Colonna M. Innate Lymphoid Cells in Mucosal Immunity. Front Immunol. 2019;10:861. doi:10.3389/fimmu.2019.00861
138. Jarade A, Garcia Z, Marie S, et al. Inflammation triggers ILC3 patrolling of the intestinal barrier. Nat Immunol. 2022;23(9):1317–1323. doi:10.1038/s41590-022-01284-1
139. Shen Y, Cui N-Q. Clinical observation of immunity in patients with secondary infection from severe acute pancreatitis. Inflamm Res. 2012;61(7):743–748. doi:10.1007/s00011-012-0467-1
140. Yin G, Zhao C, Pei W. Crosstalk between macrophages and innate lymphoid cells (ILCs) in diseases. Int Immunopharmacol. 2022;110:108937. doi:10.1016/j.intimp.2022.108937
141. Shi S, Ye L, Jin K, Xiao Z, Yu X, Wu W. Innate Lymphoid Cells: emerging Players in Pancreatic Disease. Int J Mol Sci. 2022;23(7):3748. doi:10.3390/ijms23073748
142. Yang Y, Palm NW. Immunoglobulin A and the microbiome. Curr Opin Microbiol. 2020;56:89–96. doi:10.1016/j.mib.2020.08.003
143. Corthésy B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev. 2013;12(6):661–665. doi:10.1016/j.autrev.2012.10.012
144. Zhang H, Qi C, Zhao Y, et al. Depletion of gut secretory immunoglobulin A coated Lactobacillus reuteri is associated with gestational diabetes mellitus-related intestinal mucosal barrier damage. Food Funct. 2021;12(21):10783–10794. doi:10.1039/d1fo02517a
145. Maccio-Maretto L, Piqueras V, Barrios BE, Romagnoli PA, Denning TL, Correa SG. Luminal bacteria coated with IgA and IgG during intestinal inflammation as a new and abundant stimulus for colonic macrophages. Immunology. 2022;167(1):64–76. doi:10.1111/imm.13518
146. Michaud E, Waeckel L, Gayet R, et al. Alteration of microbiota antibody-mediated immune selection contributes to dysbiosis in inflammatory bowel diseases. EMBO Mol Med. 2022;14(8):e15386. doi:10.15252/emmm.202115386
147. Zhong Y, Cai D, Cai W, Geng S, Chen L, Han T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin Nutr. 2009;28(5):575–580. doi:10.1016/j.clnu.2009.04.026
148. Huang L, Zeng Y, Duan L, et al. Optimal timing of free total rhubarb anthraquinones on immune regulation in rats with severe acute pancreatitis. J Ethnopharmacol. 2023;308:116266. doi:10.1016/j.jep.2023.116266
149. Xiong E, Li Y, Min Q, et al. MZB1 promotes the secretion of J-chain-containing dimeric IgA and is critical for the suppression of gut inflammation. Proc Natl Acad Sci U S A. 2019;116(27):13480–13489. doi:10.1073/pnas.1904204116
150. Rochereau N, Roblin X, Michaud E, et al. NOD2 deficiency increases retrograde transport of secretory IgA complexes in Crohn’s disease. Nat Commun. 2021;12(1):261. doi:10.1038/s41467-020-20348-0
151. Rochereau N, Drocourt D, Perouzel E, et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013;11(9):e1001658. doi:10.1371/journal.pbio.1001658
152. Zhang H, Wang Y, Qu M, et al. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin Transl Med. 2023;13(1):e1170. doi:10.1002/ctm2.1170
153. Zhai Y-J, Feng Y, Ma X, Ma F. Defensins: defenders of human reproductive health. Hum Reprod Update. 2023;29(1):126–154. doi:10.1093/humupd/dmac032
154. Ehmann D, Wendler J, Koeninger L, et al. Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments. Proc Natl Acad Sci U S A. 2019;116(9):3746–3751. doi:10.1073/pnas.1817376116
155. Zhao Y, Chen F, Wu W, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11(3):752–762. doi:10.1038/mi.2017.118
156. Breugelmans T, Oosterlinck B, Arras W, et al. The role of mucins in gastrointestinal barrier function during health and disease. Lancet Gastroenterol Hepatol. 2022;7(5):455–471. doi:10.1016/S2468-1253(21)00431-3
157. Pouw RB, Ricklin D. Tipping the balance: intricate roles of the complement system in disease and therapy. Semin Immunopathol. 2021;43(6):757–771. doi:10.1007/s00281-021-00892-7
158. Benis N, Wells JM, Smits MA, et al. High-level integration of murine intestinal transcriptomics data highlights the importance of the complement system in mucosal homeostasis. BMC Genomics. 2019;20(1):1028. doi:10.1186/s12864-019-6390-x
159. Henry MW, Miller AO. A Single Dose of Antibiotics Before Removal of Orthopaedic Implants Used to Treat Below-The-Knee Fractures Did Not Reduce Surgical Site Infections at 30 Days. J Bone Joint Surg Am. 2018;100(16):1434. doi:10.2106/JBJS.18.00634
160. Xu L-T, Xu H-L, M-S F. Association between glucose-regulated protein and neutrophil apoptosis in severe acute pancreatitis. Int J Clin Exp Pathol. 2015;8(8):9300–9306.
161. Kahn A, Sottiaux M, Appelboom-Fondu J, Blum D, Rebuffat E, Levitt J. Long-term development of children monitored as infants for an apparent life-threatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83(5):668–673.
162. J-P L, Yang J, Huang J-R, et al. Immunosuppression and the infection caused by gut mucosal barrier dysfunction in patients with early severe acute pancreatitis. Front Biosci (Landmark Ed). 2013;18(3):892–900. doi:10.2741/4150
163. Manohar M, Jones EK, Rubin SJS, et al. Novel Circulating and Tissue Monocytes as Well as Macrophages in Pancreatitis and Recovery. Gastroenterology. 2021;161(6):2014–2029.e14. doi:10.1053/j.gastro.2021.08.033
164. Xu D, Xie R, Xu Z, et al. mTOR-Myc axis drives acinar-to-dendritic cell transition and the CD4+ T cell immune response in acute pancreatitis. Cell Death Dis. 2020;11(6):416. doi:10.1038/s41419-020-2517-x
165. Qiu Z, Yu P, Bai B, et al. Regulatory B10 cells play a protective role in severe acute pancreatitis. Inflamm Res. 2016;65(8):647–654. doi:10.1007/s00011-016-0947-9
166. Dervenis C, Smailis D, Hatzitheoklitos E. Bacterial translocation and its prevention in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2003;10(6):415–418. doi:10.1007/s00534-002-0727-5
167. Qin H-L, Z-D S, Gao Q, Lin Q-T. Early intrajejunal nutrition: bacterial translocation and gut barrier function of severe acute pancreatitis in dogs. Hepatobiliary Pancreat Dis Int. 2002;1(1):150–154.
168. Sendler M, Weiss F-U, Golchert J, et al. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology. 2018;154(3):704–718.e10. doi:10.1053/j.gastro.2017.10.018
169. Rogers AP, Mileto SJ, Lyras D. Impact of enteric bacterial infections at and beyond the epithelial barrier. Nat Rev Microbiol. 2023;21(4):260–274. doi:10.1038/s41579-022-00794-x
170. Kim J-E, Li B, Fei L, et al. Gut microbiota promotes stem cell differentiation through macrophage and mesenchymal niches in early postnatal development. Immunity. 2022;55(12):2300–2317.e6. doi:10.1016/j.immuni.2022.11.003
171. Donaldson DS, Pollock J, Vohra P, Stevens MP, Mabbott NA. Microbial Stimulation Reverses the Age-Related Decline in M Cells in Aged Mice. iScience. 2020;23(6):101147. doi:10.1016/j.isci.2020.101147
172. Borbet TC, Pawline MB, Li J, et al. Disruption of the early-life microbiota alters Peyer’s patch development and germinal center formation in gastrointestinal-associated lymphoid tissue. iScience. 2023;26(6):106810. doi:10.1016/j.isci.2023.106810
173. Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14. doi:10.1186/s40779-017-0122-9
174. Hou Q, Ye L, Liu H, et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25(9):1657–1670. doi:10.1038/s41418-018-0070-2
175. Goguyer-Deschaumes R, Waeckel L, Killian M, Rochereau N, Paul S. Metabolites and secretory immunoglobulins: messengers and effectors of the host-microbiota intestinal equilibrium. Trends Immunol. 2022;43(1):63–77. doi:10.1016/j.it.2021.11.005
176. Tan J, Ni D, Taitz J, et al. Dietary protein increases T-cell-independent sIgA production through changes in gut microbiota-derived extracellular vesicles. Nat Commun. 2022;13(1):4336. doi:10.1038/s41467-022-31761-y
177. Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. doi:10.1136/gutjnl-2020-322260
178. Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16(2):164–177. doi:10.15252/embr.201439263
179. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi:10.1126/science.1110591
180. Sharkey KA, Mawe GM. The enteric nervous system. Physiol Rev. 2023;103(2):1487–1564. doi:10.1152/physrev.00018.2022
181. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi:10.1136/gutjnl-2015-309990
182. Haase S, Haghikia A, Wilck N, Müller DN, Linker RA. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology. 2018;154(2):230–238. doi:10.1111/imm.12933
183. Huang C, Chen J, Wang J, et al. Dysbiosis of Intestinal Microbiota and Decreased Antimicrobial Peptide Level in Paneth Cells during Hypertriglyceridemia-Related Acute Necrotizing Pancreatitis in Rats. Front Microbiol. 2017;8:776. doi:10.3389/fmicb.2017.00776
184. Pagliari D, Saviano A, Newton EE, et al. Gut Microbiota-Immune System Crosstalk and Pancreatic Disorders. Mediators Inflamm. 2018;2018:7946431. doi:10.1155/2018/7946431
185. Li X, He C, Li N, et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes. 2020;11(6):1774–1789. doi:10.1080/19490976.2020.1770042
186. Peng J-S, Liu Z-H, Li C-J, et al. Development of a real-time PCR method for the detection of bacterial colonization in rat models of severe acute pancreatitis. Chin Med J (Engl). 2010;123(3):326–331.
187. Wang Z, Liu J, Li F, et al. Mechanisms of Qingyi Decoction in Severe Acute Pancreatitis-Associated Acute Lung Injury via Gut Microbiota: targeting the Short-Chain Fatty Acids-Mediated AMPK/NF-κB/NLRP3 Pathway. Microbiol Spectr. 2023;11(4):e0366422. doi:10.1128/spectrum.03664-22
188. Tan C, Ling Z, Huang Y, et al. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas. 2015;44(6):868–875. doi:10.1097/MPA.0000000000000355
189. Zhang XM, Zhang ZY, Zhang CH, Wu J, Wang YX, Zhang GX. Intestinal Microbial Community Differs between Acute Pancreatitis Patients and Healthy Volunteers. Biomed Environ Sci. 2018;31(1):81–86. doi:10.3967/bes2018.010
190. Lei Y, Tang L, Liu S, et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome. 2021;9(1):115. doi:10.1186/s40168-021-01065-2
191. Zhou H, Gao J, Wu W, et al. Octreotide ameliorates intestinal dysmotility by interstitial cells of Cajal protection in a rat acute necrotizing pancreatitis model. Pancreas. 2011;40(8):1226–1233. doi:10.1097/MPA.0b013e318220afab
192. Wang H, Li C, Jiang Y, Li H, Zhang D. Effects of Bacterial Translocation and Autophagy on Acute Lung Injury Induced by Severe Acute Pancreatitis. Gastroenterol Res Pract. 2020;2020:8953453. doi:10.1155/2020/8953453
193. Piao X, Liu B, Sui X, et al. Picroside II Improves Severe Acute Pancreatitis-Induced Intestinal Barrier Injury by Inactivating Oxidative and Inflammatory TLR4-Dependent PI3K/AKT/NF-κB Signaling and Improving Gut Microbiota. Oxid Med Cell Longev. 2020;2020:3589497. doi:10.1155/2020/3589497
194. Pan X, Fang X, Wang F, et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharmacol. 2019;176(23):4446–4461. doi:10.1111/bph.14806
195. Kumar M, Kissoon-Singh V, Coria AL, Moreau F, Chadee K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2017;312(1):G34–G45. doi:10.1152/ajpgi.00298.2016
196. Zhu L, Zhang D, Zhu H, et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe-/- mice. Atherosclerosis. 2018;268:117–126. doi:10.1016/j.atherosclerosis.2017.11.023
197. Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: current understanding. Indian J Gastroenterol. 2016;35(3):153–166. doi:10.1007/s12664-016-0647-y
198. Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12(4):843–850. doi:10.1038/s41385-019-0160-6
199. Zhou B, Yuan Y, Zhang S, et al. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front Immunol. 2020;11:575. doi:10.3389/fimmu.2020.00575
200. Mjösberg J, Rao A. Lung inflammation originating in the gut. Science. 2018;359(6371):36–37. doi:10.1126/science.aar4301
201. Liu J, Zhang X, Cheng Y, Cao X. Dendritic cell migration in inflammation and immunity. Cell Mol Immunol. 2021;18(11):2461–2471. doi:10.1038/s41423-021-00726-4
202. Zundler S, Becker E, Schulze LL, Neurath MF. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut. 2019;68(9):1688–1700. doi:10.1136/gutjnl-2018-317977
203. Nan X, Zhao W, Liu W-H, et al. Bifidobacterium animalis subsp. lactis BL-99 ameliorates colitis-related lung injury in mice by modulating short-chain fatty acid production and inflammatory monocytes/macrophages. Food Funct. 2023;14(2):1099–1112. doi:10.1039/d2fo03374g
204. Tian X, Hellman J, Horswill AR, Crosby HA, Francis KP, Prakash A. Elevated Gut Microbiome-Derived Propionate Levels Are Associated With Reduced Sterile Lung Inflammation and Bacterial Immunity in Mice. Front Microbiol. 2019;10:159. doi:10.3389/fmicb.2019.00159
205. Wang Y-H, Yan -Z-Z, Luo S-D, et al. Gut microbiota-derived succinate aggravates acute lung injury after intestinal ischaemia/reperfusion in mice. Eur Respir J. 2023;61(2):2200840. doi:10.1183/13993003.00840-2022
206. Liu Q, Tian X, Maruyama D, Arjomandi M, Prakash A. Lung immune tone via gut-lung axis: gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am J Physiol Lung Cell Mol Physiol. 2021;321(1):L65–L78. doi:10.1152/ajplung.00421.2020
207. Golomb SM, Guldner IH, Zhao A, et al. Multi-modal Single-Cell Analysis Reveals Brain Immune Landscape Plasticity during Aging and Gut Microbiota Dysbiosis. Cell Rep. 2020;33(9):108438. doi:10.1016/j.celrep.2020.108438
208. Pu Q, Lin P, Gao P, et al. Gut Microbiota Regulate Gut-Lung Axis Inflammatory Responses by Mediating ILC2 Compartmental Migration. J Immunol. 2021;207(1):257–267. doi:10.4049/jimmunol.2001304
209. Erttmann SF, Swacha P, Aung KM, et al. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. 2022;55(5):847–861.e10. doi:10.1016/j.immuni.2022.04.006
210. Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human Gut Microbiota and Gastrointestinal Cancer. Genomics Proteomics Bioinf. 2018;16(1):33–49. doi:10.1016/j.gpb.2017.06.002
211. Zhu Y, Mei Q, Fu Y, Zeng Y. Alteration of gut microbiota in acute pancreatitis and associated therapeutic strategies. Biomed Pharmacother. 2021;141:111850. doi:10.1016/j.biopha.2021.111850
212. Mei Q-X, Hu J-H. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol Sin. 2021;42(6):942–953. doi:10.1038/s41401-020-00581-5
213. W-J G, Liu J-C. Probiotics in patients with severe acute pancreatitis. Crit Care. 2014;18(4):446. doi:10.1186/cc13968
214. Lau HCH, Sung JJ-Y, Yu J. Gut microbiota: impacts on gastrointestinal cancer immunotherapy. Gut Microbes. 2021;13(1):1–21. doi:10.1080/19490976.2020.1869504
215. Dong J, Ping L, Cao T, et al. Immunomodulatory effects of the Bifidobacterium longum BL-10 on lipopolysaccharide-induced intestinal mucosal immune injury. Front Immunol. 2022;13:947755. doi:10.3389/fimmu.2022.947755
216. Mandelbaum N, Zhang L, Carasso S, et al. Extracellular vesicles of the Gram-positive gut symbiont Bifidobacterium longum induce immune-modulatory, anti-inflammatory effects. NPJ Biofilms Microbiomes. 2023;9(1):30. doi:10.1038/s41522-023-00400-9
217. Zhang M, Zheng Y, Sun Z, et al. Change in the Gut Microbiome and Immunity by Lacticaseibacillus rhamnosus Probio-M9. Microbiol Spectr. 2023;11(2):e0360922. doi:10.1128/spectrum.03609-22
218. Xie Z, Li M, Qian M, Yang Z, Han X. Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients. 2022;14(21):4475. doi:10.3390/nu14214475
219. Stoeva MK, Garcia-So J, Justice N, et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021;13(1):1–28. doi:10.1080/19490976.2021.1907272
220. van Minnen LP, Timmerman HM, Lutgendorff F, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141(4):470–480. doi:10.1016/j.surg.2006.10.007
221. Muftuoglu MAT, Isikgor S, Tosun S, Saglam A. Effects of probiotics on the severity of experimental acute pancreatitis. Eur J Clin Nutr. 2006;60(4):464–468. doi:10.1038/sj.ejcn.1602338
222. Lutgendorff F, Trulsson LM, van Minnen LP, et al. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1111–G1121. doi:10.1152/ajpgi.00603.2007
223. Horst NL, Marques RG, Diestel CF, et al. Effects of probiotic supplementation on markers of acute pancreatitis in rats. Curr Ther Res Clin Exp. 2009;70(2):136–148. doi:10.1016/j.curtheres.2009.04.004
224. Mundi MS, Shah M, Hurt RT. When Is It Appropriate to Use Glutamine in Critical Illness? Nutr Clin Pract. 2016;31(4):445–450. doi:10.1177/0884533616651318
225. Jabłońska B, Mrowiec S. Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards. Nutrients. 2021;13(5):1498. doi:10.3390/nu13051498
226. Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10(11):1564. doi:10.3390/nu10111564
227. Dong S, Zhao Z, Li X, Chen Z, Jiang W, Zhou W. Efficacy of Glutamine in Treating Severe Acute Pancreatitis: a Systematic Review and Meta-Analysis. Front Nutr. 2022;9:865102. doi:10.3389/fnut.2022.865102
228. Wu D, Su S, Zha X, et al. Glutamine promotes O-GlcNAcylation of G6PD and inhibits AGR2 S-glutathionylation to maintain the intestinal mucus barrier in burned septic mice. Redox Biol. 2023;59:102581. doi:10.1016/j.redox.2022.102581
229. Lu T, Li Q, Lin W, et al. Gut Microbiota-Derived Glutamine Attenuates Liver Ischemia/Reperfusion Injury via Macrophage Metabolic Reprogramming. Cell Mol Gastroenterol Hepatol. 2023;15(5):1255–1275. doi:10.1016/j.jcmgh.2023.01.004
230. Weimann A, Felbinger TW. Gastrointestinal dysmotility in the critically ill: a role for nutrition. Curr Opin Clin Nutr Metab Care. 2016;19(5):353–359. doi:10.1097/MCO.0000000000000300
231. Newberry C, Schucht J. Use of Enteral Nutrition for Gastrointestinal Bleeding Prophylaxis in the Critically Ill: review of Current Literature. Curr Nutr Rep. 2018;7(3):116–120. doi:10.1007/s13668-018-0232-3
232. Zhang -M-M, Cheng J-Q, Y-R L, Zhai H-J, Chen Y-N, X-T W. Effects of enteral nutrition and parenteral nutrition on gut epithelial tight junction and barrier function in rats after surgical stress. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40(4):615–618.
233. Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3(1):99–108. doi:10.1016/1074-7613(95)90162-0
234. Wan X, Bi J, Gao X, et al. Partial Enteral Nutrition Preserves Elements of Gut Barrier Function, Including Innate Immunity, Intestinal Alkaline Phosphatase (IAP) Level, and Intestinal Microbiota in Mice. Nutrients. 2015;7(8):6294–6312. doi:10.3390/nu7085288
235. Zhang J, W-Q Y, Wei T, et al. Effects of Short-Peptide-Based Enteral Nutrition on the Intestinal Microcirculation and Mucosal Barrier in Mice with Severe Acute Pancreatitis. Mol Nutr Food Res. 2020;64(5):e1901191. doi:10.1002/mnfr.201901191
236. Fan J, Meng Q, Guo G, et al. Effects of early enteral nutrition supplemented with arginine on intestinal mucosal immunity in severely burned mice. Clin Nutr. 2010;29(1):124–130. doi:10.1016/j.clnu.2009.07.005
237. Nakashima I, Horibe M, Sanui M, et al. Impact of Enteral Nutrition Within 24 Hours Versus Between 24 and 48 Hours in Patients With Severe Acute Pancreatitis: a Multicenter Retrospective Study. Pancreas. 2021;50(3):371–377. doi:10.1097/MPA.0000000000001768
238. Yang C, Wang T, Chen J, et al. Traditional Chinese Medicine Formulas Alleviate Acute Pancreatitis: pharmacological Activities and Mechanisms. Pancreas. 2021;50(10):1348–1356. doi:10.1097/MPA.0000000000001931
239. Wei T-F, Zhao L, Huang P, et al. Qing-Yi Decoction in the Treatment of Acute Pancreatitis: an Integrated Approach Based on Chemical Profile, Network Pharmacology, Molecular Docking and Experimental Evaluation. Front Pharmacol. 2021;12:590994. doi:10.3389/fphar.2021.590994
240. Yang D-Y, Duan S-B, Aili J-T. Effect of qingyi decoction in treating severe acute pancreatitis and its impacts on blood level of tumor necrosis factor-alpha, interleukin-6 and interleukin-8. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29(12):1122–1124.
241. Chen W, Yang X, Huang L, et al. Qing-Yi decoction in participants with severe acute pancreatitis: a randomized controlled trial. Chin Med. 2015;10:11. doi:10.1186/s13020-015-0039-8
242. Ji CH, Tang CW, Feng WM, Bao Y, Yao LQ. A Chinese Herbal Decoction, Huoxue Qingyi Decoction, Promotes Rehabilitation of Patients with Severe Acute Pancreatitis: a Retrospective Study. Evid Based Complement Alternat Med. 2016;2016:3456510. doi:10.1155/2016/3456510
243. Li J, Zhang S, Zhou R, Zhang J, Li Z-F. Perspectives of traditional Chinese medicine in pancreas protection for acute pancreatitis. World J Gastroenterol. 2017;23(20):3615–3623. doi:10.3748/wjg.v23.i20.3615
244. Wang G-Y, Shang D, Zhang G-X, et al. Qingyi decoction attenuates intestinal epithelial cell injury via the calcineurin/nuclear factor of activated T-cells pathway. World J Gastroenterol. 2022;28(29):3825–3837. doi:10.3748/wjg.v28.i29.3825
245. Zhang J-W, Zhang G-X, Chen H-L, et al. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World J Gastroenterol. 2015;21(12):3537–3546. doi:10.3748/wjg.v21.i12.3537
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.