Back to Journals » Drug Design, Development and Therapy » Volume 16
Integrated Network Pharmacology and Metabolomics Analysis to Reveal the Potential Mechanism of Siwu Paste on Aplastic Anemia Induced by Chemotherapy Drugs
Authors He D , Dan W, Du Q, Shen BB, Chen L, Fang LZ, Kuang JJ, Tang CY, Cai P, Yu R, Zhang SH, Huang JH
Received 9 July 2021
Accepted for publication 9 March 2022
Published 28 April 2022 Volume 2022:16 Pages 1231—1254
DOI https://doi.org/10.2147/DDDT.S327433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Dan He,1 Wan Dan,1 Qing Du,1 Bing-Bing Shen,1 Lin Chen,1 Liang-zi Fang,1 Jian-Jun Kuang,1 Chun-yu Tang,2 Ping Cai,1 Rong Yu,1,3 Shui-han Zhang,1 Jian-hua Huang1,3
1Hunan Academy of Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, 410013, People’s Republic of China; 2Hunan Times Sunshine Pharmaceutical Co., Ltd., Changsha, Hunan, 425007, People’s Republic of China; 3Hunan Key Laboratory of TCM Prescription and Syndromes Translational Medicine Hunan, Changsha, Hunan, 410208, People’s Republic of China
Correspondence: Shui-han Zhang; Jian-hua Huang, Hunan Academy of Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, 410013, People’s Republic of China, Tel +86 13637400650 ; +86 18692265317, Email [email protected]; [email protected]
Purpose: This study aimed to reveal the multicomponent synergy mechanisms of SWP based on network pharmacology and metabolomics for exploring the relationships of active ingredients, biological targets, and crucial metabolic pathways.
Materials: Network pharmacology, including TRRUST, GO, and KEGG, enrichment was used to discover the active ingredients and potential regulation mechanisms of SWP. LC-MS and multivariate data analysis method were further applied to analyze serum metabolomics profiling for discovering the potential metabolic mechanisms of SWP on AA induced by Cyclophosphamide (CTX) and 1-Acetyl-2-phenylhydrazine (APH).
Results: A total of 27 important bioactive ingredients meeting the ADME (absorption, distribution, metabolism, and excretion) screening criteria from SWP were selected. Interaction networks were constructed and validated based on the 10 associated ingredients with the relevant targets. A total of 125 biomarkers were found by Metabolomics approach, which associated with the development of AA, mainly involved in amino acid metabolism and lipid metabolism. While SWP can reverse the above 12 metabolites changed by AA. Network analysis revealed the synergistic effects of SWP through the 43 crucial pathways, including Sphingolipid signaling pathway, Sphingolipid metabolism, Arginine and proline metabolism, VEGF signaling pathway, Estrogen signaling pathway.
Conclusion: The study suggested that SWP is a useful alternative for the treatment of AA induced by CTX + APH. Its potential mechanisms are to improve hematopoietic microenvironment and promote bone marrow hematopoiesis therapies.
Keywords: aplastic anemia, Siwu Paste, network pharmacology, metabolomics, integration analysis
Introduction
Aplastic anemia (AA), a bone marrow hematopoietic failure (BMF) syndrome, is characterized by attenuated bone marrow hematopoietic cell proliferation, decreased peripheral whole blood cell and immune dysfunction.1 It has high prevalence in the Asian population, especially young adults.2 Chemotherapy is the most common cause of AA. Although the immunosuppressive therapy provide an strategy for patients who are not suitable for allogeneic hematopoietic stem cell transplant, some patients after treated with a single therapeutic method show relapse or poor efficacy.3,4 Therefore, expanding an alternative and more effective therapy for AA is needed. Moreover, the pathogenesis of AA induced by chemotherapy drugs is complex, and combination therapy may supply better service for it than monotherapy.
Siwu Paste (SWP), a popular prescription for tonifying blood, has over 1000 years history in the clinical application of Traditional Chinese Medicine (TCM). It described as good effects in promoting blood circulation, ameliorating anemia and pain, with the feature of, including four herbs: Angelica sinensis (Oliv.) Diels (Dang Gui, DG), Ligusticum chuanxiong Hort. (Chuan Xiong, CX), Cynanchum otophyllum Schneid.(Bai Shao, BS) and Rehmanniae radix preparata (Shu Di Huang, SDH), in the ratio of 10:6:10:15, respectively.5 Our previous research suggested that SWP may alleviates hematopoietic functions via “multi-component and multichannel”, such as regulating the bone marrow hematopoiesis, improving blood rheology, inhibiting inflammation and exerting estrogen-like effects.6,7 Compared with other manners, paste is more convenient to carry. And it has better treatment compliance and lower economic burden in treatment of AA induced by chemotherapy drugs. Moreover, SWP at dose of 22.68g/kg has similar efficacy as Fu-Fang E’jiao Jiang.6 Although, the efficacy of SWP has been demonstrated, the potential mechanism of therapeutic effects of SWP on AA remains puzzled. It limits the application of SWP in experiment as well as clinic. Thus, a comprehensive study is necessary for discovering the relationships among the active ingredient, potential targets, and their molecular mechanisms of SWP in treating AA.
As a promising approach to discover TCM, network pharmacology links the chemical space of drugs to the biomolecular pathways of disease through network footprints. Li et al, conducted a network pharmacology analysis of Danggui Buxue Decoction and they found that the four chemical components (Z-ligustilide, E-butylidenephthalide, Z-butylidenephthalide and ferulic acid) played a major role in the blood enrichment effect.8 Wang et al, have demonstrated that tetramethylpyrazine and paeoniflorin enable the vessel to sprout by up-regulating ESRα mRNA expression.9 These studies provide a better basis for our understanding of AA, but network analysis alone cannot truly reflect the impact of multi-component SWP on the occurrence and development of AA. As a systematic method, metabolomics provides a series of particular and sensitive markers for disease diagnosis and drug treatment. The differences between AA patients and healthy, and the therapeutic effects of TCM on AA were studied. Zhong et al, analyzed the difference of serum metabolites between 40 healthy control and 40 AA patients. Comparing with the control groups, they found that the levels of aminoacyl-tRNA biosynthesis, ATP binding cassette transporters, and amino acid biosynthesis were increased markedly in AA, while the TCA cycles was decreased dramatically.10 Liet al, employed GC/MS technologyTechnology to provide an evidence that Angelica sinensis intervene 5 metabolic pathways (Arachidonic acid metabolism, citrate cycle (TCA), glycine, serine and threonine metabolism, arginine and L-proline metabolism and valine, leucine and isoleucine biosynthesis) to repair the metabolic disorders resulted from AA.11 He et al, speculate that SWP down-regulate the levels of 3 amino acids (L-Methionine, L-Tyrosine, and L-Phenylalanine) in plasma metabolites compared with AA model group, but it up-regulate the Hippuric acid, Deoxycholic acid and Sphingosine-1-phosphate which are potential therapeutic pathway for AA.12 Thus, integrated network pharmacology and metabolomics Analysis may provide us an in-depth understanding for SWP in treating AA.
Our study was the first to identify the combinational rules of SWP from molecular and systematic level by integrated network pharmacology and metabolomics analysis, along with pharmacology experiments, to elucidate its therapeutic mechanism for AA induced by chemotherapy drugs.
Materials and Methods
Equipment, Chemicals and Reagents
The SWP products were generously provided by Hunan Jiudian Pharmaceutical Co., Ltd. (Hunan, China). In order to obtain reliable experiment results, the preparation and quality control (QC) of SWP were monitored according to our previous research.6 2-Chloro-L-phenylalanine, as internal standards for metabolomics analysis, was purchased from Shanghai HC Biotech Co., Ltd. (Shanghai, China) and Avanti (USA) respectively. Q-Exactive LC-MS/MS (Thermo Fisher Scientific) Acetonitrile, methanol, formic acid and LC-MS grade water were purchased from CNW Technologies GmbH (Düsseldorf, Germany).
Animal
Our tests adhere to the Chinese national laws and local guidelines. Animal experiment has been authorized by the Animal Ethics Committee at Hunan Academy of Chinese Medicine (No.20190030). Healthy Male Sprague Dawley (SD) rat (8 weeks old, weighing 180–220 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd (SCXK 2016–0002). Before the experiment, 30 rats were randomly divided into 3 groups, 10 rats in each group: control group, model group, and SWP group (SWP). During the experiment, all animals were access to food and water freely, with standard surroundings: 12 h light and shade cycle, 50–55% humidity and temperature at 20–22°C.
In this section, the model of aplastic anemia was established on the basis of previous study.6 The model group and SWP group were intraperitoneal injected with Cyclophosphamide (CTX) (Baxter International Co., Ltd., Illinois, USA) and 1-Acetyl-2-phenylhydrazine (APH) (Meilun Biotechnology Co., Ltd., Dalian, China), while the control group received an equal volume of phosphate-buffered saline. Additionally, the SWP group was orally administered 22.68g/kg/d SWP, whereas the control and model groups obtained an equal amount of distilled water, lasted for 14 days.
Peripheral Blood Routine and Histological Analysis
After 14 days, rats were sacrificed under anesthesia and blood sample was collected through the abdominal aorta. The peripheral blood (PB) routine test, including white blood cell count (WBC/109·L−1), red blood cell count (RBC/1012·L−1), hemoglobin (HGB/g·L−1), haematocrit (HCT/%), and platelet count (PLT/109·L−1), was applied to evaluate the quantity of blood cells and to diagnosis conditions of blood.13
HE staining of femur was also employed to evaluate the medullary hematopoiesis function and the changes of hematopoietic microenvironment.14 Specific methods are as follows: the fixed femur (decalcified in 5.5g EDTA-2K + 100 mL 4% paraformaldehyde for 20 days in 4°C prior to the following experiments) were embedded in paraffin, and sectioned at 5 mm followed by staining with hematoxylin/eosin. Olympus BH-2 light microscope was used to observe the stained sections of femur tissue (Tokyo, Japan).
Network Pharmacology Prediction
Compound Data Preparation and ADME Screening
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (http://lsp.nwu.edu.cn/tcmsp.php, Ver.2.3), and Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine (BATMAN) (http://bionet.ncpsb.org/batman-tcm/, updated January 2016)15,16 were used to select all candidate compounds of four herbs in SWP. In order to obtain bioactive components contributing to its therapeutic effect, all selected ingredients should meet the principle of ADME: 1. Oral bioavailability (OB) ≥30%, 2. Drug-likeness (DL ≥ 0.18.17 In addition, a widely scale data-mining strategy was adopted to replenish some bioactive ingredients, while those with poor pharmacological properties and bad drug ability compounds were removed.18
Targets Fishing and Network Construction
PharmMapper server (http://lilab-ecust.cn/pharmmapper/, updated April 10, 2019) was employed to discover the potential targets of SWP bioactive components.19 We gathered the AA-related targets from following database under the operating condition of “aplastic anemia” and “Homo sapiens”: ①GeneCards (https://www.genecards.org/, Ver. 5.0),20 ②DisGeNET (https://www.disgenet.org/, Ver. 6.0),21③OMIM (http://www.omim.org/, updated Jun 27, 2017)22 ④TTD (http://db.idrblab.org/ttd/, updated Jan 8, 2020).23 Herb-Compounds-Target-Pathway network of SWP was displayed by a visualization tool named Cytoscape (ver.3.7.0).24 The STRING (https://string-db.org/, ver. 11.0) database was employed to predict the protein-protein interaction (PPI) in SWP for treating AA, and the operating conditions were “Homo sapiens” and correlation ≥ 0.7.
GO and KEGG Enrichment Analysis for Targets
To investigate the potential biological pathways and critical targets, our study analyzed the PPI targets with Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Ontology (GO), and TRRUST (https://www.grnpedia.org/trrust/, ver.2).25 The terms of GO or KEGG with p-value ≤ 0.05 were finally screened out for the research.
Metabolomics Analysis
Collection and Preparation of Serum Sample
The following methods were employed to prepare serum samples: 100 μL aliquot of serum sample were taken from the −80°C refrigerator, thawed at 4°C for 30 minutes. To remove the protein in the sample, 10 μL internal standard (0.3 mg/mL 2-chloro-l-phenylalanine was dispersed in 300 μL ice-cold mixture contented methanol and acetonitrile (2/1, v/v). Then they were vortexed for one minute. Subsequently, the mixtures were handled with concentration under 13,000 rpm and 4 °C for 15 min. 400 μL of the clear supernatant was evaporated, reconstituted with 200 μL of 20% methanol, vortexed for 30s, and sonicated for 2 min. After centrifugation for 10 minutes (13,000 rpm, 4 °C), the supernatant was filtered through a 0.22 μm microfilter and transferred to LC vial. Finally, 10 μL of the supernatant was taken for LC-MS test. Equal volume samples from each group were mixed as Quality Control (QC) in our experiment. Aim to ensure the stability of the samples, the above samples were stored at 4 °C during the LC/MS experiment.
UPLC-Q-TOF/MS Analysis
An ACQUITY UPLC BEH C18 column (100 mm×2.1 mm, 1.7 um, Waters, USA) were used for non-targeted metabolites’ study to analyze the metabolic profiling under both positive and negative ESI ion modes. Water (containing 0.1% formic acid, v/v) (A) and methanol (B) formed the gradient elution, which procedure was optimized as follows: 0.01 min, 5% B; 1.5 min, 5% B; 3 min, 30% B; 7 min, 60% B; 9min, 90% B; 11 min, 100% B; 12 min, 100% B; 15 min, 5% B. The total procedure time was 15 min, the injection volume was 10 μL, the flow rate was 0.35 mL/min, and the column temperature was 45 °C. The following are the instrument parameters for this experiment: 320°C (+) and 320 °C (−) for ion source temperature; 3500 V (+) and 3100 V (−) for ion spray voltage; 30 PSI for curtain gas; 60–900 (+) and 60–900 (−) for mass scan; 30 eV for collision energy.
Identification and Quantitative Analysis
In the study, progenesis QI software (Waters Corporation, Milford, USA) was employed to perform standardized preprocessing of metabolomics data.26 Subsequently, the acceptable ranges of Precursor, fragment and retention time are set as 5 ppm, 10 ppm and 0.02 min, respectively. In order to obtain reliable peak information, we exclude the interference of isotopes, internal standard and noise, and limit the minimum intensity of chromatographic peak to 15% of the base peak intensity. The peaks will be eliminated when their ion intensity equal to 0 exists in more than 60% of samples. The retention time, m/z and MS/MS information are used to automatically match the metabolite information of human metabolite database (HMDB), lipid maps (v2.3) and metlin database to identify the chromatographic peak. Finally, the endogenous metabolites with P < 0.05 were considered as the differential metabolites to distinguish SWP, model and control group after Student’s test.
Target Verification of SWP in Treating AA
Virtual Docking
To decrease the complexity and improve the accuracy of the constituent-target network, Molecular Operating Environment (MOE 2015.10 software) was further employed in our study to validate the interaction between compounds and critical targets, according to previous studies.17
Elisa
Serum was obtained from the supernatant of blood sample after centrifugation at 4 °C. The levels of serum SPHK-1 (NO. 1229A15), PLD1 (NO. 1228A11), and PLD2 (NO. 1228A09) were monitored by ELISA kits according to the instructions of the manufacturer. All of the ELISA kits were purchased from ZCIBIO Technology Co., Ltd. (Shanghai, China).
Western Blot Analysis
Bone marrow cells (BMCs) were obtained for Western blot (WB) analysis. BMCs were washed out from rat femurs and tibiae using a 5 mL syringe with PBS (prechilled overnight at 4 °C). The suspension was then centrifuged at 3000 rpm under 4 °C for 15 min. The precipitate was collected, quenched in liquid nitrogen and stored at - 80 °C until analysis. WB analysis was performed as accordance with previous report.27 In brief, BMCs sample were homogenized on ice in RIPA lysis buffer (Beyotime Biotechnology, China). After the above mixture was centrifuged, the supernatant was collected. Membranes were blotted with primary antibodies, anti-rat p-STAT1 (1:500 dilution), anti-rat GRB2 (1:1000 dilution) or anti-rat GSPT1 (1:1000 dilution), overnight at 4°C. Then incubated with horseradish peroxidase bound secondary antibody for 2 hours. Antibodies p-STAT1 (NO. YP0249), and GSPT1 (NO. YT1619) were obtained from ImmunoWay Biotechnology Company (USA). Antibodies GRB2 (NO. ba32111) were purchased from Abcam (UK). Immunoreactive proteins were detected by ELX800 and quantified by ClinxChemiScope 6000 System.
Multivariate Statistical and Pathway Analysis
SPSS 23.0 software was used to analyze the experimental data. An analysis of variance (ANOVA) is performed to obtain statistical significance for comparisons between multiple parameters. The LSD test is performed under the assumption of equal variances; otherwise, Dunnett’s T3 test is carried out to data with unequal variances. Statistical significance is established at P < 0.05 or P < 0.01. We tested the normality of the data. If the data fit the normal distribution, we did homogeneity analysis of variance, otherwise we did Kruskal–Wallis test and Dunn’s test. Normally distributed data are displayed by 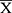 ± SD or
± SD or 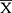 ± SEM, while skewed data are presented as median (min-max). According to our previous study, random forest and SIMCA software package (version 14.0, Umetrics, Umeå, Sweden) was used to observe the metabolic profiles and differential metabolites in different experimental groups under positive and negative mode. As described in previous studies, differential metabolites were sent to Metaboanalyst 4.0 for pathway enrichment analysis and pathway topological analysis.28
± SEM, while skewed data are presented as median (min-max). According to our previous study, random forest and SIMCA software package (version 14.0, Umetrics, Umeå, Sweden) was used to observe the metabolic profiles and differential metabolites in different experimental groups under positive and negative mode. As described in previous studies, differential metabolites were sent to Metaboanalyst 4.0 for pathway enrichment analysis and pathway topological analysis.28
Results and Discussion
Therapeutic Effect of Siwu Paste on AA Induced by CTX + APH
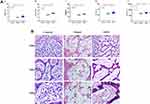
The results of peripheral blood (PB) and Histological examination indicated that we not only successfully established the aplastic anemia model, but also proved that SWP at the dose of this study had a positive effect on AA. Contrasted with control group, the level of WBC, RBC, HCT and PLT in AA model group was reduced obviously (P < 0.01). The above indices in SWP increased significantly compared with model group (P < 0.05, P < 0.01), suggesting that treatment of SWP could relieve the blood deficiency in AA rats. The details are displayed in Figure 1A and Figure S1.
HE staining clearly shown the structure of periosteum, marrow cavity and cartilage tissue in control group, and abundant cellular components (granulocytes, megakaryocytes, nucleated cells, etc.) were observed in the medullary cavity. However, AA rats had serious medullary cavity injury, including edema of vascular fibrosis and significant fibrosis. After the treatment of SWP, the pathological condition of femur was improved significantly. The myelofibrosis, medullary cavity edema and fat cells were inhibited efficiently in SWP group. It was also observed that the proliferation of nucleated cells increased actively, and the number of erythrocytes in parenchyma vessels increased (Figure 1B). Overall, all the investigated parameters were changed significantly in AA rats, but they were restored by the SWP treatments.
The pharmacological study displayed the improving anemia and recovering hematopoietic functions effects of SWP treatment on AA animals, that were conformed to previous research.6,7 It suggests that SWP may be an alternative manner for AA induced by CTX + APH.
Network Pharmacology Analysis
Identification of Siwu Paste’s Ingredients and Target Genes
To explore its specific mechanism of anti-anemia, network pharmacology analysis was performed. 230 ingredients were identified in SWP, containing 88 in Angelicae Sinensis Radix, 40 in Rehmanniae radix preparata, 81 in Ligusticum chuanxiong Hort., and 41 in Cynanchum otophyllum Schneid., and some repeating compounds were removed (Table S1). 19 active ingredients were screened from above library by ADEM criteria. According to the literature, eight compounds (Ferulic Acid, vanillin, senkyunolide-C, senkyunolide-D, senkyunolide-E, senkyunolide-A, cis-ligustilide, and senkyunolide-B) were considered to have therapeutic potential for AA, so even if they are below the standard, they are still selected for further study.8,9 Table 1 provides detailed information on 27 potentially bioactive ingredients. 27 components were imported into the Pharmmapper database in SDF format for target prediction, and their norm fitting values were used for sorting. A total of 317 targets were screened out from the top 100 of each compound. Matching 317 targets with the AA-related targets acquired from GeneCards, DisGeNET, OMIM, and TTD, our study finally identified 64 common targets which considered as the potential gene for SWP in treating AA (Table 2, Figure 2A).
 |  |  |  |  |  |
Table 1 A List of the Active Ingredients Among the Four Herbal Medicines in SWP for Network Analysis |
 |
Table 2 64 Common Targets from Protein-Protein Interaction Network |
Network Construction and Enrichment Analysis
Protein-Protein (PPI) interaction, GO and KEGG analysis were performed in 64 common targets to investigate target function and the potential mechanism of SWP on AA. A view of PPI network is displayed in Figure 2B. In this network, 7 nodes (containing ALB, AKT1, MAPK1, CASP3, HRAS, CAT, IL-2) with the value of degree > 20, were regarded as potential hub target for SWP to ameliorate the hematopoiesis disorder caused by AA.
As results shown in GO enrichment (Figure 2C), the top 4 enriched terms are positive regulation of biological process (GO:0048518), cellular protein metabolic process (GO:0044267), regulation of metabolic process (GO:0019222), and regulation of catalytic activity (GO:0050790), all of which belonged to Biological Process. Extracellular vesicle (GO:1903561), vesicle (GO:0031982), and cytosol (GO:0005829) with gene number > 7 belong to terms of GO cellular compound. The terms of GO molecular functions mainly included catalytic activity (GO:0003824) and transferase activity, transferring phosphorus-containing groups (GO:0016772). Further TRRUST analysis demonstrates that the 64 targets were primarily regulated by Sp1 transcription factor (SP1, TRR00641), v-rel reticuloendotheliosis viral oncogene homolog A (avian) (RELA, TRR00575), nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (NF-κB1, TRR00452), and jun proto-oncogene (JUN, TRR00323). Those results indicated that SWP may be mainly involved in regulation of metabolic and protein on treating AA.
In our research, there are 12 KEGG pathway (P < 0.05). The top 3 terms are FoxO signaling pathway (map04068), MAPK signaling pathway (map04010), Ras signaling pathway (map04014) which play an important role in the occurrence and development of AA (Figure 2D). A map of the “Herb-Compound-target-pathway” (including 71 nodes and 354 edges) based on KEGG analysis were established to investigate the mechanism of SWP on AA (Figure 2D). In this network, DG was considered as a principal drug herb for regulating the most multiple compounds, which meet the TCM principles that DG is the sovereign drug in SWP. Moreover, we found that Mitogen-activated protein kinase 1 (MAPK1), RAC-alpha serine/threonine-protein kinase (AKT1), Glutathione S-transferase P (GSTP1), signal transducer and activator of transcription 1-alpha/beta (STAT1), growth factor receptor-bound protein 2 (GRB2) with higher degree also act critical targets for regulating or participating in multiple herbs or signaling pathways. Take GRB2 for example, it involves in multiple pathways related to the blood system, such as FoxO signaling pathway, Phospholipase D signaling pathway (map04072), MAPK signaling pathway, Ras signaling pathway.
GRB2, which links IL3 signaling to the ERK/MAPK proliferative pathway, has been reported to play important functions during the HSPC proliferation, survival and expansion.29 STAT1 plays an important role in the regulation of erythropoiesis. Studies have shown that STAT1 deficiency leads to increased red blood cell apoptosis.30 As a stem cell factor (SCF) receptor gene, KIT play a key role in regulating the growth, survival, and differentiation of erythroid progenitors alone, as well as a synergistic effect with erythropoietin (EPO).31,32 Ratajczak et al, demonstrated that the growth of hematopoietic colony would be inhibited by more than 30%, when the mRNA expression of KIT was blocked in bone marrow mononuclear cells (MNC).33 Analysis of miRNA differential gene expression in bone marrow revealed that Ras signaling pathways and Rap1 signaling pathway were the most significantly altered pathways between AA patients and healthy controls.34 The damage of aplastic anemia to bone marrow, HSPC and metabolism is partly due to antigen mediated immune response, which eventually leads to increased oxidative stress or inflammatory response.35–38 It has been recently reported that FoxO signaling-mediated autophagy and regulation of mitochondrial activity are necessary for maintenance of hematopoiesis homeostasis in HSPC metabolic.39–41 Our results suggest that promoting erythropoiesis may be an effective strategy for SWP in the treatment of AA.
Those results provide that the herbal combination may have a great range of possible molecular targets than each individual herb, which might help to explain the synergistic of SWP effects in treatment of AA.17
Metabolic Profiles Analysis
Identification of Differential Metabolites and Multivariate Data Analysis
LC-MS was used to identify endogenous serum metabolic profile between control, model, and SWP group (Figure S2). It can be clearly seen from the random forest method under positive and negative ion modes that the above three groups have been completely separated (Figure 3A). Compared with model group, SWP were closer to control group, as well as the latter one had the trend of regression to the control group. This part indicated that our SWP have the positive regulation on AA. Meanwhile, the QC group was clustered together tightly. It indicated that our metabolic profile is stable and reliable.
In addition, the differences between each two groups were analyzed by orthogonal partial least square-discriminate analysis (OPLS-DA), and the metabolic characteristics of different groups were visualized by SIMCA. In positive (Figure 3B) and negative (Figure 3C) ion modes, there was significant separation between each two groups. If the metabolite meets the conditions of VIP > 1 and P < 0.05, it will be selected as a potential biomarker. Based on positive ions mode, there are 111 (93 up-/18 down-regulation) and 77 (45 up-/32 down-regulation) biomarkers identified respectively between control and model, and model and SWP. Analogously, 40 (28 up-/12 down-regulation) and 28 (3 up-/25 down-regulation) biomarkers were separately identified under negative ion mode in above mentioned groups (Figure 4, Table S2).
 |
Figure 4 Comparison of differentially expressed metabolites levels between control group, mode group and Siwu Paste group in positive (A) and negative-ion (B) modes. |
Metabolic Pathway Analysis
A total of 106 differential metabolites regulated by SWP were further analyzed at Metaboanalyst 4.0 to reveal their functional and metabolic characteristics. 17 metabolic pathways were enriched in our research (Table S3, Figure S3). According to the impact value > 0.1, the top six metabolic pathways, which mainly enriched to lipid metabolism, amino acid, metabolism of terpenoids and polyketides, were obtained (Figure 5). Riboflavin and retinal were involved in Riboflavin metabolism (map00740, impact = 0.5) and Retinol metabolism (map00830, impact = 0.242) respectively. Sphingolipid metabolism (map00600, impact = 0.199) regulated by sphinganine, sphingosine, SM (d18:0/16:1(9Z)), and PE (16:1 (9Z)/P-18:1 (11Z)). Glycerophosphocholine, PC (20:4 (5Z,8Z,11Z,14Z)/P-16:0), PE (16:1 (9Z)/P-18:1 (11Z)), choline, LysoPC (17:0), and LysoPC (24:1 (25Z)) were metabolite nodes in the process of Glycerophospholipid metabolism (map00564, impact = 0.168). PC (20:4 (5Z,8Z,11Z,14Z)/P-16:0) and 15(S)-HPETE were charged in Arachidonic acid metabolism (map00590, impact = 0.1). Niacinamide was participated in the Nicotinate and nicotinamide metabolism (map00760, impact = 0.194). The regulation of SWP on the above 12 metabolites is regarded as a potential biomarker for SWP to reduce AA metabolic disorder (Table 3).
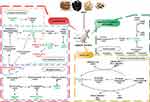 |
Figure 5 The hub metabolic pathway of the Siwu Paste the treatment of aplastic anemia. Red and green represent the up-regulation of metabolites and down-regulation of metabolites, respectively. |
 |
Table 3 12 Differentially Metabolites Analysis of Siwu Paste, Model and Control |
Study testified that Nicotinamide Riboside (NR) and Niacinamide, as nicotinamide adenine dinucleotide (NAD+) dietary supplements, recovery hematopoiesis function in mice suffering from hematological failure through reduced mitochondrial activity within HSPC.42–45 Though products of lipoxygenase in arachidonic acid metabolism are regarded as typical pro-inflammatory, recently study has been proved that 15-HPETE exhibit anti-inflammatory activity by destabilizing TNFmRNA.46,47 However, abnormal elevation of the membrane-located 15(S)-HPETE can trigger greater levels of oxidative damage to the polyunsaturated fatty acid side-chains, witch finally induces the loss of erythrocyte membranes integrity.48 In this study, the serum level of Nicotinamide Riboside, Niacinamide and 15(s)-HPETE in AA model group was significantly higher than that in control group, whereas the levels of LysoPC (17:0), LysoPC (24:1 (25Z) and PE (16:1 (9Z)/ P-18:1 (11Z)) were decreased markedly (P < 0.05). After SWP treatment, the above indexes were significantly restored (P < 0.05). Phosphatidylethanolamine (PE) regarded as a potent anti-inflammatory lipid.49 It has been confirmed that an abundant of lyso-phosphatidylcholine (LPC) such as LysoPC (17:0) and LysoPC (24:1 (25Z) in plasma, contributes to driving the activation of inflammatory responses.50 We speculate that SWP may suppress the inflammation and oxidative damage by restoring the level of PE and LPC and balancing the abnormally increased 15(s)-HPETE under the AA. SWP may also promote the metabolism of Niacinamide for maintenance of adult HSC quiescence. It provides a better hematopoietic microenvironment for promoting hematopoiesis.
Our observations also illustrated that Glycerophospholipid metabolism and Sphingolipid metabolism including PE (16:1 (9Z)/P-18:1 (11Z), Glycerophosphocholine, Sphingosine, Sphinganine, and Sphingosine-1-phosphate, were related to the dysregulation of hematopoietic.51,52 Orsini’s study provides evidence that sphingolipid was participated in TNFα-mediated modulation of TF/miR network and autophagy inhibition in HSPCs, affecting the formation of erythrocytes.53 Sphinganine is a derivative of Sphingosine. The accumulation of them has been verified to suppresses Bcl-xL expression and downregulates Bcl-2 to enhance apoptosis, which accompanied by the strong inhibition of MAPK cascade.54–57 Whatmore, Thiet et al, study indicated that a significantly decrease in plasma S1P level in Major-facilitator superfamily transporter 2b (Mfsd2b) knockout mice would result in damaged membrane of erythrocyte and disordered PB.58 They also found that sphingosine was highly accumulated when the secretion of S1P were block by knockout the Mfsd2b in red blood cell (RBC). On contrary, Sphingosine-1-phosphate (S1P), synthesized by phosphorylation of sphingosine in the presence of sphingosine kinase 1 (SphK1), was shown to have antiapoptotic properties.54 Our and Wątek’s research demonstrate that the levels of Sphingosine and Sphinganine were up-regulated significantly in the model with immune damage mediated by chemotherapeutic drugs, while the concentration of Sphingosine-1-phosphate (S1P) was significantly lower (P < 0.05).59 After the treatment of SWP, the concentration of Sphingosine and Sphinganine was declined significantly compared with model (P < 0.05). The Sphingosine-1-phosphate has elevated tendency after treated with SWP with no significance.
Network Pharmacology and Metabolomics Integration Analysis
To further clarify the molecular mechanism and potential biological pathway of SWP for treatment of AA, we comprehensively analyzed the results of network pharmacology and metabonomics. Firstly, we searched the HMDB database for 12 different metabolites obtained by metabolic pathway analysis, and then acquired the enzymes involved in the regulation of these metabolites. The 64 targets of PPI were then linked to these enzymes through string website. Finally, the network of component target gene enzyme metabolite was constructed (Figure 6). Four hub compounds (cis-ligustilide, paeoniflorin, betulinic acid and senkyunolide-B) screened from the network “Herb-Compound-Target-Pathway” were docked with three key targets (GSTP1, STAT1 and GRB2) selected from the “Metabolites-enzyme-target” network, respectively.
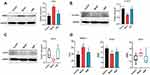
The three-dimensional structures of proteins 4K2R, 3GUR and 4U2X were obtained by searching GSTP1, GRB2 and STAT1 from PDB protein database. All of the docking scores were less than −3.8, which indicated that the proteins shown well binding activity (Table S4, Figure 7). The docking results are shown in Figure 7. It suggests that paeoniflorin, betulinic acid and senkyunolide-B may be involved in the regulation of GSTP1, STAT1 and GRB2 to support SWP in the treatment of AA caused by chemotherapy drugs. Western blot was also applied to further validate the results of virtual docking. In our study, compared with the control group, the protein expression of p-STAT1 (p <0.05) and GSTP1 (p <0.01) have been significant decreased by CTX-treatment, while the protein expression of GRB2 (p <0.01) increased significantly (Figure 8 and Figure S4). After treated with SWP, p-STAT1 protein was significantly increased (p <0.05) and GRB2 was obviously declined (p <0.05) as compared to model group.
Compared with control group, model group developed higher serum levels of SPHK-1 (p < 0.001) and PLD1 (p < 0.01), while SWP obviously reduced above indexes (p < 0.01, 0.05) (Figure 8, Figure S5). Additionally, in contrast to control group, the serum levels of PLD2 were significantly decreased in model group (p < 0.01). After SWP intervention, the serum PLD2 were elevated as compared to model group (p < 0.05). Although the role of PLD in AA remains unclear, our study suggests that PLD expression is disrupted in chemotherapy-induced AA and that SWP reverses this imbalance. In addition, it is partly consistent with the prediction of our networked pharmacology combined with metabolomics.
Although we have examined the possible role of SWP in treating AA induced by CTX + APH, there are some limitations in the present study. Due to the limitation of the sample size, we cannot estimate the efficacy of SWP in large samples. Therefore, it is necessary to enlarge the sample size in future experiments. In addition, the potential targets and biological pathways predicted by network pharmacology combined with metabolomics methods need to be validated by more in vitro experiments. Targeting lipid metabolism is also the direction we need to work on in the next step.
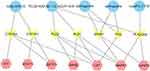 |
Figure 6 “Metabolites-enzyme-target network”: Blue circles, pink hexagon, and yellow represent metabolites, target and enzyme, respectively. |
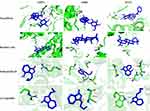 |
Figure 7 Virtual docking based on bioactive ingredients of Siwu Paste and therapeutic targets of Aplastic Anemia. |
Conclusion
In summary, our research confirmed the positive effect of SWP on aplastic anemia (AA), and enriched the possible pathway of SWP via network pharmacology and metabonomics analysis. In the component-target and PPI network, we identified 27 active compounds from SWP and 64 common proteins associated with AA. Furthermore, MAPK1, CASP3, STAT1, GRB2 and KIT were identified as the hub targets for SWP in treating AA. Metabolomics was employed to characterize the metabolic profiles of APH together with CTX-induced AA rats, attending to identified the endogenous markers that drive molecular mechanisms. SWP improved inflammation and oxidative damage of hematopoietic microenvironment predominantly by significantly down-regulated the elevated levels of 15(s)-HPETE, as well as dramatically up-regulated the levels of LysoPC (17:0), LysoPC (24:1 (25Z) and PE (16:1 (9Z)/P-18:1 (11Z)). Most importantly, SWP effected hematopoietic by declined the accumulation of Niacinamide, Sphingosine and Sphinganine. The alter of these metabolites mainly involve in Sphingolipid metabolism, Glycerophospholipid metabolism, Arachidonic acid metabolism, Nicotinate and nicotinamide metabolism. Although we have examined the possible role of SWP in treating AA, there are some limitations in the present study. Further vitro and vivo experimental validation will be involved in our next step study.
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 81673585, No. 81874493, and No. 81573956); Training Program for Excellent Young Innovators of Changsha (kq1802017); Key Research and Development Project of Changsha Science and Technology (kq1901067); Science Foundation of Hunan Province (2019JJ50345, 2020JJ5325, and 2021168); Program of Survey of Chinese Medicines of China ([2017]66); Research on the Comprehensive Development and Utilization of Characteristic Traditional Chinese Medicine Resources (2060302); Key Research and Development Project of Hunan Province Science and Technology (2020SK2029, 2020SK2101, 2022ZYC024), and Project of Changsha Technology Innovation Center (kh2004018); and the authors are thankful for the support of Hunan Province traditional Chinese medicine preparation and quality traceability engineering and technology center and 2011 Collaboration and Innovation Center for Digital Chinese Medicine in Hunan.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content (including the work of network pharmacology and metabonomic analysis); agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Chun-yu Tang is an employee of Hunan Times Sunshine Pharmaceutical Co., Ltd. The authors declare no other potential conflicts of interest for this work.
References
1. Cermak J. Aplastic anemia. Vnitr Lek. 2018;64(5):501–507. doi:10.36290/vnl.2018.070
2. Ahmed P, Chaudhry QUN, Satti TM, et al. Epidemiology of aplastic anemia: a study of 1324 cases. Hematology. 2020;25(1):48–54. doi:10.1080/16078454.2019.1711344
3. Cabannes-Hamy A, Boissel N, Peffault de Latour R, et al. The effect of age in patients with acquired aplastic anaemia treated with immunosuppressive therapy: comparison of adolescents and young adults with children and older adults. Br J Haematol. 2018;183(5):766–774. doi:10.1111/bjh.15650
4. Pierri F, Dufour C. Management of aplastic anemia after failure of frontline immunosuppression. Expert Rev Hematol. 2019;12(10):809–819. doi:10.1080/17474086.2019.1645003
5. He R, Wang HJ, Ying Z, et al. Siwu decoction improves iron deficiency anemia in infant rats and regulates iron metabolism. China J Chin Mater Med. 2017;42(5):944–950. doi:10.19540/j.cnki.cjcmm.20170121.012
6. Du Q, He D, Zeng HL, et al. Siwu Paste protects bone marrow hematopoietic function in rats with blood deficiency syndrome by regulating TLR4/NF-κB/NLRP3 signaling pathway. J Ethnopharmacol. 2020;262:113160. doi:10.1016/j.jep.2020.113160
7. He D, Wan D, Shu J, et al. Study progress on compounds, pharmacological action and clinical application of Siwu Decoction. Pharmacol Clin Chin Mater Med. 2020;36(6):221–229.
8. Shi XQ, Yue SJ, Tang YP, et al. A network pharmacology approach to investigate the blood enriching mechanism of Danggui buxue Decoction. J Ethnopharmacol. 2019;235:227–242. doi:10.1016/j.jep.2019.01.027
9. Wang Y, Guo G, Yang BR, et al. Synergistic effects of Chuanxiong-Chishao herb-pair on promoting angiogenesis at network pharmacological and pharmacodynamic levels. Chin J Integr Med. 2017;23(9):654–662. doi:10.1007/s11655-017-2408-x
10. Zhong P, Zhang J, Cui X. Abnormal metabolites related to bone marrow failure in aplastic anemia patients. Genet Mol Res. 2015;14(4):13709–13718. doi:10.4238/2015.October.28.33
11. Li PL, Sun HG, Hua YL, et al. Metabolomics study of hematopoietic function of Angelica sinensis on blood deficiency mice model. J Ethnopharmacol. 2015;166:261–269. doi:10.1016/j.jep.2015.03.010
12. He Y, Gao T, Li J, et al. Metabonomics study on the effect of Siwu Decoction for blood deficiency syndrome in rats using UPLC-Q/TOF-MS analysis. Biomed Chromatogr. 2019;33(11):e4617. doi:10.1002/bmc.4617
13. Válka J, Čermák J. Differential diagnosis of anemia. Vnitr Lek. 2018;64(5):468–475. doi:10.36290/vnl.2018.067
14. Marchesi RF, Velloso E, Garanito MP, et al. Clinical impact of dysplastic changes in acquired aplastic anemia: a systematic study of bone marrow biopsies in children and adults. Ann Diagn Pathol. 2020;45:151459. doi:10.1016/j.anndiagpath.2019.151459
15. Liu Z, Guo F, Wang Y, et al. BATMAN-TCM: a bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine. Sci Rep. 2016;6(1):21146. doi:10.1038/srep21146
16. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(1):13. doi:10.1186/1758-2946-6-13
17. He D, Huang JH, Zhang ZY, et al. A network pharmacology-based strategy for predicting active ingredients and potential targets of Liuwei Dihuang pill in treating type 2 diabetes mellitus. Drug Des Devel Ther. 2019;13(1):3989–4005. doi:10.2147/DDDT.S216644
18. Li B, Rui J, Ding X, et al. Exploring the multicomponent synergy mechanism of Banxia Xiexin Decoction on irritable bowel syndrome by a systems pharmacology strategy. J Ethnopharmacol. 2019;233:158–168. doi:10.1016/j.jep.2018.12.033
19. Wang X, Shen Y, Shiwei W, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45(1):356–360. doi:10.1093/nar/gkx374
20. Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards Suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54(1):
21. Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48(D1):D845–d855. doi:10.1093/nar/gkz1021
22. Amberger JS, Hamosh A. Searching Online Mendelian Inheritance in Man (OMIM): a knowledgebase of human genes and genetic phenotypes. Curr Protoc Bioinform. 2017;58(1):
23. Wang Y, Zhang S, Li F, et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48(D1):D1031–D1041. doi:10.1093/nar/gkz981
24. Merino J, Udler MS, Leong A, et al. A decade of genetic and metabolomic contributions to type 2 diabetes risk prediction. Curr Diab Rep. 2017;17(12):135. doi:10.1007/s11892-017-0958-0
25. Han H, Cho JW, Lee S, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46(D1):D380–D386. doi:10.1093/nar/gkx1013
26. Jia L, Zuo T, Zhang C, et al. Simultaneous Profiling and holistic comparison of the metabolomes among the flower buds of Panax ginseng, Panax quinquefolius, and Panax notoginseng by UHPLC/IM-QTOF-HDMS(E)-based metabolomics analysis. Molecules. 2019;24(11):2188. doi:10.3390/molecules24112188
27. Ying M, Zheng B, Yu Q, et al. Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice. Food Chem Toxicol. 2020;138:111244. doi:10.1016/j.fct.2020.111244
28. Huang JH, He D, Chen L, et al. GC-MS based metabolomics strategy to distinguish three types of acute pancreatitis. Pancreatology. 2019;19(5):630–637. doi:10.1016/j.pan.2019.05.456
29. Frelin C, Ofran Y, Ruston J, et al. Grb2 regulates the proliferation of hematopoietic stem and progenitors cells. Biochim Biophys Acta Mol Cell Res. 2017;1864(12):2449–2459. doi:10.1016/j.bbamcr.2017.09.018
30. Halupa A, Bailey ML, Huang K, et al. A novel role for STAT1 in regulating murine erythropoiesis: deletion of STAT1 results in overall reduction of erythroid progenitors and alters their distribution. Blood. 2005;105(2):552–561. doi:10.1182/blood-2003-09-3237
31. Munugalavadla V, Kapur R. Role of c-Kit and erythropoietin receptor in erythropoiesis. Criti Rev Oncol Hematol. 2005;54(1):63–75. doi:10.1016/j.critrevonc.2004.11.005
32. Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004;18(7):1119–1176. doi:10.1038/sj.leu.2403383
33. Ratajczak MZ, Luger SM, Deriel K, et al. Role of the KIT protooncogene in normal and malignant human hematopoiesis. Proc Natl Acad Sci USA. 1992;89(5):1710–1714. doi:10.1073/pnas.89.5.1710
34. Adhikari S, Mandal P. Integrated analysis of global gene and microRNA expression profiling associated with aplastic anaemia. Life Sci. 2019;228(1):47–52. doi:10.1016/j.lfs.2019.04.045
35. Kordasti S, Costantini B, Seidl T, et al. Deep phenotyping of Tregs identifies an immune signature for idiopathic aplastic anemia and predicts response to treatment. Blood. 2016;128(9):1193–1205. doi:10.1182/blood-2016-03-703702
36. Pascutti MF, Erkelens MN, Nolte MA. Impact of Viral Infections on hematopoiesis: from beneficial to detrimental effects on bone marrow output. Front Immunol. 2016;7:364. doi:10.3389/fimmu.2016.00364
37. Giudice V, Banaszak LG, Gutierrez-Rodrigues F, et al. Circulating exosomal microRNAs in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2018;103(7):1150–1159. doi:10.3324/haematol.2017.182824
38. Bissinger R, Bhuyan AAM, Qadri SM, et al. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. Febs j. 2019;286(5):826–854. doi:10.1111/febs.14606
39. Ansó E, Weinberg SE, Diebold LP, et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat Cell Biol. 2017;19(6):614–625. doi:10.1038/ncb3529
40. Mouchiroud L, Houtkooper RH, Moullan N, et al. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi:10.1016/j.cell.2013.06.016
41. Warr MR, Binnewies M, Flach J, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494(7437):323–327. doi:10.1038/nature11895
42. Vannini N, Campos V, Girotra M, et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell. 2019;24(3):405–418.e7. doi:10.1016/j.stem.2019.02.012
43. Moon J, Kim HR, Shin MG. Rejuvenating aged hematopoietic stem cells through improvement of mitochondrial function. Ann Lab Med. 2018;38(5):395–401. doi:10.3343/alm.2018.38.5.395
44. Yang Y, Sauve AA. NAD(+) metabolism: bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016;1864(12):1787–1800. doi:10.1016/j.bbapap.2016.06.014
45. Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32(1):12–19. doi:10.1016/j.tibs.2006.11.006
46. Rock C, Moos PJ. Selenoprotein P protects cells from lipid hydroperoxides generated by 15-LOX-1. Prostaglandins Leukot Essent Fatty Acids. 2010;83(4–6):203–210. doi:10.1016/j.plefa.2010.08.006
47. Ferrante JV, Ferrante A. Novel role of lipoxygenases in the inflammatory response: promotion of TNF mRNA decay by 15-hydroperoxyeicosatetraenoic acid in a monocytic cell line. J Immunol. 2005;174(6):3169–3172. doi:10.4049/jimmunol.174.6.3169
48. Calzada C, Rice-Evans C. Ruptured erythrocytes inhibit the oxidation of membranes by 15-hydroperoxy-eicosatetraenoic acid. FEBS Lett. 1993;329(1–2):111–115. doi:10.1016/0014-5793(93)80204-8
49. Ireland R, Schwarz B, Nardone G, et al. Unique Francisella phosphatidylethanolamine acts as a potent anti-inflammatory lipid. J Innate Immun. 2018;10(4):291–305. doi:10.1159/000489504
50. Taylor LA, Arends J, Hodina AK, et al. Plasma lyso-phosphatidylcholine concentration is decreased in cancer patients with weight loss and activated inflammatory status. Lipids Health Dis. 2007;6:17. doi:10.1186/1476-511X-6-17
51. Pernes G, Flynn MC, Lancaster GI, et al. Fat for fuel: lipid metabolism in haematopoiesis. Clin Transl Immunology. 2019;8(12):e1098. doi:10.1002/cti2.1098
52. Bansal P, Dahate P, Raghuwanshi S, et al. Current updates on role of lipids in hematopoiesis. Infect Disord Drug Targets. 2018;18(3):192–198. doi:10.2174/1871526518666180405155015
53. Orsini M, Chateauvieux S, Rhim J, et al. Sphingolipid-mediated inflammatory signaling leading to autophagy inhibition converts erythropoiesis to myelopoiesis in human hematopoietic stem/progenitor cells. Cell Death Differ. 2019;26(9):1796–1812. doi:10.1038/s41418-018-0245-x
54. Young MM, Kester M, Wang HG. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res. 2013;54(1):5–19. doi:10.1194/jlr.R031278
55. Shirahama T, Sakakura C, Sweeney EA, et al. Sphingosine induces apoptosis in androgen-independent human prostatic carcinoma DU-145 cells by suppression of bcl-X(L) gene expression. FEBS Lett. 1997;407(1):97–100. doi:10.1016/S0014-5793(97)00304-9
56. Sakakura C, Sweeney EA, Shirahama T, et al. Suppression of bcl-2 gene expression by sphingosine in the apoptosis of human leukemic HL-60 cells during phorbol ester-induced terminal differentiation. FEBS Lett. 1996;379(2):177–180. doi:10.1016/0014-5793(95)01508-6
57. Jarvis WD, Fornari FA
58. Vu TM, Ishizu AN, Foo JC, et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature. 2017;550(7677):524–528. doi:10.1038/nature24053
59. Wątek M, Durnaś B, Wollny T, et al. Unexpected profile of sphingolipid contents in blood and bone marrow plasma collected from patients diagnosed with acute myeloid leukemia. Lipids Health Dis. 2017;16(1):235.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.