Back to Journals » Drug Design, Development and Therapy » Volume 17
Integrated Network Pharmacology and Experimental Approach to Investigate the Protective Effect of Jin Gu Lian Capsule on Rheumatoid Arthritis by Inhibiting Inflammation via IL-17/NF-κB Pathway
Authors Chen T, Li S, Lian D, Hu Q, Hou H, Niu D, Li H, Song L, Gao Y, Chen Y, Hu X, Li J, Ye Z, Peng B , Zhang G
Received 22 June 2023
Accepted for publication 17 November 2023
Published 13 December 2023 Volume 2023:17 Pages 3723—3748
DOI https://doi.org/10.2147/DDDT.S423022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Tengfei Chen,1,* Sihan Li,1,* Dongyin Lian,1 Qin Hu,2 Hongping Hou,1 Delian Niu,1 Han Li,1 Ling Song,1 Yunhang Gao,1 Ying Chen,1 Xiaoru Hu,3 Jianrong Li,1 Zuguang Ye,1 Bo Peng,1 Guangping Zhang1
1Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2College of Life Sciences and Bio-Engineering, Beijing University of Technology, Beijing, People’s Republic of China; 3National Institute for Food and Drug Control, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Peng; Guangping Zhang, Tel +8610 64056575, Email [email protected]; [email protected]
Purpose: This study aimed to investigate the main pharmacological action and underlying mechanisms of Jin Gu Lian Capsule (JGL) against rheumatoid arthritis (RA) based on network pharmacology and experimental verification.
Methods: Network pharmacology approaches were performed to explore the core active compounds of JGL, key therapeutic targets, and signaling pathways. Molecular docking was used to predict the binding affinity of compounds with targets. In vivo experiments were undertaken to validate the findings from network analysis.
Results: A total of 52 targets were identified as candidate JGL targets for RA. Sixteen ingredients were identified as the core active compounds, including, quercetin, myricetin, salidroside, etc. Interleukin-1 beta (IL1B), transcription factor AP-1 (JUN), growth-regulated alpha protein (CXCL1), C-X-C motif chemokine (CXCL)3, CXCL2, signal transducer and activator of transcription 1 (STAT1), prostaglandin G/H synthase 2 (PTGS2), matrix metalloproteinase (MMP)1, inhibitor of nuclear factor kappa-B kinase subunit beta (IKBKB) and transcription factor p65 (RELA) were obtained as the key therapeutic targets. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis showed that the efficacy of JGL was functionally involved in regulating immune-mediated inflammation, in which IL-17/NF-κB signaling was recommended as one of the main pathways. Molecular docking suggested that the core active compounds bound strongly to their respective targets. Experimentally, JGL treatment mitigated inflammation, showed analgesic activity, and ameliorated collagen-induced arthritis. Enzyme-linked immunosorbent assay showed that JGL effectively reduced the serum levels of cytokines, chemokines, and MMPs. Immunohistochemistry staining showed that JGL markedly reduced the expression of the targets in IL-17/NF-κB pathway including IL-17A, IL-17RA, NF-κB p65, C-X-C motif ligand 2, MMP1 and MMP13.
Conclusion: This investigation provided evidence that JGL may alleviate RA symptoms by partially inhibiting the immune-mediated inflammation via IL-17/NF-κB pathway.
Keywords: rheumatoid arthritis, Jin Gu Lian capsules, network pharmacology, experimental validation, immune-mediated inflammation
Graphical Abstract:
Introduction
Rheumatoid arthritis (RA) is a chronic, rheumatic autoimmune disease characterized by progressive articular cartilage damage, synovial hyperplasia, and systemic disease manifestations in other organs (interstitial lung disease, pericarditis, or bronchiectasis). RA can lead to severe functional disability and is associated with an increased risk of mortality.1 As an immune-mediated inflammatory disease, RA is a series of syndromes characterized by immune dysregulation that can result in chronic inflammation and irreversible joint or organ damages.2,3 Current treatment strategies for RA rely on nonsteroidal anti-inflammatory drugs (NSAIDS), glucocorticoids (GCs), disease-modifying anti-rheumatic drugs (DMARDs), and biological agents to control pain, alleviate inflammation, modulate the immune response, and prevent joint damage progression.4 Despite advances in therapeutic approaches, obtaining efficacious strategies for RA is still a clinical challenge owing to the poor effectiveness of these drugs, serious long-term adverse effects, and high financial burden.4 Therefore, identification of alternative therapeutic agents for RA is urgently required to improve disease outcomes.
Traditional herbal medicines (THM) have a significantly beneficial effect on RA treatment in terms of their low risk of adverse events over the long-term treatment period, their essential characteristics as a holistic and personalized therapy, and their effectiveness for patients who do not respond to conventional medication.5 Based on a combination of materials in a polyherbal formulation, THM show a superiorly synergistic effect on improving rheumatoid symptoms by their multifaceted actions on the complex pathology involved in RA, including immunoregulation, inflammation, angiogenesis, and oxidative stress.6 Therefore, THM holds great potential to be considered as desirable candidates for managing RA. Jin Gu Lian prescription is a representative traditional medicine formula used to treat rheumatic diseases clinically in Southeast China for decades of years. It has been extensively accepted by clinicians because of its superiorly therapeutic efficacy and few adverse effects.7 Jin Gu Lian Capsule (JGL), derived from this classic folk prescription, is included in the Compilation of National Standard for Traditional Chinese Medicines (2002 edition). Clinical studies revealed that it could ameliorate RA symptoms, including swelling and stiffness of the joint, muscle soreness, and joint pain, as well as slow disease activity in approximately 93.33% of patients.8 Previous studies have reported that multiple JGL herbal medicines exert therapeutic effects on RA. Alangium chinense relaxed skeletal muscle and exhibited anti-inflammatory activity by attenuating pro-inflammatory cytokines, inhibiting nuclear factor kappa B (NF-κB) signaling9 and improving oxidative stress.10 Gaultheria leucocarpa var. yunnanensis regulated the balance between pro-inflammatory and anti-inflammatory activities during RA progression.11 Psammosilene tunicoides demonstrated anti-RA effects by alleviating NOD-like receptor protein 3 (NLRP3)-mediated inflammatory responses.12 Sargentodoxa cuneata can ameliorate arthralgic pain and bone destruction by inhibiting cyclooxygenase, suppressing cartilage proteoglycan synthesis, and modulating inflammatory cytokines.13 However, considering the complex composition of JGL, there remains no detailed research in terms of its therapeutic characteristics and the mechanism underlying its amelioration of RA symptoms.
Given the multi-component, multi-pathway, and multi-target characteristics of Chinese herbal medicine, it is difficult to perform a detailed and comprehensive study of complex Chinese medicine based on the “one gene, one drug, and one disease” paradigm.14,15 Network pharmacology explains the foundation of complex disease by curing causal mechanisms for a systemic and holistic perspective, and it is a promising approach to understanding multiple drugs acting mechanistically on key network proteins in a synergistic manner.14,16 The idea and methods of network pharmacology hold great advantages to address the complex system of traditional Chinese medicine, which is also featured by holistic, personalized and multicomponent theory.17 Network pharmacology has made sustainable achievements and generated breakthroughs in a wide spectrum of traditional medicine research,15 including analyzing the causal mechanisms of action of herbal formulae,18 deciphering biological network of diseases and syndromes,17,19 understanding the molecular basis of traditional natures of herbs or herb formulae,20 and guiding the discovery of herbal active ingredients.17 Many researches on herbs or herbal formulae have benefited from network pharmacology to analyze the systematic mechanisms of action in the treatment of RA, such as ShexiangZhuifeng and Sidaxue.18,21
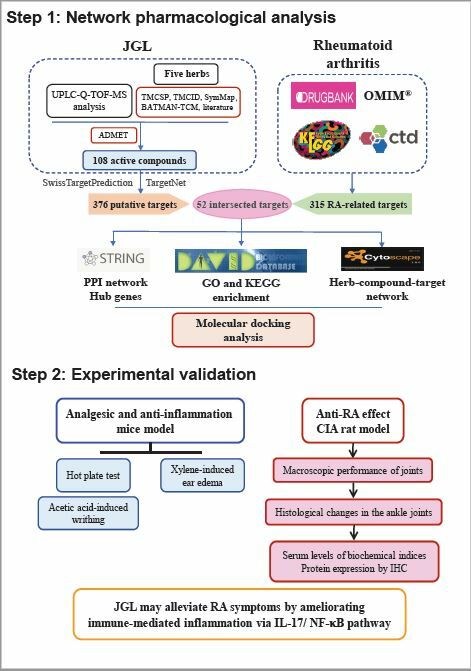
To investigate the main pharmacological action and underlying mechanisms of JGL against RA, we conducted an integrative approach of network-based computational prediction coupled with experimental verification in animal models of RA.
Materials and Methods
Collection of Compounds for Jin Gu Lian Capsule (JGL) and Screening of Active Components
Jin Gu Lian capsule (JGL) consists of five types of herbs: Gaultheria leucocarpa var. yunnanensis (Franch.) T.Z.Hsu & R.C.Fang (Ericaceae; Gaultheriae Herba; Tou Gu Xiang in Chinese, GL), Sargentodoxa cuneata (Oliv.) Rehder & E.H.Wilson (Lardizabalaceae; Sargentodoxae Caulis; Da Xue Teng in Chinese, SC), Heptapleurum leucanthum (R.Vig.) Y.F.Deng (Araliaceae; Schefflerae Leucanthae Caulis seu Folium; Han Tao Ye in Chinese, HL), Alangium chinense (Lour.) Harms (Cornaceae; Alangii Radix; Ba Jiao Feng in Chinese, AC), and Psammosilene tunicoides W.C.Wu & C.Y.Wu (Caryophyllaceae; Psammosilenes Radix; Jin Tie Suo in Chinese, PT). Fifty grams of PT was extracted using boiling water for 3 h and filtered. The residue was dried and crushed into fine powder. The other herbs (370 g of SC, 400 g of HL, 343 g of GL, and 50 g of AC) were extracted using boiling water, filtered, mixed with the filtrate of PT, and concentrated into a thick extract at a relative density of 1.25–1.28. Finally, the thick extract was combined with the fine powder of PT to obtain the desired product of JGL.7 The constituents of JGL have been analyzed by ultra-performance liquid chromatography-quadrupole-time-of-flight tandem mass (UPLC-Q-TOF-MS) as previously described.22
The ingredients of each herb in JGL were collected from Traditional Chinese Medicine Systems Pharmacology Database (TCMSP Version 2.3, https://tcmsp-e.com/tcmsp.php),23 Traditional Chinese Medicine Integrated Database (TCMID 2.0, http://www.megabionet.org/tcmid),24 SymMap (https://www.symmap.org/),25 Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine (BATMAN-TCM, http://bionet.ncpsb.org.cn/batman-tcm/)26 and previous publications.
The potential active components were screened based on Absorption, Distribution, Metabolism, Elimination (ADME) analysis. The ingredients of Sargentodoxa cuneate were obtained from the TCMSP database and were screened according to the criteria of OB ≥ 20% and DL ≥ 0.1 based on recommendations from the TCMSP database. For the components of the other four herbs that cannot be retrieved from TCMSP and the compounds identified by UPLC-Q-TOF-MS analysis, ADME analysis was performed using FAFDrugs4 webserver (free ADME-tox filtering computations for chemical biology and early stage drug discovery) (https://fafdrugs4.rpbs.univ-paris-diderot.fr/).27 The chemicals with acceptable results for FAFDrugs4’s built-in “Drug-Like Soft filter” were included in this research as the potential active components.
Prediction of JGL Putative Targets and Collection of RA-Related Genes
Target genes of the active components were predicted using SwissTargetPrediction (http://www.swisstargetprediction.ch)28 and TargetNet (http://targetnet.scbdd.com/)29 with the “Homo sapiens” settings. The more credible target genes of each compound were screened by setting “Probability” ≥0.4 in SwissTargetPrediction and “Prob” ≥0.8 in TargetNet.
RA-related targets were retrieved from four existing databases using “rheumatoid arthritis” as the keyword, while the species was limited to “Homo sapiens”: (1) DrugBank database (https://go.drugbank.com/, version 5.0)30 Only the targets that belong to human genes in Food and Drug Administration (FDA)-approved therapeutic drugs can be included. (2) The Online Mendelian Inheritance in Man (OMIM) database (http://www.omim.org, last updated: June 30th, 2022).31 (3) Comparative Toxicogenomics Database (CTD) (http://ctdbase.org/).32 We selected the genes whose “Direct Evidence” was “marker/mechanism” or “therapeutic”, and “Inference Score” was higher than 20. (4) Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Database (https://www.genome.jp/kegg/, last updated: April 1, 2022). Finally, the retrieved results were combined and deleted duplicates.
Intersection Targets Between JGL Targets and RA-Related Genes
The potential targets of JGL and RA-related targets were imported into VENNY 2.1 and overlapped to obtain the potential targets of JGL for the treatment of RA. The network was visualized using Cytoscape 3.8.0.
Protein–Protein Interaction (PPI) Network Construction and Topological Analysis
PPI network was constructed by connecting the intersections of the compound and disease targets with other human proteins interacting with them according to PPI data from the Search Tool for the Retrieval of Interacting Genes database (STRING, https://string-db.org/, version 11.5).33 The organism was set to Homo sapiens and the minimum required interaction score was >0.7. The PPI network in the TSV-format file was imported into Cytoscape 3.8.0, and the cytoHubba plugin was used to extract hub genes using the Maximal Clique Centrality (MCC) method. MCC is a relatively new algorithm and has a better performance on the precision of predicting essential proteins among the 11 topological analysis methods provided by cytoHubba, such as Degree, Maximum Neighborhood Component, Density of Maximum Neighborhood Component, EcCentricity, Closeness, Betweenness, etc.34
Gene Ontology (GO) and KEGG Pathway Enrichment Analysis
Functional and pathway enrichment analyses of potential targets of JGL against RA were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) in 2021 (https://david.ncifcrf.gov/).35 The threshold of P < 0.05 was set to identify key GO and KEGG pathways.
Network Construction
To elucidate the associations between compounds and targets, the herbs of JGL, active compounds in JGL, and the candidate therapeutic targets were imported into Cytoscape 3.8.0 to construct a “herbs-compounds-RA-targets” interaction network. The network was analyzed with a plug-in network analyzer and the topology parameters were used to identify the core active compounds of JGL with a degree value more than the average degree.
To explain the relationship between targets and pathways, a network between JGL therapeutic targets and the RA-related KEGG pathways was built and analyzed using Cytoscape 3.8.0. According to the topology parameters, the greater degree value of a target indicated the greater importance in KEGG pathways. The top 10 enriched genes were selected according to the degree value.
Molecular Docking
Molecular docking was performed to investigate the direct-binding efficiencies of the main active compounds of JGL and the key targets predicted by the PPI network and KEGG enrichment analysis. The 3D structure of human genes was retrieved from the Protein Data Bank (PDB, https://www.rcsb.org/). The files of core active compounds were downloaded in mol2 format. AutoDock Tools (Version 1.5.6, https://ccsb.scripps.edu/mgltools/) was used to preprocess the structure of the protein by removing water, adding polar hydrogen, calculating the Gasteiger charges, and adjusting Grid box size. Hydrogen atoms, Gasteiger charges, and rotatable bonds were assigned to the ligands. Molecular docking was performed using AutoDock Vina (The Scripps Research Institute, version 4.2.6), and the values of the binding energy were calculated. A heatmap was generated to visualize the value of the binding energy of molecular docking. The binding energy <0 kcal/mol indicates that the conformation of ligands and receptor proteins can be spontaneously bound. The lower the binding energy is, the stronger the affinity is and the higher the possibility of interaction between them. The binding energy of less than −4.25 kcal/mol represents a good docking affinity, and less than −7 kcal/mol represents a strong docking affinity.36
Experimental Validations
Drugs
Clinically used Jin Gu Lian capsules were provided by Guizhou Yibai Pharmaceutical Co. Ltd. (Batch number: 191004, Production Date: 2019.10.16, Valid period: 2021.09). Methotrexate (MTX, 2.5 mg per tablet, Batch number: 033628024) was purchased from Xinyi Medical Ltd. (Shanghai, China). Bovine Immunization Grade Type II collagen (CII, 2 mg/mL) and Incomplete Freund’s Adjuvant (IFA, 5 mL) were obtained from Chondrex Inc. (Woodinville, WA, USA).
Animals
Male or female CD-1 mice (18–22 g) and Male Sprague-Dawley (SD, 7–9 weeks) rats were obtained from Beijing Vital Laboratory Animal Technology (Beijing, China). Animal studies were performed following the Institutional Animal Care and Use Committee guidelines and approved by the Institutional Animal Ethical and Welfare Committee of the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences (approval numbers:2020B130, 2020B131, and 2020B137). Animals were maintained in a specific pathogen-free laboratory at the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, based on the requirements in line with Laboratory Animal-Requirements of Environment and Housing Facilities (GB 14925–2010, National Laboratory Animal Standardization Technical Committee of China). All animals were fed water and standard maintenance food ad libitum. The acclimation period for animals was three to five days.
Drug Administration
The clinical daily dose of JGL is 0.114 g/kg in terms of crude drug weight for a 60 kg weighing adult. According to our previous studies, the doses of JGL for analgesic and anti-inflammatory studies in mice were designed as 0.58, 1.73, and 5.2 g/kg in the present study, which were equivalent to 5, 15, and 45 times the normal human dose in clinical prescription. The doses selected for the anti-arthritic effects against collagen-induced arthritis (CIA) in rats were grounded on those in mice. According to the equivalent-dose ratio converted by interspecies body surface area, the equivalent dose of rats is 0.7 times of that for mice. Hence, the doses of JGL were set to 0.4, 1.2 and 3.6 g/kg in CIA model, which were equivalent to 3.3, 10, 30 times the normal human dose in clinical prescription. All drugs were dissolved in 0.5% carboxymethylcellulose sodium (CMC-Na).
Aspirin obtained from Beijing Shuguang Pharmaceutical Co., LTD (Chinese medicine approval number: H11021029) was used as a positive control in mice. The clinical dosage of aspirin in humans is about 20 mg/kg recommended by Drug Instruction. According to the formula of body surface area conversion between human and mice, the equivalent dose of mice is about 184 mg/kg (9.2 times of human dose), so the dose of aspirin was designed as 0.19 g/kg in this study. In CIA model, MTX at the dose of 0.2 mg/kg was used as a positive control according to the previous report.37
Xylene-Induced Mouse Ear Edema
The effects of JGL on acute topical inflammation were analyzed by using the xylene-induced mouse ear edema model, which is a simple and classic model for acute exudative inflammation with local vasodilation, increased capillary permeability, and inflammatory cell infiltration caused by the release of histamine, bradykinin, and other inflammatory mediators.38 The method was performed as described previously with some slight modification.39 Male mice were randomly divided into six groups with ≥9 mice in each group, which included normal control (treated with 0.5% CMC-Na), xylene model (treated with 0.5% CMC-Na), positive control (0.19 g/kg aspirin), and JGL at three different doses. All mice were orally gavaged once daily for 7 consecutive days. On Day 7, one hour after the treatment, inflammation was induced in mice (except for the normal control group) as ear edema by 20 μL of xylene smeared on both surfaces of the right ear, whereas the left ear was left untreated. After 30 min, the mice were sacrificed. The ears were collected using a biopsy punch with a diameter of 9 mm and weighed. Ear edema was defined as the difference in weight between the two ears.
Hot Plate Test
Female mice were divided randomly into five groups, including 0.5% CMC-Na (vehicle control), aspirin (0.19 g/kg), or JGL (0.58, 1.73, or 5.2 g/kg in terms of crude drug weight), with no less than 9 mice in each group. The mice were orally gavaged once daily for 7 consecutive days. Hot plate test was performed to evaluate the nociceptive response to heat one hour after the last treatment on day 7. The hot-plate temperature was maintained at (55 ± 0.5) °C, and the cutoff time was 30 seconds. The nociceptive response time (s) was defined as the latency for hind paw licking (or jumping), which is the time between the placement of mice on the hot plate and the occurrence of either licking of the hind paws or jumping off from the surface.
Acetic Acid-Induced Abdominal Writhing Response
Male mice were randomly divided into five groups and fasted overnight. Mice were orally administered with 0.5% CMC-Na (vehicle control), aspirin (0.19 g/kg), or JGL (0.58, 1.73, or 5.2 g/kg in terms of crude drug weight) once daily for 7 consecutive days. One hour after the last treatment on day 7, mice were intraperitoneally injected with 1.0% acetic acid aqueous solution (10mL/kg). The writhe was defined as the contraction of the abdomen and pelvic rotation, followed by the extension of the hind limbs. The latency to the beginning of the first writhe and the number of writhes were recorded within 20 min after the injection of acetic acid.
Induction of Collagen-Induced Arthritis (CIA) and JGL Treatment
Male rats were randomly divided into six groups: normal control (treated with 0.5% CMC-Na), CIA model (treated with 0.5% CMC-Na), methotrexate (MTX, positive control, 0.2 mg/kg) or three doses of JGL. A collagen-induced arthritis model was established according to the previously reported protocol.40 Briefly, the CII was mixed and thoroughly emulsified with an equal volume of IFA. For immunization, 0.2 mL of collagen emulsion was injected intradermally into the base of the tail, which was defined as Day 0. The rats were given a booster injection on Day 7. Rats in the normal control group were injected with an equal volume of saline instead of CIA. All rats were orally gavaged once daily from Day 1 and continued for 42 days. Ankle width (mediolateral diameter) was measured twice a week using a caliper. On Day 42, the paw edema was defined as the volume of the right-hind paw.
Histopathological Examination
At the end of the experiment (Day 43), the rats were anesthetized with pentobarbital sodium (48 mg/kg, i.p.) and then sacrificed. The hind limbs, including the paws and ankles, were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) and embedded in paraffin. Five micrometer thick sections were cut and stained with hematoxylin and eosin (H&E staining).
Immunohistochemistry (IHC) Assay
Ankle joint sections were analyzed using standard IHC techniques as described previously. After antigen retrieval, the tissues were incubated with anti-rat antibodies against interleukin (IL)-17A(AB214588, Abcam, 1:300), IL-17RA (bs-2606R, Bioss antibodies, 1:200), NF-κB p65 (8242, Cell Signaling Technology,1:500), matrix metalloproteinase (MMP)1 (10371-2-AP, Proteintech Group, Inc,1:300), MMP13(AB219620, Abcam, 1:500), or C-X-C motif ligand 2 (CXCL2, bs-1162R, Bioss antibodies, 1:200) at 4°C overnight. Following washing, the sections were processed with rabbit HRP-polymer kit (cat. no. PV-6001; ZSGB-BIO, China) and developed using DAB. The images were acquired under a light microscope, with at least five random high-power fields (HPF) in each section. For IL-17A and IL-17RA, the number of positive cells was counted per HPF (magnification ×40). For the other four indices, the positively stained area (Area) and the integrated optical density (IOD) were measured by ImageJ software. The relative expression was calculated using the average optical density (AOD)=IOD/Area.
Enzyme-Linked Immunosorbent Assays (ELISAs)
Blood samples were collected from the abdominal aortas. Serum was separated by centrifugation at 3500 × g for 20 min at room temperature, collected into a tube, and stored at −80°C. Serum levels of anti-collagen type II (anti-CII) were detected using a Type II Collagen Detection ELISA kit (Chondrex Inc., Woodinville, WA, USA). Serum levels of anti-cyclic citrullinated peptide (CCP) and rheumatoid factor (RF) were measured using ELISA kits (Euroimmun Medizinische Labordiagnostika, Luebeck, Germany). Serum levels of MMP1, MMP3 and MMP13 were detected using ELISA kits (Dakewe Biotech Co. Ltd., China). Serum levels of cytokines and chemokines were measured using the V-PLEX Plus Pro-inflammatory Panel 2 Rat Kit in the MESO QUICKPLEX SQ 120 system (Meso Scale Diagnostics, Rockville, MD, USA) and MILLIPLEX® MAP kits (Merck Millipore, Whitehouse Station, NJ, USA). All measurements were performed according to the manufacturer’s instructions.
Statistical Analyses
Data were expressed as the mean ± SEM. Statistical analyses were performed using Prism 8.0.2 (GraphPad Software, La Jolla, CA, USA). Significant differences of multiple groups were determined using one-way ANOVA followed by Dunnett post-hoc test or Kruskal–Wallis test followed by Dunn’s post-hoc test for multiple comparisons. P <0.05 was considered significant.
Results
Identification of Putative Targets of JGL Against RA
A total of 248 compounds in JGL were collected by searching the TCMSP, TCMID, SymMap, BATMAN-TCM databases, as well as previous publications. Additionally, UPLC-Q-TOF-MS analysis was performed to identify the constituents of JGL. Total ion current chromatograms for the negative and positive ionization modes were shown in Figure S1. Forty-three compounds were identified according to the chemical formulae, fragment ions, retention times, and literature comparison (Table S1). All retrieved components were combined and deleted duplicates.
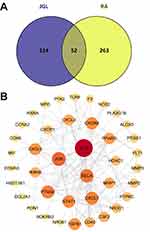
A total of 108 compounds were filtered by ADME prediction and selected as active candidates with acceptable drug-likeness properties for further analysis (Table S2). As shown in Table S3, 376 potential target genes hit by these 108 chemicals were predicted based on the similarities in drug structures and functions in the SwissTargetPrediction and TargetNet databases. Next, RA-related genes were obtained by searching four different databases. We collected 116 known targets from DrugBank database, 10 targets from OMIM database, 124 targets with an “Inference Score” more than 20 from CTD database and 101 targets in the RA pathway from KEGG database. After removing duplicates, 315 RA disease targets were identified (Table S4). Next, 376 active component targets and 315 RA disease targets were visualized using a Venn diagram (Figure 1A). A total of 52 intersected targets were identified as putative core targets of JGL for the treatment of RA.
Among these, 24 putative targets were approved with the known drugs from Drugbank database (Table 1). JGL herbs shared the targets of aryl hydrocarbon receptor (AHR), tumor necrosis factor receptor superfamily member 5 (CD40/TNFRSF5), glucocorticoid receptor (NR3C1) and Toll-like receptor 9 (TLR9) with the known immunosuppressive or immunomodulating agents, including leflunomide, ruplizumab, hydrocortisone, prednisolone, chloroquine, and hydroxychloroquine, some of which are the first-line treatment for RA.4 Besides these targets mentioned above, several putative targets of JGL, such as aldose reductase (AKR1B1), aldo-keto reductase family 1 member B10 (AKR1B10), interleukin-1 beta (IL1B), inducible nitric oxide synthase (NOS2), nuclear receptor subfamily 0 group B member 1 (NR0B1) and prostaglandin G/H synthase 1/2 (PTGS1/2), were identified as therapeutic targets for inflammation in RA, which have been approved by the known drugs, including sulindac, canakinumab, dexamethasone, celecoxib, ibuprofen, etc. Moreover, JGL herbs share aldo-keto reductase family 1 member C1 (AKR1C1), arachidonate 5-lipoxygenase (ALOX5), cyclin A (CCNA2), C-X-C chemokine receptor type 1 (CXCR1), Endothelin-1 receptor (EDNRA), phospholipase A2 (PLA2G1B), peroxisome proliferator-activated receptor gamma (PPARG), ribosomal protein S6 kinase alpha-3 (RPS6KA3), and transient receptor potential cation channel subfamily V member 1 (TRPV1) with the approved analgesics through anti-inflammatory or non-inflammatory mechanisms, such as acetylsalicylic acid, sulfasalazine, ketoprofen, niflumic acid, and acetaminophen, etc. In JGL, Sargentodoxa cuneate and Gaultheria leucocarpa var. yunnanensis shared inhibitor of nuclear factor kappa-B kinase subunit beta (IKBKB) with auranofin, an approved anti-rheumatic agent that prevents the destruction of bones and cartilage. Gaultheria leucocarpa var. yunnanensis, Heptapleurum leucanthum Psammosilene tunicoides, and Sargentodoxa cuneata target carbonic anhydrase 2 (CA2) and carbonic anhydrase 3 (CA3). These data suggested that the putative targets of JGL are involved in immunosuppression, attenuation of inflammation, mitigation of pain, and prevention of bone destruction.
 |
Table 1 The 24 Putative Anti-RA Targets of Jin-Gu-Lian Capsules Approved with the Known Drugs from Drugbank Database |
PPI Network Construction and Screening of Hub Genes
The candidate 52 therapeutic targets were imported into the STRING database to obtain the protein–protein interaction network. The PPI network included 40 nodes and 116 edges (Figure 1B). The major hub genes were screened by cytoHubba using MCC algorithm. As a result, a total of 14 nodes, the MMC score of which was no less than 4 folds of the median score, were identified as major hub genes. The hub genes were sequentially ordered by score as follows: IL1B, growth-regulated alpha protein (CXCL1), transcription factor AP-1 (JUN), CXCL2, transcription factor p65 (RELA), C-X-C motif chemokine 3 (CXCL3), PTGS2, C-X-C chemokine receptor type 4 (CXCR4), CXCR1, signal transducer and activator of transcription 1 (STAT1), PPARG, MMP1, histone deacetylase 1/2 (HDAC1), and IKBKB.
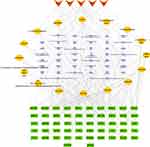
Construction of Herb-Compound-Target Network and Screening of Core Active Components in JGL
We constructed a network to visualize the interaction of JGL herbs, compounds and candidate therapeutic targets using Cytoscape 3.8.0 software. The network included 116 nodes and 306 edges (Figure 2). According to the degree value, 16 components with the degree values no less than twice of average value were selected as the core active compounds of JGL. They are quercetin (JGL1), myricetin (JGL2), salidroside (JGL3), anabasine (JGL4), fumaric acid (JGL5), protocatechuic acid (JGL6), emodin (JGL7), 7-hydroxycadalene (JGL9), 2.7-dihydroxycadalene (JGL10), kaempferol (JGL11), venoterpine (JGL12), (-)-catechin (JGL13), 2.7-dihydroxy-4-isopropyl-6-methylnaphthalene-1-carboxylic acid methyl ester (JGL14), β-sitosterol (JGL15), (5S,8R)-2-hydroxy-3,8-dimethyl-5-vinyl-5,6,7,8-tetrahydronaphthalene-1,4-dione (JGL16). Detailed information about the topology parameters of these compounds was shown in Table S5.
GO and KEGG Pathway Enrichment Analysis
We used DAVID database to perform GO and KEGG pathway enrichment analyses to interpret the potential significance of the functional units in the biological system network. A total of 209 related GO enrichment terms were identified, including 139 biological processes (BP), 22 cellular components (CC), and 48 molecular functions (MF). The top 10 significantly enriched GO terms in the BP, CC, and MF categories are shown in Figure 3A. These main proteins and genes are associated with physiological processes and molecular functions, including inflammation, such as regulation of inflammatory responses, regulation of cell response to lipopolysaccharide, activation of MAP kinase activity, regulation of neutrophil chemotaxis, activation of NF-κB transcription factor activity, and regulation of chemokine receptor binding; cell hyperplasia, such as activation of fibroblast proliferation and smooth muscle cell proliferation; and pain, such as regulation of neuronal cell body. These results suggest that putative targets of JGL may affect a variety of physiological functions in anti-RA therapies.
Furthermore, KEGG enrichment results in 47 significantly enriched KEGG pathways (P<0.01), and the top 15 pathways were selected visually (Figure 3B, Table S6). The enrichment results showed that the candidate targets of JGL against RA were mainly involved in pathways of rheumatoid arthritis, the immune-mediated inflammation (such as IL-17 signaling pathway, TNF signaling pathway, NF-κB signaling pathway, cytokine–cytokine receptor interaction, chemokine signaling pathway, NOD-like receptor signaling pathway, Th17 cell differentiation, inflammatory bowel disease, Toll-like receptor signaling pathway, and C-type lectin receptor signaling pathway), and osteoclast differentiation. Notably, the regulation of the pathways related to inflammation and the immune system was the most enriched functional module, indicating that JGL ameliorated RA by intervening in immune-mediated inflammatory responses in the disease progression. Among them, IL-17 and NF-κB pathways exhibited high confidence levels. We would further experimentally validate the pharmacological effects and potential mechanisms of action of JGL against RA.
Among the top 15 KEGG pathways, 12 KEGG pathways were related to RA progression. We construct a network between JGL therapeutic targets and the 12 RA-related KEGG pathways (Figure S2). According to the topology parameters, the genes with a degree value no less than the median score were selected as the KEGG enriched targets. They were sequentially ordered by score as follows: IL1B, IKBKB, RELA, JUN, STAT1, CXCL1, CXCL3, CXCL2, PTGS2, TGFB1, MMP1, MMP3, CD40, granulocyte-macrophage colony-stimulating factor (CSF2).
Molecular Docking Analysis
We performed a molecular docking analysis to evaluate the interaction between compounds and targets, predict the binding affinity, and validate the results of the network analysis. The 16 core active compounds in the “herb-compound-target” network were selected as ligands. The intersection of Hub genes and KEGG-enriched genes in the pathway-target network was used as the receptors: IL1B (PDB: 1RWN), JUN (PDB:1JUN), CXCL1 (PDB:1MGS), CXCL3 (PDB:6WZK), CXCL2 (PDB:3N52), STAT1 (PDB:3WWT), PTGS2 (PDB:5IKR), MMP1 (PDB:2CLT), IKBKB (PDB:4KIK), and RELA (IκBα/NF-κB complex, PDB: 1IKN).
The docking results demonstrated that the core active compounds of JGL had a good capacity to spontaneously bind to each key target. Detailed information on the minimum binding energy (kcal/mol) on molecular docking was shown in Figure 4. One hundred and sixty groups of compound molecules and target proteins were subjected to molecular docking. One hundred and fifty-five out of 160 groups with binding energy less than −4.25 kcal/mol were considered to have a good binding affinity. Among them, 73 groups with a score < −7 kcal/mol represented a strong binding affinity. The top 15 compound-target complexes with the lowest binding affinities are myricetin-PTGS2 (−9.7 kcal/mol), quercetin-IKBKB (−9.5 kcal/mol), myricetin-IKBKB (−9.4 kcal/mol), kaempferol-PTGS2 (−9.4 kcal/mol), quercetin-PTGS2 (−9.3 kcal/mol), (-)-catechin-PTGS2 (−9.3 kcal/mol), emodin-MMP1 (−9.3 kcal/mol), kaempferol-IKBKB (−9.2 kcal/mol), 2.7-dihydroxycadalene-IKBKB (−9.1 kcal/mol), quercetin-MMP1 (−9.0 kcal/mol), myricetin-MMP1 (−8.9 kcal/mol), kaempferol-MMP1 (−8.9 kcal/mol), emodin-RELA (−8.7 kcal/mol), quercetin-RELA (−8.5 kcal/mol), and myricetin-RELA (−8.3 kcal/mol). According to the average binding energy of core active compounds to the key targets, myricetin, quercetin, kaempferol, (-)-catechin, emodin, β-sitosterol, 2.7-dihydroxycadalene, 2.7-dihydroxy-4-isopropyl-6-methylnaphthalene-1-carboxylic acid methyl ester, salidroside, 7-hydroxycadalene were the top 10 ingredients with the best binding activity to the key targets which were evidenced by the lowest binding energy of −8.1, −8.1, −7.8, −7.6, −7.5, −7.5, −6.9, −6.8, −6.7, −6.6 kcal/mol, respectively. These compounds may be the candidate drug molecules for the treatment of JGL against RA.
In addition, the average value of binding affinity between target proteins (RELA, IKBKB, PTGS2, MMP1, IL-1β, and CXCL2) and the active compounds was −7.2, −7.5, −7.8, −7.4, −7.0, −7.0 kcal/mol, respectively, showing that these 5 key targets formed stable complexes with 16 core active compounds. These results verified that the therapeutic effect of JGL for RA treatment may be mediated by NF-κB signaling pathway from molecular docking level.
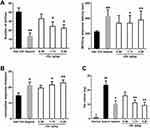
JGL Showed Analgesic Activity in Hot Plate Model and Acetic Acid-Induced Writhing Model
Analgesics have been widely used to relieve pain and stiffness during the treatment of rheumatoid arthritis. Acetic acid-induced writhing and hot-plate tests were performed to examine the analgesic activity of JGL. In the acetic acid-induced writhing test, JGL treatment significantly prolonged the latency to the beginning of the first writhe and significantly decreased the number of writhes. The percent reduction of writhes was increased by 15.2%, 34.7% and 43.4% at the doses of 0.58 g/kg, 1.73 g/kg or 5.2 g/kg, respectively (Figure 5A). In the hot plate test, the nociceptive response time of mice in JGL treatment was markedly extended compared to that of the vehicle control (P<0.05, P<0.01) (Figure 5B). These data indicated that JGL was effective in ameliorating the pain induced by thermal and chemical stimulation.
JGL Alleviated Xylene-Induced Mouse Ear Edema
We assessed the anti-inflammatory effects of JGL using xylene-induced mouse ear edema. As shown in Figure 5C, xylene application enhanced the degree of ear edema compared with that in normal control mice. JGL treatment resulted in a notable reduction in ear edema in a dose-dependent manner in comparison with xylene only treated mice.
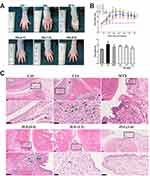
JGL Possessed Significant Anti-Arthritic Effects Against CIA in Rats
We further verified the efficacy of JGL against RA using a collagen-induced rat model. We measured the ankle diameters and arthritis scores of the rats throughout the experimental period, and paw volumes on the last day of the experiment. Although the clinical manifestations of arthritis appeared on different days after immunization, subcutaneous injection of type II collagen induced significant macroscopic changes of arthritis such as redness, erythema, and swelling of the paws. As shown in Figure 6A and B, the ankle diameters and paw volumes of CIA rats were significantly higher than those of normal rats. Administration of JGL at doses of 0.4, 1.2 and 3.6 g/kg significantly reduced ankle diameters and decreased paw volumes in a dose-dependent manner compared to vehicle-treated CIA rats.
Histological analysis in the ankle joints was performed to assess the protective effects of JGL against RA. As shown in Figure 6C, significant synovial hyperplasia, infiltration of inflammatory neutrophils, and articular cartilage destruction and erosion were observed in the CIA rats. These RA symptoms regarding joint damage were alleviated in rats administered with JGL compared to those in CIA rats. Taken together, these morphological and histopathological data indicated the beneficial effects of JGL treatment on collagen-induced arthritis.
JGL Inhibited CIA-Induced Release of Serum Biochemical Indices
To elucidate the potential molecular mechanism of JGL predicted from the above network pharmacology analysis, we detected the levels of autoantibodies, cytokines, chemokines, and MMPs in the serum of CIA rats. As shown in Figure 7, compared to control rats, CIA rats exhibited overproduction of specific autoantibodies, including anti-CII, RF, and anti-CCP (Figure 7A); enhanced secretion of MMP1, MMP3, and MMP13 (Figure 7B); significantly increased levels of inflammatory cytokines and chemokines, including interleukin (IL)-1β, IL-4, IL-5, IL-12p70, IL-17A, tumor necrosis factor-α (TNF-α), CXCL1, and CXCL2 (Figure 7C). Compared to CIA rats, a significant reduction in the serum levels of all these biochemical markers was observed in JGL-treated rats in a dose-dependent manner (P < 0.05).
JGL Inhibited the Expression of IL-17A, IL-17RA, NF-κB p65, CXCL2, MMP1 and MMP13 in Synovial Tissues
According to the above network and molecular docking analysis, the major targets of JGL against RA were significantly enriched into IL-17/NF-κB signaling pathway, which plays an essential role in immune-mediated inflammatory diseases.41,42 We next detected the effect of JGL on the IL-17/NF-κB pathway to experimentally validate the anti-RA mechanism of JGL. In particular, we examined the expression of IL-17A, IL-17RA, NF-κB p65, CXCL2, MMP1 and MMP13 in synovial tissues across CIA and control rats using immunohistochemistry staining. As shown in Figure 8, the expression of IL-17A, IL-17RA, NF-κB p65, CXCL2, MMP1 and MMP13 in the synovium of CIA rats was much higher when compared with that in control rats. Comparatively, 3.6 g/kg of JGL treatment significantly inhibited the expression of these proteins in arthritic rats. In general, these results from immunohistochemical and ELISA assays suggested that JGL suppressed the activation of IL-17/NF-κB pathway in CIA rats.
Discussion
Network pharmacology is a powerful weapon to address the complex system of traditional medicine. In this study, 52 JGL putative targets were predicted for the treatment of RA, and 23 of these genes were the targets of several approved anti-RA drugs from the Drugbank database, which were used as anti-inflammatory, immunosuppressive, and analgesic agents. JGL shared NR3C1, TLR9, PTGS1/2, IL1B, and IKBKB with FDA-approved anti-RA drugs, such as hydrocortisone, chloroquine, hydroxychloroquine, diflunisal, oxaprozin, canakinumab, and auranofin, among which hydrocortisone, chloroquine, and hydroxychloroquine are the first-line treatment for RA.4 Sargentodoxa cuneate and Gaultheria leucocarpa var. yunnanensis in JGL could target IKBKB, a gene that approved by the anti-rheumatic agent auranofin to prevent the progressive deterioration of bone and cartilage tissues in RA.4 Additionally, herbs in JGL target carbonic anhydrases, which have specific functions in bone resorption and osteoclast differentiation.43 Moreover, JGL herbs could target some genes associated with pain, a manifestation of rheumatoid arthritis (RA) that might result from inflammation or non-inflammatory mechanisms caused by joint damage and central neuronal processing,44 such as AKR1C1, CCNA2, EDNRA, TRPV1, etc. These results showed that JGL’s putative targets are involved in immunosuppression, attenuation of inflammation, mitigation of pain, and prevention of bone destruction.
GO analysis demonstrated that the 52 candidate targets of JGL identified against RA were significantly enriched in various biological processes involved in inflammation, immunomodulation, activation of fibroblast proliferation, smooth muscle cell proliferation, and organization of the extracellular matrix, which play important roles in RA progression. These findings also suggested that the putative targets of JGL were associated with immunosuppression and attenuation of inflammation, pathological pain, and joint damage. The predicted performance derived from network pharmacology was consistent with that of the animal models. In the present study, JGL treatment inhibited inflammation and mitigated central and peripheral pain, as evidenced by the extended nociceptive response time in the hot plate test as well as a decreased number of writhes and prolonged latency time to the beginning of the first writhe in the acetic acid-induced writhing test. In collagen-induced RA, JGL treatment ameliorated swelling, reduced the infiltration of inflammatory cells, inhibited synovial hyperplasia, and mitigated cartilage destruction in ankle joints. Our results were in accordance with previous reports that JGL showed anti-inflammatory effects in an LPS-induced inflammatory rat model,45 alleviated Freund’s complete adjuvant (CFA)-induced inflammatory pain by reducing serum levels of 5-hydroxytryptamine (5-HT) and prostaglandin E2 (PGE2) in mice46 and suppressed the degeneration of articular cartilage of the knee in a rabbit osteoarthritis model.47,48
KEGG pathway analysis showed that JGL putative targets were closely related to IL-17 signaling pathway, NF-κB signaling pathway, TNF signaling pathway, Toll−like receptor signaling pathway, Th17 cell differentiation, cytokine–cytokine receptor interaction, and chemokine signaling pathway, among which IL-17 and NF-κB pathways exhibited high confidence levels. In RA, infiltration of “pathogenic” Th17 cells in synovial tissue releases abundant IL-17 and active inflammation through IL-17R receptor.49 Increased levels of IL-17 in joints are correlated with the disease severity,50 and it has been demonstrated to be a potential therapeutic target, resulting in several trials being carried out.51 NF-κB pathway, a downstream signaling from IL-17 receptor, initiates inflammatory responses in R.42 IL-17/NF-κB signaling pathway has been widely proved to play a pivotal role in the pathogenesis of RA by inducing pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6), inducing chemokine-mediated recruitment of leukocytes, degrading articular cartilage, and activating keratinocytes and synoviocytes.41,42,52,53 Recently, targeting immune pathways and control inflammation has been recognized as the best outcomes in the immune-mediated inflammatory diseases.54 In current analysis of PPI and “target-KEGG” networks, 10 genes were identified as the key targets for JGL treatment against RA. Among them, RELA, IKBKB, PTGS2, MMP1, IL-1β, CXCL2, CXCL1, and CXCL3 are closely related to IL-17/NF-κB signaling pathway. IL-1 is a key cytokine in the differentiation of effector T cells and inducer of inflammation which is associated with RA severity.2 TNF-α, a cytokine derived from macrophages, neutrophils, and activated T cells, induces cytokine production in immune cells, activates fibroblasts, as well as stimulates osteoclasts with subsequent formation of bone erosions in RA.3 Chemokines CXCL1 and CXCL2 mediate the recruitment of leukocytes to the synovial membrane and participate in synovial hyperplasia, activation of endothelial cells for angiogenesis, and regulation of osteoclastogenesis.53,55 All these data reminded us of the important role of IL-17/NF-κB pathway in JGL treatment against RA. Next, we performed molecular docking analysis and in vivo experiments to validate the network prediction. Five of the key targets (RELA, IKBKB, PTGS2, MMP1, IL1B and CXCL2) was found to correspond to multiple components in JGL as evidenced by strong binding affinity with all core active compounds of JGL. Meanwhile, serum levels of IL-17A, IL-1β, TNF-α, CXCL1 and CXCL2 were markedly increased in CIA rats. Administration of JGL at doses of 1.2 or 3.6 mg/kg significantly reduced the levels of these cytokines and chemokines. A previous study also reported that JGL treatment decreased the expression levels of IL-1β and IL-18 in rat osteoarthritis or collagen-induced mouse arthritis.12,48 Considering that IL-17/NF-κB pathway leads to a boost in the production of MMPs to promote the degradation of cartilage matrix and bone resorption in RA,41 we observed the expression of MMP1, MMP3, and MMP13 in CIA rats. Oral administration of JGL resulted in a dose-dependent reduction in serum levels of MMP1, MMP3, and MMP13. A previous study also demonstrated the inhibitory effect of Gaultheria leucocarpa, the main ingredient of JGL, and suppressed the expression of MMP9 in IL-1β-induced human fibroblast-like synoviocytes of RA.11 Parallel to our results in ELISA assays, JGL treatment suppressed the up-regulated expression of IL-17A, IL-17RA, NF-κB p65, CXCL2, MMP1 and MMP13 using immunohistochemistry staining. These findings suggested that JGL may ameliorate the pathological changes of RA, such as inflammation, synovial hyperplasia, and cartilage destruction, partly by regulating IL-17/NF-κB signaling pathway.
Apart from all the mentioned cytokines, IL-4 and IL-12 showed strong synergism in the onset or progress of rheumatoid arthritis.56,57 In addition to the well-established regulation of the inflammatory process in RA by IL-4 directly promoting Th2 differentiation from Th0 cells, IL-4 indirectly contributes to the activation of Th1-driven arthritis by enhancing IL-12 production in dendritic cells.57 According to the KEGG analysis, we discovered that there was also considerable confidence in Th1 and Th2 differentiation pathway for the treatment of JGL on RA. In the present collagen-induced RA, serum levels of IL-4 and IL-12 were significantly increased. Compared to CIA rats, JGL treatment significantly decreased the levels of IL-4 and IL-12, as well as ameliorated the severity of arthritis. Our findings were consistent with previous results showing that deficiency or neutralization of IL-4 and IL-12 resulted in reduced symptoms of arthritis and less susceptibility to the development of the disease in collagen-induced arthritis.56,57
Autoantibodies, a hallmark for RA, may form immune complexes in the joints leading to the activation of immune cells, augment the immune response, and contribute to chronic inflammation and bone destruction.58 High level of IL-17 results in the development of two main autoantibodies, RF and anti-CCP.59 IL-4 and IL-5 have been demonstrated to induce B1 B-cell activation, which contributes to autoantibodies in the pathogenesis of RA.56,58,60 In the present CIA rat model, JGL treatment significantly attenuated the serum levels of anti-CII, RF, and anti-CCP.
Notably, all data observed in this experimental study showed that JGL treatment markedly mitigated joint swelling and alleviated the number of inflammatory or immune cells in the ankle joints, such as lymphocytes and neutrophils infiltrating the synovium, which occurred in accordance with the reduced serum levels of cytokines, chemokines, and autoantibodies, as well as the decreased levels of IL-17A, IL-17RA, NF-κB p65 and CXCL2 in synovial tissues. These results suggested that JGL treatment against RA may be associated with modulating the immune responses and ameliorating the inflammation via IL-17A/NF-κB signaling pathway to some extent. Besides serum autoantibodies, some key targets of this pathway have a potential possibility to be used as serum indices for the evaluation of JGL clinical efficacy, such as IL-1 and TNF-α, which are closely related to the disease severity of RA,2,3 as well as IL-17A and IL-12, which have been approved as the therapeutic targets in other immune-mediated diseases.3 These speculations should be further supported by trials to establish their clinical relevance and potential as JGL treatment options against RA.
Owing to the complex active ingredients of TCM, it is difficult to identify the key pharmacodynamic components of TCM formulae. Network pharmacology and molecular docking have provided novel insights to reveal the pharmacodynamic material basis of the complex chemical substance system.17,20 In this study, based on the degree values in “herb-compound-target” network, 16 chemicals of JGL were selected as core active compounds. The molecular docking results suggested that 10 compounds, including myricetin, quercetin, kaempferol, (-)-catechin, emodin, β-sitosterol, 2,7-dihydroxycadalene, 2,7-dihydroxy-4-isopropyl-6-methylnaphthalene-1-carboxylic acid methyl ester, salidroside, 7-hydroxycadalene, could be stably combined with the key targets associated with the immune-mediated inflammation of RA. These compounds may be considered as the potential candidate pharmacodynamic components of JGL against RA. Previous studies have shown great potential of some components as agents for the RA treatment. Quercetin can inhibit the pathological process of RA by regulating autoimmune responses through modulation of Th17/Treg imbalance and Th17 cell differentiation, suppressing inflammation of synovial membrane, mitigating synovial hyperplasia, and reducing bone destruction via lowering MMPs and osteoclasts formation.61 Salidroside has been attributed to a variety of biological activities in RA treatment, such as inhibiting proliferation, migration and invasion of RA-fibroblast-like synoviocytes, alleviating inflammation, and ameliorating arthritis-induced cognition deficits via Rho/ROCK/NF-κB signaling.62,63 Myricetin was reported to contribute to RA treatment because of its immune modulation, antioxidation, anti-inflammation and amelioration of cartilage degradation.64 Emodin inhibited spinal inflammatory reaction, alleviated arthritis pain,65 and suppressed inflammatory responses by controlling differentiation and maturation of T lymphocytes, dendritic cells, and regulatory T cells, reducing neutrophil infiltration in synovial tissues.66,67 Catechin upregulated expression of PGE2 receptor (EP2), modulated cyclic adenosine monophosphate (cAMP) levels and inhibited secretion of IL-1 and TNF-α in rats with adjuvant arthritis.68 Therefore, all these findings offer the possibility that myricetin, quercetin, salidroside, (-)-catechin, and emodin may be the candidate active components in JGL. Follow-up experiments are needed to confirm the specific role of these potential candidate components in the overall efficacy of JGL.
Although we have explained the mechanism of JGL against RA to some extent using a combination of network pharmacology and experimental verification, there are still some issues that need to be investigated in future studies. First, the ingredients of JGL used for target prediction were filtered by the ADME properties, and the quantitative data of specific ingredients were not considered. It is difficult to explain the relationship between the content of components and their therapeutic benefits. Therefore, we cannot accurately predict the importance of the core active compounds in the anti-RA efficacy of JGL. Second, the identified compounds in this study might not be truly comparable to the JGL prescription due to the compatible interactions among different fractions of traditional herbal medicine. Future studies are required to identify the main components of JGL that may exert anti-RA effects, identify the serum-absorbed constituents of JGL, and determine their content in JGL formula and blood exposure levels. Additional experimental validation of the multi-target anti-RA effect of this prescription is also required. Third, the interaction between 10 potential candidate components and their corresponding respective targets has not been fully explained. Follow-up research should validate the specifically binding relationship of compounds and targets, as well as carry out experimental verification to explore the mechanism of these bioactive candidates. Last, although we found that IL-17/NF-κB pathway may be the essential mechanism for JGL treatment and the core active compounds can interact with the key targets well in this pathway, we should not ignore other potentially enriched KEGG pathways worthy of further study, particularly those which have crosstalk with IL-17/NF-κB pathway and involve in the immune-mediated inflammation.
Conclusions
In summary, therapeutic effects and related mechanisms of JGL against RA were elucidated by network workflow and experimental validations. We demonstrated that JGL alleviated RA symptoms by partially inhibiting the immune-mediated inflammation via the IL-17/NF-κB pathway. This investigation not only contributes to the exploration of the mechanisms underlying the action of JGL but also offers experimental evidence for its clinical application in the treatment of RA. In addition, this study also suggests that the workflow combining network pharmacology with experimental validation provides a promising strategy to address the complex system of traditional medicine by identifying core pharmacodynamic ingredients, excavating the therapeutic targets and related pathways, as well as deciphering the pharmacological mechanisms.
Ethics Statements
The network pharmacology-based study was reviewed by Ethics Committee of Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, and the ethnic approval has been waived.
Acknowledgments
We sincerely appreciate the efforts of all authors who contributed to this study.
Funding
This study was supported by the National Key R&D Program of China (2018YFC1708105) and the Scientific and Technological Innovation Project of the China Academy of Chinese Medical Sciences (CI2021A04615 and CI2021A04905).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Finckh A, Gilbert B, Hodkinson B, et al. Global epidemiology of rheumatoid arthritis. Nature Rev Rheumatol. 2022;18(10):591–602. doi:10.1038/s41584-022-00827-y
2. Monteleone G, Moscardelli A, Colella A, et al. Immune-mediated inflammatory diseases: common and different pathogenic and clinical features. Autoimmunity Rev. 2023;22(10):103410. doi:10.1016/j.autrev.2023.103410
3. Schett G, McInnes IB, Neurath MF. Reframing Immune-Mediated Inflammatory Diseases through Signature Cytokine Hubs. N Eng j Med. 2021;385(7):628–639. doi:10.1056/NEJMra1909094
4. Mueller AL, Payandeh Z, Mohammadkhani N, et al. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: new Treatment Strategies. Cells. 2021;10(11):3017. doi:10.3390/cells10113017
5. Jo HG, Seo J, Lee D. Clinical evidence construction of East Asian herbal medicine for inflammatory pain in rheumatoid arthritis based on integrative data mining approach. Pharmacol Res. 2022;185:106460. doi:10.1016/j.phrs.2022.106460
6. Wang Y, Chen S, Du K, et al. Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. J Ethnopharmacol. 2021;279:114368. doi:10.1016/j.jep.2021.114368
7. Zheng L, Zhou T, Liu H, et al. WS-10093(ZD)-0093-2002, Compilation of National Standard for Traditional Chinese Medicines, Branch Volume of Brain Meridians, Limbs and Brain System. China Food Drug Adm. 2002.
8. Gong CY, Ao HR, Wang MM, et al. [Clinical study of Jin Gu Lian capsule in the treatment of rheumatic obstruction syndrome rheumatoid arthritis]. Chin J Clin Rat Drug Use. 2018;11(6A):18–19.
9. Gao F, Zhang S. Salicin inhibits AGE-induced degradation of type II collagen and aggrecan in human SW1353 chondrocytes: therapeutic potential in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019;47(1):1043–1049. doi:10.1080/21691401.2019.1591427
10. Zhai KF, Duan H, Khan GJ, et al. Salicin from Alangium chinense Ameliorates Rheumatoid Arthritis by Modulating the Nrf2-HO-1-ROS Pathways. Journal of Agricultural and Food Chemistry. 2018;66(24):6073–6082. doi:10.1021/acs.jafc.8b02241
11. Wang X, Sun Y, Ling L, et al. Gaultheria leucocarpa var. yunnanensis for Treating Rheumatoid Arthritis-An Assessment Combining Machine Learning-Guided ADME Properties Prediction, Network Pharmacology, and Pharmacological Assessment. Front Pharmacol. 2021;12:704040. doi:10.3389/fphar.2021.704040
12. He ZM, Huang ZP, Wei YC, et al. Therapeutic mechanism of Psammosilene tunicoides extract on rheumatoid arthritis based on NLRP3 inflammasome]. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi =. China j Chinese materia medica. 2021;46(17):4504–4510. doi:10.19540/j.cnki.cjcmm.20210609.701
13. Zhang W, Sun C, Zhou S, et al. Recent advances in chemistry and bioactivity of Sargentodoxa cuneata. J Ethnopharmacol. 2021;270:113840. doi:10.1016/j.jep.2021.113840
14. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nature Chemical Biol. 2008;4(11):682–690. doi:10.1038/nchembio.118
15. Yuan Z, Pan Y, Leng T, et al. Progress and Prospects of Research Ideas and Methods in the Network Pharmacology of Traditional Chinese Medicine. J Pharm Pharm Sci. 2022;25:218–226. doi:10.18433/jpps32911
16. Nogales C, Mamdouh ZM, List M, et al. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–150. doi:10.1016/j.tips.2021.11.004
17. Wang X, Wang ZY, Zheng JH, et al. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19(1):1–11. doi:10.1016/S1875-5364(21)60001-8
18. Wang J, Chen Q, Sheng R, et al. Integration of transdermal chemistry and network pharmacology to decipher the mechanism of ShexiangZhuifeng analgesic plaster to treat rheumatoid arthritis. Phytomedicine. 2023;108:154507. doi:10.1016/j.phymed.2022.154507
19. Yang HY, Liu ML, Luo P, et al. Network pharmacology provides a systematic approach to understanding the treatment of ischemic heart diseases with traditional Chinese medicine. Phytomedicine. 2022;104:154268. doi:10.1016/j.phymed.2022.154268
20. Xia F, Liu C, Wan JB. Characterization of the cold and hot natures of raw and processed Rehmanniae Radix by integrated metabolomics and network pharmacology. Phytomedicine. 2020;74:153071. doi:10.1016/j.phymed.2019.153071
21. Wu N, Yuan T, Yin Z, et al. Network Pharmacology and Molecular Docking Study of the Chinese Miao Medicine Sidaxue in the Treatment of Rheumatoid Arthritis. Drug Design Dev Therapy. 2022;16:435–466. doi:10.2147/dddt.S330947
22. Lian D, Chen T, Yan L, et al. Protective effect of compatible herbs in Jin-Gu-Lian formula against Alangium chinense-induced neurotoxicity via oxidative stress, neurotransmitter metabolisms, and pharmacokinetics. Front Pharmacol. 2023;14:1133982. doi:10.3389/fphar.2023.1133982
23. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminf. 2014;6:13. doi:10.1186/1758-2946-6-13
24. Huang L, Xie D, Yu Y, et al. TCMID 2.0: a comprehensive resource for TCM. Nucleic Acids Res. 2018;46(D1):D1117–D20. doi:10.1093/nar/gkx1028
25. Wu Y, Zhang F, Yang K, et al. SymMap: an integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019;47(D1):D1110–D17. doi:10.1093/nar/gky1021
26. Liu Z, Guo F, Wang Y, et al. BATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci Rep. 2016;6:21146. doi:10.1038/srep21146
27. Lagorce D, Bouslama L, Becot J, et al. FAF-Drugs4: free ADME-tox filtering computations for chemical biology and early stages drug discovery. Bioinformatics. 2017;33(22):3658–3660. doi:10.1093/bioinformatics/btx491
28. Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–W64. doi:10.1093/nar/gkz382
29. Yao ZJ, Dong J, Che YJ, et al. TargetNet: a web service for predicting potential drug-target interaction profiling via multi-target SAR models. J Computer Aided Mol Design. 2016;30(5):413–424. doi:10.1007/s10822-016-9915-2
30. Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D82. doi:10.1093/nar/gkx1037
31. Hamosh A, Scott AF, Amberger JS, et al. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(Database issue):D514–7. doi:10.1093/nar/gki033
32. Davis AP, Grondin CJ, Johnson RJ, et al. Comparative Toxicogenomics Database (CTD): update 2021. Nucleic Acids Res. 2021;49(D1):D1138–D43. doi:10.1093/nar/gkaa891
33. Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–d12. doi:10.1093/nar/gkaa1074
34. Chin CH, Chen SH, Wu HH, et al. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8 Suppl 4(Suppl 4):S11. doi:10.1186/1752-0509-8-S4-S11
35. Sherman BT, Hao M, Qiu J, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022. doi:10.1093/nar/gkac194
36. Gaillard T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 Benchmark. J Chem Inf Model. 2018;58(8):1697–1706. doi:10.1021/acs.jcim.8b00312
37. Nho JH, Lee HJ, Jung HK, et al. Effect of Saururus chinensis leaves extract on type II collagen-induced arthritis mouse model. BMC Complement Altern Med. 2019;19(1):2. doi:10.1186/s12906-018-2418-z
38. Lee YY, Saba E, Irfan M, et al. The anti-inflammatory and anti-nociceptive effects of Korean black ginseng. Phytomedicine. 2019;54:169–181. doi:10.1016/j.phymed.2018.09.186
39. Zhang Z, Li L, Huang G, et al. Embelia Laeta aqueous extract suppresses acute inflammation via decreasing COX-2/iNOS expression and inhibiting NF-kappaB pathway. J Ethnopharmacol. 2021;281:114575. doi:10.1016/j.jep.2021.114575
40. Griffiths MM, Cannon GW, Corsi T, et al. Collagen-induced arthritis in rats. Methods Mol Med. 2007;136:201–214. doi:10.1007/978-1-59745-402-5_15
41. Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Sem immunopathol. 2017;39(4):365–383. doi:10.1007/s00281-017-0619-z
42. Manou-Stathopoulou S, Lewis MJ. Diversity of NF-κB signalling and inflammatory heterogeneity in Rheumatic Autoimmune Disease. Sem Immunol. 2021;58:101649. doi:10.1016/j.smim.2022.101649
43. Angeli A, Kartsev V, Petrou A, et al. Substituted furan sulfonamides as carbonic anhydrase inhibitors: synthesis, biological and in silico studies. Bioorg. Chem. 2023;138:106621. doi:10.1016/j.bioorg.2023.106621
44. McWilliams DF, Walsh DA. Pain mechanisms in rheumatoid arthritis. Clin exp rheumatol. 2017;35 Suppl 107(5):94–101.
45. Fu ZL, Pu J, Li GL, et al. Study on anti-inflammatory effect and mechanism of Jinggulian Capsule on inflammatory model rats. China Pharmacy. 2021;32(9):56.
46. Du MD, Qiu DW, Xu JY. Pathological mechanism of JinGuLian-Capsule on rheumatoid arthiritis. World J Traditional Chine Orthopedics. 2004;6(2):7.
47. Peng X, Yang J, You Q, et al. Effect of Psammosilene Capsule gavage on the expression of apoptosis-related proteins in chondrocytes of a rabbit osteoarthritis model. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24(8):1188–1194.
48. Zeng L, Liu Y, Xiong H, et al. Preventive Effect of Jingulian Capsule on Knee Osteoarthritis in Rabbits. Chin J Trad Med Traum & Orthop. 2016;24(7):7–11.
49. Takeuchi T. Cytokines and cytokine receptors as targets of immune-mediated inflammatory diseases-RA as a role model. Inflammation Regeneration. 2022;42(1):35. doi:10.1186/s41232-022-00221-x
50. Akhter S, Tasnin FM, Islam MN, et al. Role of Th17 and IL-17 Cytokines on Inflammatory and Auto-immune Diseases. Curr. Pharm. Des. 2023;29(26):2078–2090. doi:10.2174/1381612829666230904150808
51. Kunwar S, Dahal K, Sharma S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatol Int. 2016;36(8):1065–1075. doi:10.1007/s00296-016-3480-9
52. Taams LS. Interleukin-17 in rheumatoid arthritis: trials and tribulations. J exp med. 2020;217(3). doi:10.1084/jem.20192048
53. Szekanecz Z, Vegvari A, Szabo Z, et al. Chemokines and chemokine receptors in arthritis. Front bioscience. 2010;2(1):153–167. doi:10.2741/s53
54. McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21(10):680–686. doi:10.1038/s41577-021-00603-1
55. Wang X, Sun L, He N, et al. Increased expression of CXCL2 in ACPA-positive rheumatoid arthritis and its role in osteoclastogenesis. Clin Exp Immunol. 2021;203(2):194–208. doi:10.1111/cei.13527
56. Chen Z, Bozec A, Ramming A, et al. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nature Rev Rheumatol. 2019;15(1):9–17. doi:10.1038/s41584-018-0109-2
57. Hochrein H, O’Keeffe M, Luft T, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J exp med. 2000;192(6):823–833. doi:10.1084/jem.192.6.823
58. van Delft MAM, Huizinga TWJ. An overview of autoantibodies in rheumatoid arthritis. J Autoimmunity. 2020;110:102392. doi:10.1016/j.jaut.2019.102392
59. Xu X, Hsu HC, Chen J, et al. Increased expression of activation-induced cytidine deaminase is associated with anti-CCP and rheumatoid factor in rheumatoid arthritis. Scandinavian j Immunol. 2009;70(3):309–316. doi:10.1111/j.1365-3083.2009.02302.x
60. Iwaszko M, Biały S, Bogunia-Kubik K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells. 2021;10(11). doi:10.3390/cells10113000
61. Tang M, Zeng Y, Peng W, et al. Pharmacological Aspects of Natural Quercetin in Rheumatoid Arthritis. Drug Design Dev Therapy. 2022;16:2043–2053. doi:10.2147/dddt.S364759
62. Qin Y, Su J. Salidroside suppresses cell growth and inflammatory response of fibroblast-like synoviocytes via inhibition of phosphoinositol-3 kinase/threonine kinase signaling in rheumatoid arthritis. Zeitschrift fur Rheumatologie. 2023. doi:10.1007/s00393-023-01431-5
63. Zhu L, Chen T, Chang X, et al. Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-κB pathway. Neuropharmacology. 2016;103:134–142. doi:10.1016/j.neuropharm.2015.12.007
64. Yuan X, Liu Y, Hua X, et al. Myricetin ameliorates the symptoms of collagen-induced arthritis in mice by inhibiting cathepsin K activity. Immunopharmacol Immunotoxicol. 2015;37(6):513–519. doi:10.3109/08923973.2015.1096942
65. Cheng DW, Yue YF, Chen CX, et al. Emodin alleviates arthritis pain through reducing spinal inflammation and oxidative stress. Molecular Pain. 2022;18:17448069221146398. doi:10.1177/17448069221146398
66. Cheng L, Chen J, Rong X. Mechanism of Emodin in the Treatment of Rheumatoid Arthritis. Evidence Based Complementary Alternative Med. 2022;2022:9482570. doi:10.1155/2022/9482570
67. Hui P, Zhou S, Cao C, et al. The elucidation of the anti-inflammatory mechanism of EMO in rheumatoid arthritis through an integrative approach combining bioinformatics and experimental verification. Front Pharmacol. 2023;14:1195567. doi:10.3389/fphar.2023.1195567
68. Tang LQ, Wei W, Wang XY. Effects and mechanisms of catechin for adjuvant arthritis in rats. Adv Therapy. 2007;24(3):679–690. doi:10.1007/bf02848793
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.