Back to Journals » Journal of Inflammation Research » Volume 17
Integrated Analysis Identified TGFBI as a Biomarker of Disease Severity and Prognosis Correlated with Immune Infiltrates in Patients with Sepsis
Authors Shi M , Wei Y, Guo R, Luo F
Received 21 December 2023
Accepted for publication 26 March 2024
Published 15 April 2024 Volume 2024:17 Pages 2285—2298
DOI https://doi.org/10.2147/JIR.S456132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tara Strutt
Mingjie Shi,1,2 Yue Wei,3 Runmin Guo,1,2 Fei Luo1,2
1Key Laboratory of Research in Maternal and Child Medicine and Birth Defects, Guangdong Medical University, Foshan, Guangdong, People’s Republic of China; 2Matenal and Child Research Institute, Shunde Women and Children’s Hospital (Maternity and Child Healthcare Hospital of Shunde Foshan), Guangdong Medical University, Foshan, People’s Republic of China; 3Department of Ultrasound, Shunde Women and Children’s Hospital (Maternity and Child Healthcare Hospital of Shunde Foshan), Guangdong Medical University, Foshan, People’s Republic of China
Correspondence: Fei Luo, Shunde Women and Children’s Hospital (Maternity and Child Healthcare Hospital of Shunde Foshan), Guangdong Medical University, Foshan, People’s Republic of China, Email [email protected]
Background: Sepsis is a major contributor to morbidity and mortality among hospitalized patients. This study aims to identify markers associated with the severity and prognosis of sepsis, providing new approaches for its management and treatment.
Methods: Data were mined from the Gene Expression Omnibus (GEO) databases and were analyzed by multiple statistical methods like the Spearman correlation coefficient, Kaplan-Meier analysis, Cox regression analysis, and functional enrichment analysis. Candidate indicator’ associations with immune infiltration and roles in sepsis development were evaluated. Additionally, we employed techniques such as flow cytometry and neutral red staining to evaluate its impact on macrophage functions like polarization and phagocytosis.
Results: Twenty-eight genes were identified as being closely linked to the severity of sepsis, among which transforming growth factor beta induced (TGFBI) emerged as a distinct marker for predicting clinical outcomes. Notably, reductions in TGFBI expression during sepsis correlate with poor prognosis and rapid disease progression. Elevated expression of TGFBI has been observed to mitigate abnormalities in sepsis-related immune cell infiltration that are critical to the pathogenesis and prognosis of the disease, including but not limited to type 17 T helper cells and activated CD8 T cells. Moreover, the protein-protein interaction network revealed the top ten genes that interact with TGFBI, showing significant involvement in the regulation of the actin cytoskeleton, extracellular matrix-receptor interactions, and phagosomes. These are pivotal elements in the formation of phagocytic cups by macrophages, squaring the findings of the Human Protein Atlas. Additionally, we discovered that TGFBI expression was significantly higher in M2-like macrophages, and its upregulation was found to inhibit lipopolysaccharide-induced polarization and phagocytosis in M1-like macrophages, thereby playing a role in preventing the onset of inflammation.
Conclusion: TGFBI warrants additional exploration as a promising biomarker for assessing illness severity and prognosis in patients with sepsis, considering its significant association with immunological and inflammatory responses in this condition.
Keywords: sepsis, septic shock, TGFBI, prognosis, biomarker
Introduction
Sepsis is a life-threatening condition characterized by an abnormal response of the body to an infection caused by bacteria, viruses, or other pathogens.1 It begins with an inflammatory reaction and immune system activation. Symptoms such as a high temperature, racing heart, and difficulty breathing may manifest at this stage, while vital signs like blood pressure and organ function are often unaffected. The inflammation can worsen and spread throughout the body if the infection is not adequately controlled, eventually leading to severe sepsis, which is characterized by symptoms of organ dysfunction such as decreased urine output, altered state of consciousness, and hypotension, posing a serious threat to the patient’s life.2,3 Septic shock is the most serious stage, characterized by persistent hypotension and poor response to fluid resuscitation, which often leads to multi-organ failure of the heart, lungs, kidneys, liver and other organs, and is prone to serious complications such as refractory hypotension, metabolic acidosis, and coagulation disorders, and is the main cause of disability and death in hospitalized patients.2,4 Hence, the management of sepsis centres around promptly identifying and intervening to halt its advancement towards severe sepsis or shock, thereby diminishing the likelihood of grave sequelae and mortality.
Recent years have seen an uptick in the amount of studies focusing on sepsis biomarkers, which have revealed markers not only for predicting the overall diagnosis and prognosis of sepsis but also for predicting harm to numerous systems.5–8 Investigating these biomarkers is crucial for learning about the pathophysiology of sepsis, achieving early diagnosis, tracking the development of the disease, and determining how well treatment worked.9 Currently commonly used, such as C-reactive protein (CRP) and interleukin-6 (IL-6) have inherent limitations in terms of sensitivity and specificity, which restrict their clinical applications. CRP is a protein that increases in response to acute-phase reactions, and its heightened concentration is strongly linked to inflammatory processes.10,11 IL-6, being a cytokine, can serve as an indicator of the magnitude of an inflammatory response due to its concentration fluctuations.12,13 Increased concentrations of lactate, however, are linked to insufficient oxygen supply to tissues and disruptions in metabolism, and are frequently employed to evaluate the level of severity in patients with sepsis.14,15 Along with these more established markers, researchers are on the lookout for more sensitive and specific novel markers to enhance diagnostic accuracy, detect high-risk patients at an earlier stage, track the progression of diseases, and assess the effectiveness of treatments. Syndecan-1 and platelets, for instance, are predictors of disseminated intravascular coagulation and mortality in patients with sepsis.16,17 In our study, we employed multiple publicly accessible datasets from the Gene Expression Omnibus (GEO) database to identify biomarkers associated with disease severity and prognosis in patients with sepsis to distinguish between individuals requiring intensified therapy for sepsis and those who do not, as well as subsequently assessed their association with septic immune cell infiltration and potential clinical applicability, hoping that new guidance will be provided for their clinical diagnosis and management.
Materials and Methods
Microarray Datasets Collection and Data Process
We have comprehensively analyzed the Microarray datasets of sepsis subjects derived from peripheral blood mononuclear cells (PBMC), which were downloaded GEO (https://www.ncbi.nlm.nih.gov/geo/) databases. Firstly, Limma packages with normalizeBetweenArrays function was utilized to merge and emend two datasets (GSE13904 and GSE66099), the combined dataset contained 65 normal controls, 70 sepsis, and 287 septic shock samples. GSE65682 containing 42 healthy controls and 479 sepsis and/or septic shock samples with clinic information was then used to explore the prognostic ability of candidate genes and their relationship with immune infiltration. GSE26378 and GSE13347 were also applied as external cohorts for sepsis and septic shock, respectively, to evaluate the expression levels and prediction abilities of candidate genes (Supplementary Table 2).
Identification of Differentially Expressed Genes (DEGs) Associated with Sepsis Progression and Functional Enrichment Analysis
Probes were converted into gene symbols in each dataset using the platform’s annotation file. In cases where numerous probes were mapped to the same gene symbol, the gene expression value was determined by calculating the mean value of these probes. DEGs among septic shock, sepsis and control were analyzed via the “limma package” in R software (http://bioconductor.org/packages/release/bioc/html/limma.html), with the following cutoff for adjustment: p value < 0.05 and |FC (fold changes) > 0.5|. The intersections of the three sets of DEGs are visualized by Venn diagrams, and their annotations and functional enrichment analysis on Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) were executed by “clusterProfiler” and “enrichplot” packages, respectively. The cutoff was determined to be a P value < 0.05.
Identifying and Exploring the Diagnostic and Prognostic Capabilities of Candidate DEGs
The GSE65682 dataset served a dual purpose in our study. On one hand, it was utilized to confirm the expression levels of the identified candidate DEGs. In another, samples with completed clinical data were applied to identify the potential prognosis-associated DEGs by lasso regression analyses, univariate-and multivariate Cox regression analyses. To investigate the clinical prognostic significance of TGFBI, the packages (survival and survminer) were employed to conduct univariate- and multifactor Cox regression analyses within the GSE65682 dataset, based on several clinic factors (age, diabetes, gender and endotype) and TGFBI expression.
Immune Cell Infiltration Evaluation and Correlation Analysis
The single-sample gene set enrichment analysis (ssGSEA, http://software.broadinstitute.org/gsea/msigdb/index.jsp), as a prominent and innovative enrichment algorithm that has emerged in recent years, has been widely employed in medical research to evaluate the immunological microenvironment of patients. In our study, we leveraged the ggplot2 package to create a volcano map, a visual representation that effectively illustrates the distribution of 23 types of immune cell infiltrations across various samples. Furthermore, this map was instrumental in demonstrating the influence of TGFBI expression on the distribution patterns of these immune cell infiltrations, providing valuable insights into the immunological impacts of TGFBI within the patient microenvironment.
Protein-Protein Interaction Network Analysis and Potential Function Exploration
The protein-protein network comprises a collection of proteins that engage in interactions with one another, thereby contributing to various fundamental biological processes like signal transduction, regulation of gene expression, metabolism of energy and materials, and control of the cell cycle. The integration of information regarding the functions of various proteins within cells can be effectively accomplished by including it into databases and subsequently representing it using protein network diagrams. STRING (version 11.5, https://string-db.org/)18 was utilized to explore the PPI networks and ten hub genes of TGFBI for further understanding of its physical and functional interplay. The visualization of the results was performed via Cytoscape (v.3.7.1, https://cytoscape.org/),19 an open-source network visualization tool, in order to enhance the performance of various interactions.
Cell Culture and TGFBI Overexpression
The THP-1 cells were acquired from the Central Laboratory of Central South University Xiangya and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere containing 5% carbon dioxide (CO2). First, M0 macrophage differentiation was stimulated in THP-1 cells using 100 nM phorbol 12-myristate 13-acetate (PMA, Sigma, Cat# P1585) for 24 hours. Next, the M0 macrophages were subjected to stimulation using 1 μg/mL of lipopolysaccharide (LPS, Sigma, Cat# L4516) for an additional 24 hours, or interleukin-4 (IL-4, Chamot Bio, Cat# CM006-5HP) at a concentration of 20 ng/mL for an additional 48 hours to induce the differentiation of M1- or M2-like macrophages, respectively. The expression of marker genes associated with M0 (CD11b and CD68), M1 (CD86, iNOS), and M2 (CD163, CD206) macrophages was examined to assess whether THP-1 cells were properly polarized. TGFBI lentivirus was engineered and packaged at Shenggong Biological Engineering (Shanghai) Co., Ltd, and its overexpression effectiveness was evaluated by immunoblotting (Affinity, DF7015).
Quantitative Polymerase Chain Reaction (q-PCR)
Total RNA from the intervened cells was isolated with TRIzol™ Reagent (Thermo Scientific, USA). The assessment of RNA purity and quantity was conducted utilizing the Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). The cDNA was synthesized using a 1st Strand cDNA Synthesis SuperMix (gDNA Purge) (NovoProtein, China). qPCR was carried out using the PowerUp™ SYBR™ Green Kit (Thermo Scientific, USA) and QuantStudio 5 machine (Thermo Scientific, USA), adhering to the guidelines provided by the manufacturer. An internal control, GAPDH, was chosen. All primer sequences used in q-PCR are listed in Supplementary Table 1.
Flow Cytometry Analysis
Cultured target cells were harvested and pelleted before being suspended in FACS buffer (PBS, 1% BSA, 2.5 mM EDTA, and 0.01% sodium azide) at a volume of 2.5 mL. The cell pellets were resuspended in FcR blocking reagent (diluted 1:10 in FACS buffer; Miltenyi Biotec, Leiden, Netherlands) and afterwards incubated for a duration of 10 minutes at a temperature range of 2–8°C to inhibit the activity of FcR receptors on THP-1-derived macrophages. Cell pellets were centrifuged, and then stained with PE-labeled anti-F4/80 (5 μL/106 cells; clone; BM8; Bio-Rad) for 20 minutes at 2–8°C to identify macrophages among the dead cells. Staining macrophages with FITC-labeled anti-CD11c (0.5 μL/106 cells; clone: N418; Bio-Green) for 20 minutes at 2–8°C enables the identification of M1 subtype macrophages. Following the staining of cell pellets, they were washed twice with a 1 Perm/Wash Buffer, and then analyzed on a flow cytometer. To properly recognise positive F4/80 and CD11c signals, the gating strategy was devised to ensure that the overlap with the isotype control signal did not exceed 5%. Flow cytometry (FACScantoII System, BD Biosciences, San Jose, CA, USA) was utilized to identify macrophage polarization, and the data was analyzed with FlowJo.
Neutral Red Uptake Assay
The effect of transforming growth factor beta induced (TGFBI) on phagocytosis of THP-1 cells was assessed by a neutral red uptake assay. The appropriate number of target cells were cultured in six-well plates, treated with 100 nM PMA for 24 hours induced into macrophages, and then added 1 μg/mL LPS intervention for 24 hours. The cell cultures were then removed and washed twice with PBS solution. Staining solution was added at the proper concentration to guarantee complete cell coverage, and the cells were stained for 5 minutes before being washed three times in PBS solution before being observed and photographed.
Enzyme Linked Immunosorbent Assay (ELISA)
Human IL-6 and IL-10 ELISA kits were purchased from Meimian Industrial Co., Ltd., Jiangsu, China; all the other chemicals used were of analytical grade. Their contents were determined via commercially available kits in accordance with the instructions provided by the manufacturer. All samples were analyzed in duplicate, and the data are reported as either medians or means.
Statistical Analysis
All statistical analyses were performed using R software (version 4.1.0) with multiple packages like Limma, ggplot2, survminer, ect., and GraphPad Prism software (version 8.4.3). Receiver operating characteristic (ROC) curves were used to evaluate TGFBI’s diagnostic accuracy, with results given as the area under the ROC curves (AUROC) and 95% confidence intervals (CIs). The optimal cut-off value, sensitivity, and specificity of the candidate gene were determined by maximizing the Youden’s index. Spearman correlation coefficient was employed to assess the bonds between TGFBI and immune cells. All hypothetical tests conducted in this study were two-tailed, and a significance level of P value < 0.05 was used to determine statistical significance.
Results
Identification of Common DEGs in Sepsis and Sepsis Shock
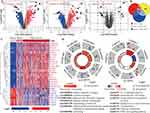
Sepsis is a complex syndrome where infection triggers a dysregulated host response, potentially leading to life-threatening organ damage, and is a clinical condition characterized by high rates of morbidity and mortality. We selected two datasets encompassing cases of sepsis and septic shock, and the study process and the detailed characteristics are shown in Figure 1 and Supplementary Table 2. Initially, we used |logFC| ≥ 0.5 and adjust P-value < 0.05 as screening criteria to identify progression genes in sepsis to septic shock. This condition led to the identification of 2209 genes aberrantly expressed in sepsis, 2571 in septic shock, and 35 in the progression from sepsis to septic shock (Figure 2A). Ultimately, 28 genes were pinpointed as key players in the development and progression of sepsis (Figure 2B). Of these, 20 genes showed gradual increases the onset of sepsis or progression to septic shock, while the remaining 8 genes demonstrated the opposite trend (Figure 2C). As a preliminary step toward elucidating the processes these genes participate in, we used GeneMANIA (http://genemania.org/) to identify 25 additional genes with potentially similar functions (Supplementary Figure 1). Subsequent enrichment analysis revealed their primary involvement in the defense response to fungus and the IL-17 signaling pathway (Figure 2D), shedding light on the molecular mechanisms underlying sepsis and its progression.
 |
Figure 1 A flow diagram exhibiting the process analysis of the study. |
An Assessment of the Candidate Genes’ Expression and Identification of the Target TGFBI with a Prognosis
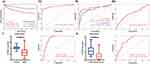
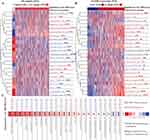
Next, extensive validation of these 28 gene expressions using the external datasets (GSE65682) found them to all be significantly different in expression, with trends corroborating our initial findings (Figure 3A). Five genes (CECR1, G0S2, HLA-DMB, MME and TGFBI) were incorporated into the Lasso regression analyses that followed the univariate Cox regression analyses, exhibiting that their association with patients’ overall survival (P-value < 0.05, Figure 3B). A multivariate Cox proportional hazards regression analysis was later conducted to identify the target TGFBI as an independent predictor for predicting the overall survival of the patients (HR 0.73, 95% CI 0.62–0.87, P < 0.001; Figure 3B). TGFBI and other clinical parameters (such as age, gender, diabetes and endotype) were evaluated in relation to patient survival, using samples with comprehensive clinical data. Both univariate (HR 0.74, 95% CI 0.63–0.88, P < 0.001; Figure 3C) and multivariate (HR 0.77, 95% CI 0.65–0.92, P < 0.01; Figure 3C) Cox regression analyses showed a significant correlation between TGFBI expression and overall survival in patients with sepsis. Meanwhile, the Kaplan-Meier analysis indicated that high TGFBI expression positively correlated with prognosis, showing significantly better overall survival compared to those with low expression (Figure 4Ai). More, the AUROC for TGFBI in diagnosing sepsis patients was notably high at 0.916 (95% CI 0.852–0.980), with a sensitivity of 0.814 and specificity of 0.952 (Figure 4Aii). TGFBI also had a significant predictive ability to distinguish patients with sepsis (AUROC 0.848, 95% CI 0.788–0.908; Figure 4Bi) and septic shock (AUROC 0.786, 95% CI 0.705–0.866; Figure 4Bi) patients, as shown by the merged datasets. However, its predictive capacity was moderately effective in differentiating between patients with and without septic shock (AUROC 0.623, 95% CI 0.545–0.701; Figure 4Bii). Furthermore, we confirmed the readily observable differential expression levels accompanied by the favorable predictive power of TGFBI in two additional sepsis (GSE134347, Figure 4C) and septic shock (GSE26378, Figure 4D) datasets.
Effects of TGFBI on Immunological Homeostasis Changes During Sepsis
Sepsis typically involves an initial phase characterized by systemic inflammation, followed by a phase of immunosuppression and organ dysfunction. The formation and course of this condition are significantly influenced by immune cells, which also play a crucial role in determining patient prognosis. Here, we used the ssGSEA algorithm to explore the variations in immune cell expression between normal donors and sepsis patients. Additionally, an examination was conducted to assess the potential association between TGFBI and these immune cells. Patients with sepsis exhibited profound immunological dysregulation, with a marked decrease in the proportion of 14 immune cells represented by innate dendritic cells and a substantial increase in the proportion of 9 immune cells, including activated dendritic cells. This imbalance in immune cell regulation highlights the complex and severe alterations in the immune system associated with sepsis, underlining the critical need for targeted therapeutic strategies to address these immune dysfunctions. Intriguingly, we discovered that cells involved in mediating inflammatory factor secretion, such as activated dendritic cells and type 17 T helper cells, were significantly upregulated in sepsis patients. In contrast, immunomodulatory cells, like innate dendritic cells, activated and innate B cells, were notably suppressed (Figure 5A). On the other hand, patients with sepsis who expressed high levels of TGFBI exhibited improved outcomes due to increased infiltration of immunomodulatory cells like dendritic cells, activated and innate B cells, activated CD8 T cells, natural killer T cells, etc., and reduced infiltration of cells mediating inflammatory factor secretion, such as activated dendritic cells and type 17 T helper cells (Figure 5B). We also investigated the prognostic ability of immune cells in sepsis and their correlation with TGFBI levels. TGFBI levels correlated with specific immunocytes associated with clinical outcomes in patients with sepsis, such as type 17 T helper cells and T follicular helper cells, highlighting the pivotal role of TGFBI in modulating the immune response in sepsis (Figure 5C).
TGFBI’s Role in Polarization, Phagocytosis and Mediating Inflammation of Macrophages
To better understand the potential functions of TGFBI, we first identified top 10 genes with clear reciprocal relationships through the String database (Figure 6A). The functional enrichment of them revealed their involvement primarily in regulating actin cytoskeleton, extracellular matrix-receptor connections, phagosomes, etc. (Figure 6B). Next, a single-cell analysis from the Human Protein Atlas database revealed that TGFBI was highly expressed in macrophages, monocytes, and dendritic cells within PBMC (Figure 6C). TGFBI functions, such as regulating the actin cytoskeleton and phagosomes, are required for macrophages to form phagocytic cups, suggesting that TGFBI plays a significant role in the activation of macrophage activity. Moreover, TGFBI was found to be highly expressed in M2-type macrophages, as evidenced by the macrophage sequencing dataset (GSE159112) and then verified by q-PCR in macrophages derived from THP-1 cells (Figure 6D and Supplementary Figure 2). Flow cytometric analysis revealed that LPS treatment in TGFBI overexpressing macrophages resulted in a 32.8% reduction in F4/80+CD11c+ cells compared to the LPS-treated group (Figure 6E). Additionally, neutral red staining results also showed that TGFBI diminished the phagocytic capacity of LPS-induced M1-like macrophages (Figure 6F). Then, it was found in our TGFBI overexpressing stable-transformed cells that LPS-stimulated TGFBI-high expressing macrophages (THP-1 derived) showed decreased production of both pro-inflammatory (IL-6) and anti-inflammatory (IL-10) molecules, indicating its ability to ameliorate inflammation and boost immunological function (Figure 6G).
Discussion
Sepsis is a highly heterogeneous disease, with its development and severity influenced by the balance between cytokine storm and the intensity of immunosuppression.20,21 As a first step in identifying regulators implicated in the severity of sepsis, we used two shared datasets to search for genes that were differentially expressed in three different conditions (healthy or non-infected, sepsis, and septic shock). Twenty-eight genes, including carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), Resistin (RETN), Methyltransferase like 7B (METTL7B), and others, were ultimately found to play a role in the onset and progression of sepsis, with these genes and their regulatory counterparts primarily concentrated in the interleukin 17 signaling pathway. We next used an external database (GSE65682) to investigate the levels of these 28 genes and found expression trends that were in line with our initial observations. Many of these genes have been previously reported to be closely associated with the progress of sepsis. For instance, Catton et al22 discovered that CEACAM1, which is specifically targeted by the bacterial R28 protein, promotes postpartum sepsis development by interactions such as cervical cell adhesion, epithelial wound healing suppression, and modification of innate immune responses. Karampela et al23 noted a significant increase in RETN in critical illness and sepsis, correlating its levels with the severity and outcomes of sepsis. METTL7B, is upregulated in sepsis and regulates lipopolysaccharide-induced inflammatory responses and macrophage polarization.24 To further identify novel regulators that are more clinically relevant, we explored their prognostic capabilities in a dataset containing clinical information by multiple bioinformatic means and showed that TGFBI may be used as a standalone predictor of clinical outcome and has a high diagnostic predictive value. Additional sepsis (GSE134347) and septic shock (GSE26378) datasets confirmed its differential expression and the predictive value. Notably, the expression of TGFBI is downregulated or absent in various malignancies, such as lung,25 breast,26 ovarian,27 and prostate,28 and is associated with poor prognosis in patients as well as sensitivity to and efficacy of chemotherapy. However, the exact clinical roles and regulatory mechanisms of TGFBI in sepsis remain unclear. Additional research into TGFBI’s function may enhance sepsis diagnosis and treatment efficacy, as our findings implies that it is pivotal to the development and growth of the disease.
Matricellular TGFBI, a nonstructural protein on human chromosome 5 with a molecular weight of 68 kDa, is expressed in several cell types and organs, particularly in response to transforming growth factor β (TGF-β) signaling.29,30 TGFBI indirectly affects the biological activity of TGF-β1 by participating in cell-matrix interactions and cell migration, and thus plays a key role in various biological processes.31,32 Fibronectin, SPARC, and some collagen proteins are just a few examples of extracellular matrix proteins that it has been shown to interact with.33 As of now, while research into the relationship between TGFBI and sepsis is still relatively limited, the role and mechanisms of the TGF-β signaling pathway in sepsis have emerged as significant areas of focus in clinical and basic research, with numerous key achievements already accomplished.34–36 Our findings suggests that TGFBI may be an additional vital regulator in the TGF-β signalling pathway that contributes to the onset and progression of sepsis. An example of this is the fact that TGFBI lacks a novel protective effect against obesity, which is achieved through activation of the Notch-1 signaling pathway, as discovered by Lee et al.37 More recent research on TGFBI has shown its significant involvement in non-neoplastic disorders such osteoarthritis and corneal dystrophy, as well as in tumorigenesis and progression that varies according to tumour type and relative stage of the tumour.38–41 Cancer cell biology experiments in vitro show that overexpressing TGFBI reduces migration and colony formation in pancreatic, lung, and breast cancer cells.41–43 In a mouse model of primary breast cancer, overexpressing TGFBI similarly suppresses tumour growth.44 These findings are in line with clinical data from cancer patients, which indicate that higher expression levels of TGFBI mRNA in non-small cell lung cancer patients are linked to a better prognosis, especially when combined with adjuvant chemotherapy.41,45 One explanation for these occurrences is that TGFBI has the ability to enhance αvβ3 integrin signalling through integrin-bound Arg-Gly-Asp (RGD) motifs, which in turn mediates its inhibitory effects on carcinogenesis and progression through influencing apoptosis and angiogenesis, etc.46 As in melanoma cells, TGFBI hinders αvβ3-VEGFR2 signaling via Akt and MAPK signaling through its fourth Fas1 structural domain and RGD motifs, thereby reducing angiogenesis-induced tumor growth in vitro and in vivo.47,48 Something also crucial is that TGFBI could affect the microenvironment of the organism, which in turn can alleviate or aggravate disease progression.49,50 Studies have shown that TGFBI can modulate macrophage responses to both normal and malignant cells, while also impacting the synthesis of inflammatory cytokines.50,51 Patient immunological abnormalities, which can affect the onset and progression of sepsis, can be caused by a lack of TGFBI due to its central role in the generation and destruction of immune peptides. Several types of immunomodulatory cells, including immature and activated dendritic cells, immature and activated B cells, type 1/2/17 T helper cells, activated CD8 T cells, eosinophils, monocytes, myeloid-derived suppressor cells (MDSC), natural killer T cells, and T follicular helper cells, were disrupted in sepsis individuals but improved significantly in the patients with higher TGFBI expression. Additionally, The expression level of TGFBI was substantially correlated with the degree of immune cell infiltration, which was primarily related to the prognosis of sepsis patients. In sepsis patients, for instance, a high expression of TGFBI is tied with a poor prognosis and shows a significant inverse correlation with expression of type 17 T helper cells, which are known for being highly pro-inflammatory, characterized by their abundant production of IL-17A, IL-17F, IL-21, and IL-22.52 In instances of bacterial infections like sepsis, signals may be sent to attract eosinophils to the affected tissue, leading to a general decrease in eosinophil levels in the bloodstream.53 The presence of higher eosinophil counts in patients with higher TGFBI expression could explain why these individuals experience a milder form of sepsis and have a better prognosis compared to those with lower TGFBI expression.
Unrestrained immunological response due to excessive production of inflammatory mediators by the organism leading to immune dysfunction is at the core of sepsis pathogenesis.54,55 TGFBI’s potential link to both inflammation and immunity makes it an intriguing candidate for use in clinical trials of sepsis. Data from the single cell panel of The Human Protein Atlas suggest that TGFBI is highly expressed by PBMC macrophages, monocytes, and dendritic cells, making it a potential key modulator of the immune response. Indeed, inflammation brought on by pathogens like viruses and bacteria is likely to have a significant role in sepsis development.56 Studies have shown that TGFBI, a protein produced mainly by both monocytes and macrophages, decreases NK cell function and hence interferes with the efficacy of immunotherapy.51 Afterward, we evaluated the proteins with the most significant interactions with TGFBI using the String database and found that they were mainly involved in the regulation of the actin cytoskeleton, phagosomes, and other signaling pathways. The regulation of actin dynamics is required for macrophages to form phagocytic cups, which are necessary for the absorption of apoptotic cells and the mediation of the activity of inflammatory agents. Our findings, which were corroborated by Peng et al57 and Lecker et al,51 showed that TGFBI expression was much higher in M2 macrophages, and that its higher expression was associated with reduced inflammation, suggesting its potential clinical value in the treatment and management of sepsis.
In addition, several problems plague this study. Primarily, it is based on pre-existing data from the public database, where each dataset encompasses a limited sample size and lacks crucial predictive clinical information such as treatment specifics and underlying health conditions. Second, Secondly, the clinical relevance of TGFBI for diagnosis and prognosis remains constrained, as its application has not been further validated in a large-scale prospective study. Third, the impact of variables like gender and age on the expression levels of TGFBI is yet to be determined, highlighting the necessity for clinical validation. This ambiguity emphasizes the importance of exploring how TGFBI modulation may influence sepsis progression across various age brackets and genders, potentially worsening or alleviating the disease’s severity. Finally, and most critically, there is a pressing need for future research to elucidate and confirm the distinct regulatory mechanisms of TGFBI in the initiation and progression of sepsis to advance our understanding of TGFBI’s role in this complex condition, which is key to its clinical application as a therapeutic target or a biomarker. While our study provides valuable insights, it also highlights the necessity for more comprehensive research to fully leverage the potential of TGFBI in sepsis management.
To sum up, our study provides the first in-depth exploration of the link between TGFBI and the onset and progression of sepsis and the functional role in this process. It was discovered that TGFBI is a promising biomarker for determining the severity and prognosis of sepsis patients and that its putative relationship with immunological homeostasis provides unique insights into the therapy of sepsis. However, the effectiveness of TGFBI in assessing the severity and prognosis of sepsis, as well as its specific regulatory mechanisms, still requires further in-depth investigation and exploration. In addition to shedding light on the intricate processes of sepsis, these results serve as a valuable insight for the development of more effective clinical treatments in the future.
Data Sharing Statement
The datasets that are accessible to the public can be obtained by downloading them from the GEO database (https://www.ncbi.nlm.nih.gov/gds/) using the following accession numbers: GSE13904, GSE66099, GSE65682, GSE26378, and GSE134347.
Ethics Statement
The GEO is a publicly accessible database that houses genomic data of patients who have obtained ethical approval. Researchers can freely access and download pertinent data for the purpose of conducting research and publishing relevant scientific articles. This study did not involve any trials with humans or animals, and it underwent approval by the Ethics Committee of Shunde Women and Children’s Hospital (Maternity and Child Healthcare Hospital of Shunde Foshan), affiliated with Guangdong Medical University (LW-2023-009).
Acknowledgments
We appreciate the researchers who contributed to the GEO database by making their data publicly available.
Funding
This work was supported by grants from the Medical Research Project of Foshan Municipal Health Bureau (20210190) and Foshan Self-Financed Science and Technology Innovation Project (2320001006342).
Disclosure
The authors state that they have no financial or commercial ties that could be seen as a conflict of interest with the research.
References
1. Russell J. Management of sepsis. New Engl J Med. 2006;355(16):1699–1713. doi:10.1056/NEJMra043632
2. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi:10.1097/CCM.0b013e31827e83af
3. Gilbert J. Sepsis care bundles: a work in progress. Lancet Respir Med. 2018;6(11):821–823. doi:10.1016/s2213-2600(18)30362-x
4. Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/s0140-6736(18)30696-2
5. Scicluna B, Wiewel M, van Vught L, et al. Molecular biomarker to assist in diagnosing abdominal sepsis upon ICU admission. Am J Respir Crit Care Med. 2018;197(8):1070–1073. doi:10.1164/rccm.201707-1339LE
6. Mikacenic C, Price B, Harju-Baker S, et al. A two-biomarker model predicts mortality in the critically ill with sepsis. Am J Respir Crit Care Med. 2017;196(8):1004–1011. doi:10.1164/rccm.201611-2307OC
7. Downes KJ, Fitzgerald JC, Weiss SL. Utility of procalcitonin as a biomarker for sepsis in children. J Clin Microbiol. 2020;58(7). doi:10.1128/jcm.01851-19
8. Donadello K, Scolletta S, Covajes C, et al. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012;10(2). doi:10.1186/1741-7015-10-2
9. Faix JD. Biomarkers of sepsis. Critical Rev Clin Lab Sci. 2013;50(1):23–36. doi:10.3109/10408363.2013.764490
10. Póvoa P, Teixeira-Pinto AM, Carneiro AH. C-reactive protein, an early marker of community-acquired sepsis resolution: a multi-center prospective observational study. Critical Care. 2011;15(4):R169. doi:10.1186/cc10313
11. Salluh JI, Lisboa T. C-reactive protein in community-acquired sepsis: you can teach new tricks to an old dog. Critical Care. 2011;15(5):186. doi:10.1186/cc10301
12. Molano Franco D, Arevalo-Rodriguez I, Roqué IFM, et al. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst Rev. 2019;4(4):Cd011811. doi:10.1002/14651858.CD011811.pub2
13. de Matos Morawski F, Dias GBM, Sousa KAP, et al. Chitosan/genipin modified electrode for voltammetric determination of interleukin-6 as a biomarker of sepsis. Int J Biol Macromol. 2023;224:1450–1459. doi:10.1016/j.ijbiomac.2022.10.232
14. Yang K, Fan M. Lactate induces vascular permeability via disruption of ve-cadherin in endothelial cells during sepsis. Int J Med. 2022;8(17):eabm8965. doi:10.1126/sciadv.abm8965
15. Puskarich MA, Trzeciak S, Shapiro NI, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest. 2013;143(6):1548–1553. doi:10.1378/chest.12-0878
16. Haynes A, Ruda F, Oliver J, et al. Syndecan 1 shedding contributes to pseudomonas aeruginosa sepsis. Infect Immun. 2005;73(12):7914–7921. doi:10.1128/iai.73.12.7914-7921.2005
17. Cox D. Sepsis - it is all about the platelets. Front Immunol. 2023;14:1210219. doi:10.3389/fimmu.2023.1210219
18. Szklarczyk D, Kirsch R, Koutrouli M, et al. The string database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51:D638–D646. doi:10.1093/nar/gkac1000
19. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
20. Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14(2):121–137. doi:10.1038/nrneph.2017.165
21. Iskander K, Osuchowski M, Stearns-Kurosawa D, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93(3):1247–1288. doi:10.1152/physrev.00037.2012
22. Catton EA, Bonsor DA, Herrera C, et al. Human ceacam1 is targeted by a streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis. Pathogenesis. 2023;14(1):2275. doi:10.1038/s41467-023-37732-1
23. Karampela I, Christodoulatos GS, Dalamaga M. The role of adipose tissue and adipokines in sepsis: inflammatory and metabolic considerations, and the obesity paradox. Current Obesity Rep. 2019;8(4):434–457. doi:10.1007/s13679-019-00360-2
24. Huang D, Yan H. Methyltransferase like 7b is upregulated in sepsis and modulates lipopolysaccharide-induced inflammatory response and macrophage polarization. Bioengineered. 2022;13(5):11753–11766. doi:10.1080/21655979.2022.2068892
25. Nakazawa N, Yokobori T, Turtoi A, et al. Aso author reflections: high stromal tgfbi is a useful predictive marker for nivolumab in non-small cell lung cancer. Ann Surg Oncol. 2020;27(3):943–944. doi:10.1245/s10434-019-08057-5
26. Fico F, Santamaria-Martínez A. Tgfbi modulates tumour hypoxia and promotes breast cancer metastasis. Mol Oncol. 2020;14(12):3198–3210. doi:10.1002/1878-0261.12828
27. Steitz A, Steffes A, Finkernagel F, et al. Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (tgfbi) and tenascin c. Cell Death Dis. 2020;11(4):249. doi:10.1038/s41419-020-2438-8
28. Shah J, Shao G, Hei T, et al. Methylation screening of the TGFBI promoter in human lung and prostate cancer by methylation-specific PCR. BMC Cancer. 2008;8:284. doi:10.1186/1471-2407-8-284
29. Skonier J, Neubauer M, Madisen L, et al. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 1992;11(7):511–522. doi:10.1089/dna.1992.11.511
30. Ween MP, Oehler MK, Ricciardelli C. Transforming growth factor-beta-induced protein (tgfbi)/(βig-h3): a matrix protein with dual functions in ovarian cancer. Int J Mol Sci. 2012;13(8):10461–10477. doi:10.3390/ijms130810461
31. Mosher DF, Johansson MW, Gillis ME, et al. Periostin and tgf-β-induced protein: two peas in a pod? Crit. Rev. Biochem. Mol. Biol. 2015;50(5):427–439. doi:10.3109/10409238.2015.1069791
32. Thapa N, Lee BH, Kim IS. Tgfbip/betaig-h3 protein: a versatile matrix molecule induced by tgf-beta. Int J Biochem Cell Biol. 2007;39(12):2183–2194. doi:10.1016/j.biocel.2007.06.004
33. Tumbarello DA, Andrews MR, Brenton JD. Sparc regulates transforming growth factor beta induced (tgfbi) extracellular matrix deposition and paclitaxel response in ovarian cancer cells. PLoS One. 2016;11(9):e0162698. doi:10.1371/journal.pone.0162698
34. Sun J, Ge X, Wang Y, et al. Usf2 knockdown downregulates thbs1 to inhibit the tgf-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol Res. 2022;176:105962. doi:10.1016/j.phrs.2021.105962
35. de Pablo R, Monserrat J, Reyes E, et al. Sepsis-induced acute respiratory distress syndrome with fatal outcome is associated to increased serum transforming growth factor beta-1 levels. Eur J Internal Med. 2012;23(4):358–362. doi:10.1016/j.ejim.2011.10.001
36. Marie C, Cavaillon JM, Losser MR. Elevated levels of circulating transforming growth factor-beta 1 in patients with the sepsis syndrome. Ann Internal Med. 1996;125(6):520–521. doi:10.7326/0003-4819-125-6-199609150-00034
37. Lee SG, Chae J, Woo SM, et al. Tgfbi remodels adipose metabolism by regulating the notch-1 signaling pathway. Exp Mol Med. 2023;55(3):520–531. doi:10.1038/s12276-023-00947-9
38. Ruiz M, Toupet K, Maumus M, et al. Tgfbi secreted by mesenchymal stromal cells ameliorates osteoarthritis and is detected in extracellular vesicles. Biomaterials. 2020;226:119544. doi:10.1016/j.biomaterials.2019.119544
39. Nielsen NS, Poulsen ET, Lukassen MV, et al. Biochemical mechanisms of aggregation in tgfbi-linked corneal dystrophies. Prog Retinal Eye Res. 2020;77:100843. doi:10.1016/j.preteyeres.2020.100843
40. Han B, Cai H, Chen Y, et al. The role of tgfbi (βig-h3) in gastrointestinal tract tumorigenesis. Mol Cancer. 2015;14:64. doi:10.1186/s12943-015-0335-z
41. Pajares MJ, Agorreta J, Salvo E, et al. Tgfbi expression is an independent predictor of survival in adjuvant-treated lung squamous cell carcinoma patients. Br. J. Cancer. 2014;110(6):1545–1551. doi:10.1038/bjc.2014.33
42. Rudra-Ganguly N, Lowe C, Mattie M, et al. Discoidin domain receptor 1 contributes to tumorigenesis through modulation of tgfbi expression. PLoS One. 2014;9(11):e111515. doi:10.1371/journal.pone.0111515
43. Wen G, Partridge MA, Li B, et al. Tgfbi expression reduces in vitro and in vivo metastatic potential of lung and breast tumor cells. Cancer Lett. 2011;308(1):23–32. doi:10.1016/j.canlet.2011.04.010
44. Calaf GM, Echiburú-Chau C, Zhao YL, et al. Bigh3 protein expression as a marker for breast cancer. IntJ Mol Med. 2008;21(5):561–568.
45. Seok Y, Lee WK, Park JY, et al. Tgfbi promoter methylation is associated with poor prognosis in lung adenocarcinoma patients. Mol Cells. 2019;42(2):161–165. doi:10.14348/molcells.2018.0322
46. Cheng Y, He C, Wang M, et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduction Targeted Therapy. 2019;4:62. doi:10.1038/s41392-019-0095-0
47. Nam JO, Son HN, Jun E, et al. Fas1 domain protein inhibits vegf165-induced angiogenesis by targeting the interaction between vegfr-2 and αvβ3 integrin. Molecular Cancer Res. 2012;10(8):1010–1020. doi:10.1158/1541-7786.mcr-11-0600
48. Nam JO, Jeong HW, Lee BH, et al. Regulation of tumor angiogenesis by fastatin, the fourth fas1 domain of betaig-h3, via alphavbeta3 integrin. Cancer Res. 2005;65(10):4153–4161. doi:10.1158/0008-5472.can-04-2705
49. Ahmed AA, Mills AD, Ibrahim AE, et al. The extracellular matrix protein tgfbi induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell. 2007;12(6):514–527. doi:10.1016/j.ccr.2007.11.014
50. Nummela P, Lammi J, Soikkeli J, et al. Transforming growth factor beta-induced (tgfbi) is an anti-adhesive protein regulating the invasive growth of melanoma cells. Am J Pathol. 2012;180(4):1663–1674. doi:10.1016/j.ajpath.2011.12.035
51. Lecker L, Berlato C, Maniati E, et al. Tgfbi production by macrophages contributes to an immunosuppressive microenvironment in ovarian cancer. Cancer Res. 2021;81(22):5706–5719. doi:10.1158/0008-5472.can-21-0536
52. Annunziato F, Cosmi L, Liotta F, et al. Type 17 t helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5(6):325–331. doi:10.1038/nrrheum.2009.80
53. Merino C, Martínez F, Cardemil F, et al. Absolute eosinophils count as a marker of mortality in patients with severe sepsis and septic shock in an intensive care unit. J Crit Care. 2012;27(4):394–399. doi:10.1016/j.jcrc.2011.10.010
54. Rubio I, Osuchowski M, Shankar-Hari M, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;19(12):e422–e436. doi:10.1016/s1473-3099(19)30567-5
55. van der Poll T, Shankar-Hari M, Wiersinga W. The immunology of sepsis. Immunity. 2021;54(11):2450–2464. doi:10.1016/j.immuni.2021.10.012
56. Sweeney T, Shidham A, Wong H, et al. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci, trans med. 2015;7(287):287ra271. doi:10.1126/scitranslmed.aaa5993
57. Peng P, Zhu H, Liu D, et al. Tgfbi secreted by tumor-associated macrophages promotes glioblastoma stem cell-driven tumor growth via integrin αvβ5-src-stat3 signaling. Theranostics. 2022;12(9):4221–4236. doi:10.7150/thno.69605
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.