Back to Journals » OncoTargets and Therapy » Volume 12
Inhibition of the hedgehog pathway for the treatment of cancer using Itraconazole
Authors Li K, Fang D, Xiong Z, Luo R
Received 13 July 2019
Accepted for publication 7 August 2019
Published 23 August 2019 Volume 2019:12 Pages 6875—6886
DOI https://doi.org/10.2147/OTT.S223119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Ke Li,1 Dengyang Fang,1 Zuming Xiong,1 Runlan Luo2
1Department of General Surgery, Fuling Central Hospital of Chongqing City, Chongqing, People’s Republic of China; 2Department of Ultrasound, Fuling Central Hospital of Chongqing City, Chongqing, People’s Republic of China
Correspondence: Runlan Luo
Department of Ultrasound, Fuling Central Hospital of Chongqing City, Chongqing 408000, People’s Republic of China
Email [email protected]
Abstract: Itraconazole (ITZ) is an anti-fungal drug that has been used in clinical practice for nearly 35 years. Recently, numerous experiments have shown that ITZ possesses anti-cancer properties. The Hedgehog (Hh) pathway plays a pivotal role in fundamental processes, including embryogenesis, structure, morphology and proliferation in various species. This pathway is typically silent in adult cells, and inappropriate activity is linked to various tumor types. The most important mechanism of ITZ in the treatment of cancer is inhibition of the Hh pathway through the inhibition of smoothened receptors (SMO), glioma-associated oncogene homologs (GLI), and their downstream targets. In this review, we discuss the mechanisms of ITZ in the treatment of cancer through inhibition of the Hh pathway, which includes anti-inflammation, prevention of tumor growth, induction of cell cycle arrest, induction of apoptosis and autophagy, prevention of angiogenesis, and drug resistance. We also discuss the clinical use of ITZ in many types of cancers. We hope this review will provide more information to support future studies on ITZ in the treatment of various cancers.
Keywords: Itraconazole, cancer, hedgehog pathway, apoptosis, autophagy
Introduction
According to the Global Cancer Statistics 2018, there would have 18.1 million new cancer cases and 9.6 million deaths from cancer worldwide.1 Increasing global demographic trends and epidemiologic transitions indicate an ever-increasing cancer burden over the coming decades, particularly in low- and middle-income countries, with over 20 million new cancer cases expected annually as early as 2025.2 However, the efficacy of current commonly used treatment methods such as surgery, chemotherapy, and radiation therapy are not satisfactory. However, the newer methods including immunotherapy, targeted therapy, and stem cell transplantation are expensive and many families cannot afford them. Therefore, a cheap and effective drug is needed for the treatment of cancer.
Itraconazole (ITZ, C35H38Cl2N8O4) (Figure 1) is a broad-spectrum, antifungal agent that has been used clinically for nearly 35 years. It can be used for the treatment of fungal infections, including candidiasis, aspergillosis, and histoplasmosis, and for prophylaxis in immunosuppressive disorders,3,4 mainly through the inhibition of lanosterol 14-α-demethylase (14LDM) to reduce the production of ergosterol in fungi and cholesterol in mammals.5,6 ITZ is a relatively safe drug with clear pharmacokinetic characteristics and minimal side effects, including neutropenia, liver failure, and heart failure.4 Recently, a large number of experiments have demonstrated that ITZ possesses anti-cancer properties and has already been assessed in cancer therapy,7–9 including in basal cell carcinoma,10 prostate cancer,11 gastric cancer,12 and non-small cell lung cancer.13 The underlying mechanisms of ITZ in cancer treatment include suppressing inflammation, arresting the cell cycle, inducing apoptosis and autophagy, and inhibiting angiogenesis and drug resistance.14–16
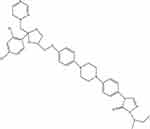 |
Figure 1 The chemical structure of Itraconazole. |
The hedgehog (Hh) pathway was originally identified in Drosophila17 and described by Nüsslein-Volhard and Wieschaus in 1980.18 Hh pathway plays a pivotal role in fundamental processes, including embryogenesis, structure, morphology, and proliferation in various species,19 it functions in the steady state of post-embryonic tissues through effects on stem cells.20 In adult tissues, the Hh pathway is typically silent, and inappropriate activity is linked to various tumor types,19 such as thoracic cancers including small-cell lung cancer,21 non-small cell lung cancer,22–24 basal cell carcinomas,25 medulloblastoma,26 cervical cancer,27 endometrial cancer,28 malignant melanoma,13 breast cancer,29 and malignant pleural mesothelioma.30 It is theorized that ITZ could effectively suppress the Hh pathway to treat cancer.12,27,31 In this review, we summarize the mechanism underlying the inhibition of the Hh pathway by ITZ and discuss its potential in the treatment and prevention of tumors.
An overview of Itraconazole and hedgehog pathway
Hedgehog pathway composition and activation
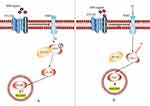
Hedgehog encodes a 45 kDa protein with a 20 kDa active N-terminal fragment that covalently binds to cholesterol.32 Three Hh ligands, including sonic hedgehog (SHh), Indian hedgehog (IHh), and Desert hedgehog (DHh), have been identified in mammals.10 SHh is the best studied and is expressed widely in tissues, while IHh is expressed in small amounts in some tissues, and DHh is expressed only in gonadal tissues. Ultimately, expression is dependent on different patterns of ligand expression, although the physiological effects may be the same.19,33 In adult tissues, the Hh pathway is mostly inactive or poorly active.34 (Figure 2A) Patched (PTCH), 12 trans-membrane protein receptors including PTCH1 and PTCH2, could inhibit the smoothened receptor (SMO), a 7-pass transmembrane G-protein coupled signal transduction molecule that contains three main domains including a seven-transmembrane helices domain, a hinge domain, and an intact extracellular cysteine-rich domain in human,35 to suppress the Hh pathway.36 The link between Hh pathway and human cancers has long been recognized.36 Once Hh is overexpressed (Figure 2B), its ligands are released to bind with PTCH immediately, thereby alleviating the inhibition of SMO by PTCH. The activated SMO is then translocated from vesicles to the primary cilium of the cell membrane, in order to activate a signaling cascade; this includes the glioma-associated oncogene homolog (GLI), which is activated through mediating the dissociation of GLI proteins from the suppressor of fused (SUFU). This allows the translocation of GLI proteins to the nucleus where they bind DNA and regulate the transcription of their target genes.37 GLI is a zinc-finger transcription factor family with three members, GLI1, GLI2, and GLI3, which play an important role in human cancer. GLI1 and GLI2 are transcriptional activator factors; GLI1 is associated with tumor progression and metastasis in human cancer;38 GLI2 is accompanied by invasive and metastatic phenotypes of cancer,39 while GLI3 is a transcriptional repressor factor, the up-regulation of which can effectively inhibit the Hh-mediated progression of tumors.40 A balance between these three factors has been proposed as a molecular code that regulates cell differentiation and compromises and participates in the maintenance of stem cells, which could have implications for cancer development.41 In addition, the over-activated Hh pathway seen in cancer is a consequence of the germline variation of GLI that induces certain cancers.42
Inhibiting the Hh pathway through SMO by ITZ
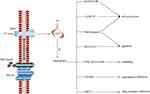
Uncontrolled activation of the Hh pathway can lead to cancers in many systems, and this can lead to the overexpression and activation of Hh ligands, SMO, and GLI.26,43 SMO plays a key role in the Hh pathway, which can regulate embryonic development and adult stem cells in animals.44 It is demonstrated that ITZ could suppress the Hh pathway by inhibiting SMO and/or GLI, especially GLI1, and their downstream targets through various mechanisms5 to inhibit the growth and proliferation of many cancers in vivo and in vitro, arrest cell cycle, inhibit angiogenesis, and induce apoptosis and autophagy (Figure 3) including in gastric cancer,12 liver cancer,45 melanoma,13 basal cell carcinoma,10 prostate cancer,11 etc. Moreover, many pre-clinical studies have also confirmed that ITZ has the capability to inhibit the Hh pathway to treat cancer.5,28,46,47 It is worth mentioning that a drug screen identified ITZ as an inhibitor of the Hh pathway at a clinically relevant concentration of 800 nM,5 and at this concentration, ITZ is very safe and has little adverse effects on human beings.
ITZ could act on SMO directly and decrease the amount of SMO on the primary cilium to suppress the Hh pathway.5 For example, ITZ could inhibit the proliferation of basal cell carcinoma, medulloblastoma and glioblastoma through combination with the C-terminal of SMO, without altering the chemical groups from other drugs.26 (Table 1) This could be a potential solution for adding ITZ in combination with other anticancer drugs to inhibit drug resistance, such as vismodegib and arsenic trioxide.7,48 In addition, ITZ could also inhibit the Hh pathway through suppressing GLI.15 ITZ could inhibit the growth of cervical cancer cells and endometrial cancer cells through significantly reducing the expression of GLI1.27,28
 |
Table 1 Some cancer types treated by ITZ in vitro through Hh pathway |
Anti-inflammation by blocking Hh pathway
The growth and progression of cancer is the result of complex signaling networks between different cell types within the cancer and its surrounding stroma. Previous research has shown that a chronic inflammatory response resulting from certain autoimmune diseases49–51 or chronic infections52 can lead to cancer. In turn, tumors could also stimulate an inflammatory reaction via the secretion of cytokines, chemokines, and growth factors that favor the recruitment of a range of infiltrating immune cell populations into the tumor microenvironment.53 While potentially able to exert tumor control, this inflammatory reaction is typically seized by the tumor to promote its own growth and progression towards metastasis.54
It has also been reported that pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and a variety of signaling pathways ligands such as transforming growth factor-beta (TGF-β) and platelet derived growth factor (PDGF) are aberrantly and abundantly expressed in tumor microenvironments.55 Meanwhile, TNF-α and IL-1β can activate the Hh pathway through increasing GLI1 in cancer cells.55–57 How the Hh pathway regulates pro-inflammatory factors has not yet been discovered correctly, the inflammation suppression of ITZ may be related to its effect on expression of GLI1 and inflammatory factors. In addition, previous studies have shown that ITZ can treat chronic rhinosinusitis through inhibiting P-glycoprotein (P-gp), and that this inhibition results in decreased inflammatory cytokine secretion.58 Kangwan et al suggested that the serum levels of IL-6, TNF-α and cyclooxygenase-2 (COX-2) were all significantly decreased after ITZ oral gavage in an animal model of colitis-associated cancer.59 Moreover, ITZ could significantly reduce the chlorhexidine gluconate-induced peritoneal thickness and inflammatory cell infiltrations in the peritoneal membrane via suppressing the activation of the SHh signaling pathway in the peritoneal tissues.8
Induction of cell cycle arrest by targeting Hh pathway
Cell cycle plays an important role in modulation of tumor growth, and previous research has shown that an arrested cell cycle could prevent unlimited proliferation of tumor cells.60,61 Tumors are a kind of periodic disease, changing the tumor cell cycle, which is important to tumor proliferation. Cell cycle is composed of four typical phases (sub-G1 phase, G1 phase, M phase and S phase) and regulated well systemically by cyclins and cyclin dependent kinases (CDKs).62 It has been shown that the Hh pathway can activate the expression of cell cycle regulators to promote cell proliferation, shorten the G1 and G2 phases of the cell cycle, and drive the cells to the final mitosis and cell cycle exit.63
Cancer cell dormancy is one of the most significant reasons for treatment failure. However, it has been reported that ITZ treats dormant cancer cells through causing irreversible tumor growth arrest, WNT pathway inhibition and synergistic activity with classical s-phase cytotoxins in colorectal cancer.64 The main mechanism is that ITZ inhibits SMO, which releases the subsequent inhibition on SUFU, and then, the derived SUFU activation in WNTHigh epithelial tumor cells prevents the nuclear localization of β-catenin causing WNT inhibition. This finally leads to senescence in both dividing and dormant cancer cells.64 In addition, ITZ could make the cell cycle of breast cancer cells arrest at the G0-G1 phase.29 ITZ could also induce gastric cancer cell cycle arrest at the G1-S phase and induce apoptosis through significantly decreasing the expression and transcription of GLI1.12 ITZ also inhibits SMO to suppress mTOR through decreasing SOX9, a Sry-like high mobility group (HMG) box transcription factor that is transcriptionally regulated by GLI.65 This eventually leads to proliferation inhibition in basal cell carcinoma.66 Furthermore, ITZ could suppress proliferation and arrest cell cycle by suppressing cyclin D1 through GLI1 in melanoma cells and multiple myeloma cells.13,48 In addition, p53 is also involved in cell cycle regulation, whether and how p53 affected by ITZ through Hh pathway are still unknown, and more studies are needed.
Induction of apoptosis and autophagy by targeting Hh pathway
Programmed cell death plays an important role in maintaining homeostasis, and it could prevent disease by removing cells destroyed by cancer, aging, and infection. Hence, it is a valid strategy for the treatment of cancer. There are three types of programmed cell death: apoptosis (type I), autophagy (type II) and necroptosis (type III).67 To date, there are some chemotherapeutic agents in the clinic to treat cancer.68
Induction of apoptosis
Apoptosis literally means “falling away” in Greek, and occurs normally in multicellular organisms. Apoptosis eliminates abnormal, damaged, or mutated cells, and plays important roles in embryonic development and adult tissue equilibrium by adjusting the physiological processes involved.69 In humans, many cells are turned over and replaced each day through apoptosis. This process maintains a balance between the death and survival of cells and tissues.70 A common feature of many cancers is the ability to escape apoptosis. This ability allows cancer cells to proliferate despite accumulated DNA damage and can confer resistance to various chemotherapy drugs.71
ITZ induces apoptosis of many kinds of cancer cells through Hh pathway inhibition, including breast cancer, colorectal cancer, melanoma, bladder cancer and gastric cancer.12,13,29,64,72 ITZ directly inhibits SMO,73 and then inhibits the expression of GLI1 and its translocation to the nucleus. Meanwhile, ITZ also directly inhibits GLI1,12 which decreases the expression of downstream gene, Bcl-2,34,74 an important anti-apoptotic member (Bcl-2, Bcl-XL, and Mcl-1) of Bcl-2 family, and increases cytochrome c (Cyt C) into the cytosol from mitochondria to induce the apoptosis of cancer cells.45,75 Moreover, ITZ also suppresses the Wnt/β-catenin signaling pathway through inhibiting SMO in cancer cells,13,64 which is related to cell growth, prognosis, invasion and metastasis.76,77 ITZ suppresses Wnt3A, Wnt4, Wnt10A and β-catenin, and then increases Axin-1, which in turn inhibits β-catenin, which finally leads to growth inhibition and cell apoptosis.13,27
In addition, ITZ could also induce apoptosis via the caspase-independent pathway. ITZ passes through the cancer cell and decreases the mitochondrial membrane, leading to increased membrane permeability, generation of reactive oxygen species (ROS), and down-regulation of Bcl-2 family members. This finally activates caspase-9 and caspase-3 cascades to cause DNA fragmentation.16,29
Induction of autophagy
Autophagy is the cellular process of lysosomal degradation in which damaged, dysfunctional, or superfluous organelles and proteins are sequestered, engulfed, and recycled to maintain cellular metabolism, viability, and homeostasis.78 Presently, there are three known subtypes of autophagy: macroautophagy as a main autophagy pathway, microautophagy, and chaperone-mediated autophagy. It has been shown that the inhibition of the Hh pathway induces autophagy of cancer cells.79 Recent evidence has also demonstrated that autophagy plays a wide range of physiological and pathophysiological roles and is associated with the pathogenesis of cancer, so the pharmacological manipulation of autophagy pathways may represent a new therapeutic strategy for cancer.80,81
ITZ inhibits the Hh pathway through inhibiting SMO and GLI1 to induce cell autophagy.29 In addition, ITZ represses the phosphorylation of class III phosphatidylinositol 3-kinase (PI3K), and AKT, a serine/threonine protein kinase belonging to the PI3K/AKT/mTOR pathway, which is required for tumorigenesis. This acts as a downstream target of the Hh pathway,82 and then inhibits mTOR through diminished trafficking of cholesterol from late endosomes and lysosomes to the plasma membrane, resulting in autophagy promotion in cancer cells.13,29,83 Moreover, ITZ also increases the expression of microtubule-associated protein 1 light chain 3 II (LC3II), which is often used as a marker for monitoring autophagy progression since it localizes to both the inner and outer membranes of phagophores and autophagosome. It also degrades P62/SQSTM1, which contributes to autophagy cell death,84 and forms autophagosome through inhibition of the Hh pathway to induce autophagy.29
Inhibition of angiogenesis
Angiogenesis is an important physiological process in tissues, especially in conditions of growth, proliferation, and wound healing because it helps deliver oxygen and nutrients to newly formed tissues. However, it is also characteristic of tumor malignancy. Metastatic cancer cells can enter the circulatory system through their own newly formed vasculature to migrate from the primary tumor and colonize other healthy tissues. Metastatic ability is tightly associated with a negative prognosis.85 The inhibition of angiogenesis is a promising avenue of anti-cancer therapy research of ITZ.86
It is reported that ITZ inhibits vascular endothelial growth factor (VEGF) signaling through inhibiting the expression and glycosylation of VEGF receptor 2, which has been demonstrated as a downstream target of Hh pathway through GLI1,87 to suppress angiogenesis in cancer cells.9,88–90 ITZ also inhibits angiogenesis and endothelial cell proliferation by targeting voltage-dependent anion channel 1 (VDAC1), which regulates mitochondrial metabolism by controlling the passage of ions and small metabolites through the outer mitochondrial membrane, to modulate the AMPK/mTOR signaling axis in endothelial cells.91
Inhibition of drug resistance
Drug resistance in cancer cells, which reduces the efficacy of chemotherapeutics and other treatments,92 is dependent on the ATP-binding cassette (ABC) transporters, which are frequently overexpressed in cancer cells.93 There are three major kinds of multidrug resistance proteins in humans: P-glycoprotein (P-gp/ABCB1/MDR1), multidrug resistance protein 1 (MRP1/ABCC1), and breast cancer resistance protein (BCRP/ABCG2/MXR/ABCP).94 It has been reported that the drug resistance of cancer cells is associated with the expression of epithelial mesenchymal transition (EMT) and the aberrantly activated Hh pathway.95 The Hh pathway could also increase the expression of ABCC1 through GLI2 in hepatoma cells; ITZ inhibits ABCC1 through suppression of Hh pathway.45 ITZ also inhibits the efflux pump to reverse resistance.3,96
Clinical use of ITZ in cancer treatment
ITZ has been used clinically for nearly 35 years as an antifungal agent. With the advent of its anticancer properties, it has also been used for the treatment of many kinds of tumors in clinical trials (Table 2).
 |
Table 2 Some examples of clinical trials of ITZ on different cancers |
Previous studies have shown that intracranial regression of an advanced basal cell carcinoma was successfully treated by ITZ with chemotherapy.6,7,10 Meanwhile, ITZ is also currently used in the treatment of high-grade neuroepithelial tumors of the central nervous system with BCOR alteration (HGNET-BCOR) in women and children.97,98 A case of biochemically recurrent prostate cancer has also been treated effectively by high dose ITZ.99 The prognosis and overall survival rate of ovarian cancer patients has improved when treated with ITZ and other chemotherapeutic drugs.96,100–102 In addition, ITZ with chemotherapy is promising for the treatment of heavily pre-treated recurrent triple-negative breast cancer.103 Combination chemotherapy with ITZ is also promising for prolonging overall survival, with acceptable toxicities in the second-line setting of pancreatic cancer.104 Additionally, ITZ has been analyzed as a second line treatment in metastatic non-squamous non-small cell lung cancer.105
Conclusion
In recent years, the development of drugs that inhibit the Hh pathway have become a new treatment for cancer due to the discovery of activated the pathway in many tumors. ITZ inhibits the Hh pathway, and this finding has provided a tremendous role in tumor therapy research. In the review, we summarize the exact mechanism by which ITZ fights with tumor by targeting the Hh pathway. ITZ blocks the Hh pathway by preventing the accumulation of receptor SMO and inhibiting the release of the transcription factor GLI. The target gene of the Hh pathway contains the anti-apoptosis factor BCL-2, while ITZ has the ability to decrease the expression of BCL-2 and promote the apoptosis of tumor cells. A large number of Hh pathways in tumor cells are activated, resulting in overexpression of GLI2, which inhibits autophagy. However, ITZ can also rescue the suppressed autophagy and promote the death of cancer cells. The mammalian cell cycle is a highly organized and canonical process that ensures the duplication of genetic material and cell division. Since the main feature of tumor cells is uncontrolled proliferation, it is not surprising that factors involved in the cell cycle change. In addition, some researchers have found that ITZ in combination with other drugs that inhibit the Hh pathway have significant effects on the treatment of cancer. Therefore, a large number of further studies should be conducted to provide a basis for reasonable combination therapy in the future. Currently, ITZ has been used in clinical trials to treat many kinds of tumors with satisfactory results. In any case, an in-depth study of ITZ could bring new hope to cancer patients in the near future.
Acknowledgments
We really appreciate the help supported by Dr. Zeyao Tang on the manuscript, a professor from the Department of Pharmacology, Dalian Medical University. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
3. Lockhart NR, Waddell JA, Schrock NE. Itraconazole therapy in a pancreatic adenocarcinoma patient: A case report. J Oncol Pharm Pract. 2016;22(3):528–532. doi:10.1177/1078155215572931
4. Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)-itraconazole as an anti-cancer agent. Ecancermedicalscience. 2015;9:521. doi:10.3332/ecancer.2015.521
5. Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17(4):388–399. doi:10.1016/j.ccr.2010.02.027
6. Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32(8):745–751. doi:10.1200/JCO.2013.49.9525
7. Yoon J, Apicelli AJ
8. Kim JS, Cho KS, Park SH, et al. Itraconazole attenuates peritoneal fibrosis through its effect on the sonic hedgehog signaling pathway in mice. Am J Nephrol. 2018;48(6):456–464. doi:10.1159/000493550
9. Nacev BA, Grassi P, Dell A, Haslam SM, Liu JO. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J Biol Chem. 2011;286(51):44045–44056. doi:10.1074/jbc.M111.278754
10. Wahid M, Jawed A, Mandal RK, et al. Vismodegib, itraconazole and sonidegib as hedgehog pathway inhibitors and their relative competencies in the treatment of basal cell carcinomas. Crit Rev Oncol Hematol. 2016;98:235–241. doi:10.1016/j.critrevonc.2015.11.006
11. Antonarakis ES, Heath EI, Smith DC, et al. Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist. 2013;18(2):163–173. doi:10.1634/theoncologist.2012-314
12. Hu Q, Hou YC, Huang J, Fang JY, Xiong H. Itraconazole induces apoptosis and cell cycle arrest via inhibiting Hedgehog signaling in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):50. doi:10.1186/s13046-017-0526-0
13. Liang G, Liu M, Wang Q, et al. Itraconazole exerts its anti-melanoma effect by suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways. Oncotarget. 2017;8(17):28510–28525. doi:10.18632/oncotarget.15324
14. Tsubamoto H, Inoue K, Sakata K, et al. Itraconazole inhibits AKT/mTOR signaling and proliferation in endometrial cancer cells. Anticancer Res. 2017;37(2):515–519. doi:10.21873/anticanres.11343
15. Pounds R, Leonard S, Dawson C, Kehoe S. Repurposing itraconazole for the treatment of cancer. Oncol Lett. 2017;14(3):2587–2597. doi:10.3892/ol.2017.6569
16. Choi CH, Ryu JY, Cho YJ, et al. The anti-cancer effects of itraconazole in epithelial ovarian cancer. Sci Rep. 2017;7(1):6552.
17. Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi:10.1101/gad.938601
18. Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi:10.1038/287795a0
19. Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi:10.1101/gad.1693608
20. Fattahi S, Pilehchian Langroudi M, Akhavan-Niaki H. Hedgehog signaling pathway: Epigenetic regulation and role in disease and cancer development. J Cell Physiol. 2018;233:5726–5735. doi:10.1002/jcp.26506
21. Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313–317. doi:10.1038/nature01493
22. Yuan Z, Goetz JA, Singh S, et al. Frequent requirement of hedgehog signaling in non-small cell lung carcinoma. Oncogene. 2007;26(7):1046–1055. doi:10.1038/sj.onc.1209860
23. Gialmanidis IP, Bravou V, Amanetopoulou SG, Varakis J, Kourea H, Papadaki H. Overexpression of hedgehog pathway molecules and FOXM1 in non-small cell lung carcinomas. Lung Cancer. 2009;66(1):64–74. doi:10.1016/j.lungcan.2009.01.007
24. Raz G, Allen KE, Kingsley C, et al. Hedgehog signaling pathway molecules and ALDH1A1 expression in early-stage non-small cell lung cancer. Lung Cancer. 2012;76(2):191–196. doi:10.1016/j.lungcan.2011.10.015
25. Pak E, Segal RA. Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev Cell. 2016;38(4):333–344. doi:10.1016/j.devcel.2016.07.026
26. Liu M, Liang G, Zheng H, Zheng N, Ge H, Liu W. Triazoles bind the C-terminal domain of SMO: illustration by docking and molecular dynamics simulations the binding between SMO and triazoles. Life Sci. 2019;217:222–228. doi:10.1016/j.lfs.2018.12.012
27. Ueda T, Tsubamoto H, Inoue K, Sakata K, Shibahara H, Sonoda T. Itraconazole modulates Hedgehog, WNT/β-catenin, as well as Akt signalling, and inhibits proliferation of cervical cancer cells. Anticancer Res. 2017;37:7.
28. Inoue K, Tsubamoto H, Sakata K, et al. Expression of Hedgehog signals and growth inhibition by itraconazole in endometrial cancer. Anticancer Res. 2016;36(1):149–153.
29. Wang X, Wei S, Zhao Y, et al. Anti-proliferation of breast cancer cells with itraconazole: Hedgehog pathway inhibition induces apoptosis and autophagic cell death. Cancer Lett. 2017;385:128–136. doi:10.1016/j.canlet.2016.10.034
30. Shi Y, Moura U, Opitz I, et al. Role of hedgehog signaling in malignant pleural mesothelioma. Clin Cancer Res. 2012;18(17):4646–4656. doi:10.1158/1078-0432.CCR-12-0599
31. Bariwal J, Kumar V, Dong Y, Mahato RI. Design of Hedgehog pathway inhibitors for cancer treatment. Med Res Rev. 2019;39(3):1137–1204. doi:10.1002/med.21555
32. Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274(5285):255–259. doi:10.1126/science.274.5285.255
33. Yang H, Cong WN, Yoon JS, Egan JM. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2015;4(2):245–252. doi:10.1002/cam4.350
34. Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci. 2018;18(1):8–20. doi:10.17305/bjbms.2018.2756
35. Bayly CI, Cieplak P, Cornell W, Kollman PA. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem. 1993;97(40):10269–10280. doi:10.1021/j100142a004
36. Xie J, Bartels CM, Barton SW, Gu D. Targeting hedgehog signaling in cancer: research and clinical developments. Onco Targets Ther. 2013;6:1425–1435. doi:10.2147/OTT.S34678
37. Hanna A, Shevde LA. Hedgehog signaling: modulation of cancer properies and tumor mircroenvironment. Mol Cancer. 2016;15:24. doi:10.1186/s12943-016-0509-3
38. Das S, Harris LG, Metge BJ, et al. The hedgehog pathway transcription factor GLI1 promotes malignant behavior of cancer cells by up-regulating osteopontin. J Biol Chem. 2009;284(34):22888–22897. doi:10.1074/jbc.M109.021949
39. Alexaki VI, Javelaud D, Van Kempen LC, et al. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst. 2010;102(15):1148–1159. doi:10.1093/jnci/djq257
40. Ulloa F, Itasaki N, Briscoe J. Inhibitory Gli3 activity negatively regulates Wnt/beta-catenin signaling. Curr Biol. 2007;17(6):545–550. doi:10.1016/j.cub.2007.01.062
41. Armas-Lopez L, Zuniga J, Arrieta O, Avila-Moreno F. The Hedgehog-GLI pathway in embryonic development and cancer: implications for pulmonary oncology therapy. Oncotarget. 2017;8(36):60684–60703. doi:10.18632/oncotarget.19527
42. Didiasova M, Schaefer L, Wygrecka M. Targeting GLI transcription factors in cancer. Molecules. 2018;23:5. doi:10.3390/molecules23051003
43. Cucchi D, Occhione MA, Gulino A, De Smaele E. Hedgehog signaling pathway and its targets for treatment in basal cell carcinoma. J Exp Pharmacol. 2012;4:173–185. doi:10.2147/JEP.S28553
44. Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5(246):re6. doi:10.1126/scisignal.2003289
45. Ding J, Zhou XT, Zou HY, Wu J. Hedgehog signaling pathway affects the sensitivity of hepatoma cells to drug therapy through the ABCC1 transporter. Lab Invest. 2017;97(7):819–832. doi:10.1038/labinvest.2017.34
46. Pace JR, DeBerardinis AM, Sail V, et al. Repurposing the clinically efficacious antifungal agent itraconazole as an anticancer chemotherapeutic. J Med Chem. 2016;59(8):3635–3649. doi:10.1021/acs.jmedchem.5b01718
47. Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23(1):23–34. doi:10.1016/j.ccr.2012.11.017
48. Huang XB, Shi Y, Wang CS, Wang XD, Cheng J, Che FF. Synergistic inhibitory effect of arsenic trioxide combined with itraconazole on Hedgehog pathway of multiple myeloma NCI-H929 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24(5):1459–1465. doi:10.7534/j.issn.1009-2137.2016.05.032
49. Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Found Symp. 2004;256:
50. Katsanos KH, Tatsioni A, Pedersen N, et al. Cancer in inflammatory bowel disease 15 years after diagnosis in a population-based European Collaborative follow-up study. J Crohns Colitis. 2011;5(5):430–442. doi:10.1016/j.crohns.2011.04.013
51. Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi:10.1016/j.ccr.2009.01.001
52. Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134(6):1445–1457. doi:10.1002/ijc.28470
53. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
54. Dominguez C, David JM, Palena C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol. 2017;47:177–184. doi:10.1016/j.semcancer.2017.08.002
55. Wang Y, Jin G, Li Q, et al. Hedgehog signaling non-canonical activated by pro-inflammatory cytokines in pancreatic ductal adenocarcinoma. J Cancer. 2016;7(14):2067–2076. doi:10.7150/jca.15786
56. Das S, Samant RS, Shevde LA. Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to Smoothened-targeting Hedgehog inhibition. J Biol Chem. 2013;288(17):11824–11833. doi:10.1074/jbc.M112.432302
57. Helm O, Held-Feindt J, Grage-Griebenow E, et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014;135(4):843–861. doi:10.1002/ijc.28736
58. Lam A, Hoang JD, Singleton A, Han X, Bleier BS. Itraconazole and clarithromycin inhibit P-glycoprotein activity in primary human sinonasal epithelial cells. Int Forum Allergy Rhinol. 2015;5(6):477–480. doi:10.1002/alr.21454
59. Kangwan N, Kim YJ, Han YM, Jeong M, Park JM, Hahm KB. Concerted actions of ameliorated colitis, aberrant crypt foci inhibition and 15-hydroxyprostaglandin dehydrogenase induction by sonic hedgehog inhibitor led to prevention of colitis-associated cancer. Int J Cancer. 2016;138(6):1482–1493. doi:10.1002/ijc.29892
60. Das U. A radical approach to cancer. Med Sci Monit. 2002;8(4):Ra79–Ra92.
61. Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M. Targeting cell cycle regulation in cancer therapy. Pharmacol Ther. 2013;138(2):255–271. doi:10.1016/j.pharmthera.2013.01.011
62. Luo R, Fang D, Hang H, Tang Z. The mechanism in gastric cancer chemoprevention by allicin. Anticancer Agents Med Chem. 2016;16(7):802–809.
63. Prykhozhij SV. In the absence of Sonic hedgehog, p53 induces apoptosis and inhibits retinal cell proliferation, cell-cycle exit and differentiation in zebrafish. PLoS One. 2010;5(10):e13549. doi:10.1371/journal.pone.0013549
64. Popova SA, Buczacki SJA. Itraconazole perturbs colorectal cancer dormancy through SUFU-mediated WNT inhibition. Mol Cell Oncol. 2018;5(4):e1494950.
65. Eberl M, Klingler S, Mangelberger D, et al. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med. 2012;4(3):218–233. doi:10.1002/emmm.201100201
66. Kim AL, Back JH, Chaudhary SC, Zhu Y, Athar M, Bickers DR. SOX9 transcriptionally regulates mTOR-induced proliferation of basal cell carcinomas. Mol Cell Oncol. 2018;138(8):1716–1725.
67. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. doi:10.1186/s12943-014-0278-9
68. Radogna F, Dicato M, Diederich M. Cancer-type-specific crosstalk between autophagy, necroptosis and apoptosis as a pharmacological target. Biochem Pharmacol. 2015;94(1):1–11. doi:10.1016/j.bcp.2014.12.018
69. Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34(3):165–175.
70. Fulda S, Debatin KM. Exploiting death receptor signaling pathways for tumor therapy. Biochim Biophys Acta. 2004;1705(1):27–41. doi:10.1016/j.bbcan.2004.09.003
71. Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins (Basel). 2010;2(10):2428–2466. doi:10.3390/toxins2102428
72. Mamtani R, Yang YX, Scott FI, Lewis JD, Boursi B. Association of itraconazole, a hedgehog inhibitor, and bladder cancer. J Urol. 2016;196(2):343–348. doi:10.1016/j.juro.2016.01.089
73. Dirix L. Discovery and exploitation of novel targets by approved drugs. J Clin Oncol. 2014;32(8):720–721. doi:10.1200/JCO.2013.53.7118
74. Liu P, Chen L. Inhibition of sonic hedgehog signaling blocks cell migration and growth but induces apoptosis via suppression of FOXQ1 in natural killer/T-cell lymphoma. Leuk Res. 2018;64:1–9. doi:10.1016/j.leukres.2017.11.001
75. Bhattacharya R, Kwon J, Ali B, et al. Role of hedgehog signaling in ovarian cancer. Clin Cancer Res. 2008;14(23):7659–7666. doi:10.1158/1078-0432.CCR-08-1414
76. Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H. Specific targeting of Wnt/beta-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31(10):1844–1853. doi:10.1093/carcin/bgq169
77. Webster MR, Weeraratna AT. A Wnt-er migration: the confusing role of beta-catenin in melanoma metastasis. Sci Signal. 2013;6(268):pe11. doi:10.1126/scisignal.2004114
78. McCormick J, Knight RA, Barry SP, et al. Autophagy in the stress-induced myocardium. Front Biosci (Elite Ed). 2012;4:2131–2141.
79. Jimenez-Sanchez M, Menzies FM, Chang YY, Simecek N, Neufeld TP, Rubinsztein DC. The Hedgehog signalling pathway regulates autophagy. Nat Commun. 2012;3:1200. doi:10.1038/ncomms2212
80. Marinkovic M, Sprung M, Buljubasic M, Novak I. Autophagy Modulation in Cancer: Current Knowledge on Action and Therapy. Oxid Med Cell Longev. 2018;2018:8023821. doi:10.1155/2018/8023821
81. Wang Y, Han C, Lu L, Magliato S, Wu T. Hedgehog signaling pathway regulates autophagy in human hepatocellular carcinoma cells. Hepatology. 2013;58(3):995–1010. doi:10.1002/hep.26394
82. Kim AL, Back JH, Zhu Y, et al. AKT1 activation is obligatory for spontaneous BCC tumor growth in a murine model that mimics some features of basal cell nevus syndrome. Cancer Prev Res (Phila). 2016;9(10):794–802. doi:10.1158/1940-6207.CAPR-16-0066
83. Liu R, Li J, Zhang T, et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking. Autophagy. 2014;10(7):1241–1255. doi:10.4161/auto.28912
84. Tasdemir E, Galluzzi L, Maiuri MC, et al. Methods for assessing autophagy and autophagic cell death. Methods Mol Biol. 2008;445:29–76. doi:10.1007/978-1-59745-157-4_3
85. Zhang S, Cao Z, Tian H, et al. SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo. Clin Cancer Res. 2011;17(13):4439–4450. doi:10.1158/1078-0432.CCR-10-3109
86. Aftab BT, Dobromilskaya I, Liu JO, Rudin CM. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71(21):6764–6772. doi:10.1158/0008-5472.CAN-11-0691
87. Di Mauro C, Rosa R, D’Amato V, et al. Hedgehog signalling pathway orchestrates angiogenesis in triple-negative breast cancers. Br J Cancer. 2017;116(11):1425–1435. doi:10.1038/bjc.2017.116
88. Shi W, Nacev BA, Aftab BT, Head S, Rudin CM, Liu JO. Itraconazole side chain analogues: structure-activity relationship studies for inhibition of endothelial cell proliferation, vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, and hedgehog signaling. J Med Chem. 2011;54(20):7363–7374. doi:10.1021/jm200944b
89. Zhang L, Liu Z, Yang K, et al. Tumor progression of non-small cell lung cancer controlled by albumin and micellar nanoparticles of itraconazole, a multitarget angiogenesis inhibitor. Mol Pharm. 2017;14(12):4705–4713. doi:10.1021/acs.molpharmaceut.7b00855
90. Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ
91. Head SA, Shi W, Zhao L, et al. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proc Natl Acad Sci U S A. 2015;112(52):E7276–E7285. doi:10.1073/pnas.1512867112
92. Tovar V, Cornella H, Moeini A, et al. Tumour initiating cells and IGF/FGF signalling contribute to sorafenib resistance in hepatocellular carcinoma. Gut. 2017;66(3):530–540. doi:10.1136/gutjnl-2015-309501
93. Li W, Zhang H, Assaraf YG, et al. Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat. 2016;27:14–29. doi:10.1016/j.drup.2016.05.001
94. Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9(1):105–127. doi:10.2217/14622416.9.1.105
95. Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2011;55(4):838–845. doi:10.1016/j.jhep.2010.12.043
96. Tsubamoto H, Sonoda T, Yamasaki M, Inoue K. Impact of combination chemotherapy with itraconazole on survival for patients with recurrent or persistent ovarian clear cell carcinoma. Anticancer Res. 2014;34(4):2007–2014.
97. Paret C, Russo A, Otto H, et al. Personalized therapy: CNS HGNET-BCOR responsiveness to arsenic trioxide combined with radiotherapy. Oncotarget. 2017;8(69):114210–114225. doi:10.18632/oncotarget.23174
98. Appay R, Macagno N, Padovani L, et al. HGNET-BCOR tumors of the cerebellum: clinicopathologic and molecular characterization of 3 cases. Am J Surg Pathol. 2017;41(9):1254–1260. doi:10.1097/PAS.0000000000000866
99. Suzman DL, Antonarakis ES. High-dose itraconazole as a noncastrating therapy for a patient with biochemically recurrent prostate cancer. Clin Genitourin Cancer. 2014;12(2):e51–e53. doi:10.1016/j.clgc.2013.11.015
100. Tsubamoto H, Ito Y, Kanazawa R, et al. Benefit of palliative chemotherapy and hospice enrollment in late-stage ovarian cancer patients. J Obstet Gynaecol Res. 2014;40(5):1399–1406. doi:10.1111/jog.12320
101. Kajiyama H, Shibata K, Mizuno M, et al. Postrecurrent oncologic outcome of patients with ovarian clear cell carcinoma. Int J Gynecol Cancer. 2012;22(5):801–806. doi:10.1097/IGC.0b013e3182540145
102. Yoshino K, Enomoto T, Fujita M, et al. Salvage chemotherapy for recurrent or persistent clear cell carcinoma of the ovary: a single-institution experience for a series of 20 patients. Int J Clin Oncol. 2013;18(1):148–153. doi:10.1007/s10147-011-0357-5
103. Tsubamoto H, Sonoda T, Inoue K. Impact of itraconazole on the survival of heavily pre-treated patients with triple-negative breast cancer. Anticancer Res. 2014;34(7):3839–3844.
104. Tsubamoto H, Sonoda T, Ikuta S, Tani S, Inoue K, Yamanaka N. Combination chemotherapy with itraconazole for treating metastatic pancreatic cancer in the second-line or additional setting. Anticancer Res. 2015;35(7):4191–4196.
105. Rudin CM, Brahmer JR, Juergens RA, et al. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2013;8(5):619–623. doi:10.1097/JTO.0b013e31828c3950
106. You M, Varona-Santos J, Singh S, Robbins DJ, Savaraj N, Nguyen DM. Targeting of the hedgehog signal transduction pathway suppresses survival of malignant pleural mesothelioma cells in vitro. J Thorac Cardiovasc Surg. 2014;147(1):508–516. doi:10.1016/j.jtcvs.2013.08.035
107. Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152(4):452–456. doi:10.1001/jamadermatol.2015.5473
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.