Back to Journals » Journal of Inflammation Research » Volume 16
Inflammatory and Nutritional Scoring System for Predicting Prognosis in Patients with Newly Diagnosed Multiple Myeloma
Authors Zhang L, Chen S, Wang W, Wang Y, Liang Y
Received 18 September 2022
Accepted for publication 24 December 2022
Published 5 January 2023 Volume 2023:16 Pages 7—17
DOI https://doi.org/10.2147/JIR.S390279
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Monika Sharma
Limei Zhang,1,2,* Shuzhao Chen,1,2,* Weida Wang,1,2 Yun Wang,1,2 Yang Liang1,2
1Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China; 2Department of Hematologic Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Liang; Yun Wang, Department of Hematologic Oncology, Sun Yat-sen University Cancer, State Key Laboratory of Oncology in South China, 651 Dongfeng Road East, Guangzhou, Guangdong, 510060, People’s Republic of China, Email [email protected]; [email protected]
Purpose: We aimed to assess the prognostic value of pretreatment inflammatory and nutritional parameters for predicting overall survival (OS) in patients with newly diagnosed multiple myeloma (NDMM), and to build a new scoring system using the most important variables.
Methods: We retrospectively analyzed baseline clinical and laboratory data for patients with NDMM, who were randomly grouped into training and validation cohorts at a ratio of 8:2. The Inflammatory Nutritional Score (INS) was developed based on the least absolute shrinkage and selection operator (LASSO) Cox regression. The INS and other independent prognostic factors were entered into a multivariate Cox model and merged to generate a nomogram model for predictive optimization. Performance and predictive accuracy were assessed using the concordance index (C-index), calibration plots, and time-dependent receiver operating characteristic (ROC) curves.
Results: In total, 442 eligible patients were enrolled. Six inflammatory/nutritional variables, including the Nutritional Risk Index (NRI), body mass index (BMI), neutrophil-lymphocyte ratio (NLR), monocyte-lymphocyte ratio (MLR), platelet-lymphocyte ratio (PLR), and albumin-alkaline phosphatase ratio (AAPR), were integrated to construct the INS using the LASSO Cox model. The predictive nomogram constructed following the multivariate Cox analysis included INS, performance status, lactate dehydrogenase, age, and C-reactive protein. The model exhibited good predictive performance, with a C-index of 0.708 in the training cohort and 0.749 in the validation cohort. Moreover, the calibration curves also demonstrated excellent consistency between predicted and observed survival in both cohorts. In the time-dependent ROC analysis, our nomogram model exhibited better performance than other staging systems for multiple myeloma.
Conclusion: The INS represents an independent prognostic signature in patients with NDMM. Our novel nomogram based on INS may aid in predicting survival probability and stratifying risk.
Keywords: multiple myeloma, survival, prognostic, nomogram
Introduction
Multiple myeloma (MM) is a malignant blood cancer featuring an abnormal accumulation of monoclonal plasma cells in the bone marrow, which can lead to hypercalcemia, renal dysfunction, anemia, and bone destruction.1 The survival outcomes of MM are heterogeneous, with some patients remaining alive for more than 10 years and others dying within a few months of diagnosis.2 Considering the heterogeneity of MM, staging systems such as the Durie–Salmon staging system (DS), International Staging System (ISS), revised International Staging System (R-ISS), and Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) cannot ideally cover all patients with MM.3–6
Recent research has increasingly focused on the associations among nutrition, inflammation, immunity, and malignancy.7 Accordingly, studies have reported a close correlation between MM and inflammation, suggesting that myeloma may develop following infection via the complex interplay among pathogens, chronic inflammation, and immune deregulation.8 Therefore, some peripheral blood biomarkers associated with inflammation may serve as prognostic signatures for MM. Indeed, some studies have demonstrated that some inflammatory indices, including the neutrophil-lymphocyte ratio (NLR) and monocyte-lymphocyte ratio (MLR), are relevant to survival outcomes in patients with MM.9–12 Dosani et al found that the absolute lymphocyte count (ALC) - absolute monocyte count (AMC) ratio (ALC/AMC) in the peripheral blood (PB) was a strong prognostic immune biomarker in patients with newly diagnosed MM (NDMM), a higher ALC/AMC value at diagnosis suggesting a longer survival, and may imply the immunologic status of these patients.9 Romano et al’s study also confirmed NLR and LMR as predictors of survival in patients with MM treated upfront with novel agents.10 Additional studies have highlighted associations between some nutritional indices, such as the Controlling Nutritional Status (CONUT), Nutritional Risk Index (NRI), and body mass index (BMI), and survival in patients with MM.13–17 The prognostic nutritional index (PNI) is a nutritional index derived from serum albumin and lymphocyte counts, which was originally used to assess preoperative nutritional status, surgical risk, and postoperative complications in surgical patients.18 A Chinese study found that low PNI suggested poor prognosis and was an independent prognostic factor in patients with NDMM.19
The ISS has verified the importance of albumin in the context of MM prognosis,4 and plasma levels of alkaline phosphatase (ALP) often increase in the presence of bone lesions.20 Fractures are also common skeletal-related events in patients with MM exhibiting extensive bone destruction, and serum ALP levels may be increased in patients with fractures. Although the albumin (Alb)–ALP ratio (AAPR) has been probed as a predictive factor in patients with various types of cancers,21,22 its prognostic value for MM remains to be explored.
Therefore, based on the evidence of the baseline inflammatory and nutritional parameters we mentioned above, our study aimed to assess the prognostic value of baseline inflammatory and nutritional parameters for predicting overall survival (OS) in patients with NDMM. Using the data obtained from such analyses, we then aimed to build a new scoring system (ie, Inflammatory Nutritional Score [INS]), which was combined with other independent prognostic factors to develop a nomogram for predicting individual survival outcomes in patients with NDMM.
Patients and Methods
Patients
This retrospective study enrolled patients with NDMM treated between July 2009 and July 2021 at Sun Yat-sen University Cancer Center (SYSUCC). Clinical and laboratory data were extracted from the medical records database of SYSUCC. Patients lacking complete baseline data were excluded from the study, as were those under the age of 18 and those with other types of cancer.
Data Collection and Definition of Parameters
Clinical and laboratory data were collected prior to treatment. The cut-off values for the following continuous variables were determined based on previously reported findings/upper limits: hemoglobin (HGB, 120 g/L), β2_microglobulin (β2_MG, 3.5 mg/L),9 creatinine (CRE, 177 µmol/L),14 and lactate dehydrogenase (LDH, 250 U/L).The eight inflammatory/nutritional indices were calculated as follows: NRI = 1.489 × albumin (g/L) + 41.7 × (weight/usual body weight);23 BMI = weight (kg)/(height in meters)^2;17 MLR = M/L; NLR = N/L; platelet-lymphocyte ratio (PLR) = P/L, PNI = albumin (g/L) + 5 × L (109/L), AAPR = albumin (g/L)/ALP (μ/L) (P: platelet count, M: monocyte count, N: neutrophil count, L: lymphocyte [109/L]). CONUT scores were determined by three basic parameters, serum albumin level, serum cholesterol, and peripheral lymphocyte count, which can be used for classification into four groups, as shown in Table S1 (available in Supplementary Materials). The best cut-off values of the above eight inflammatory/nutritional indices were determined based on maximally selected rank statistics (R package “maxstat”) for OS. The optimal cut-off values for age, C-reactive protein (CRP), and calcium (Ca) were also calculated using maximally selected rank statistics. The best cut-off values of the above variables were as follows: NRI (89), BMI (21.4), MLR (0.53), NLR (1.09), PLR (55), PNI (34.4), CONUT (5), AAPR (0.4), age (50 years), CRP (7.6 mg/L), and Ca (2.5 mmol/L). Thus, the continuous variables were changed into categorical variables, and patients were separated into two groups based on the cut-off values. As transplantation is an important factor, it was also included in our study.
Statistical Analysis
All eligible patients were randomly divided into the training and validation cohorts using the R package “caret.” Continuous variables are shown as medians and interquartile ranges (IQRs), while categorical variables are described as frequencies and percentages. The chi-square test was used to compare data between the two cohorts. Variables above and below the cut-off values were scored as 1 and 0, respectively.
To avoid the potential for multicollinearity, the least absolute shrinkage and selection operator (LASSO) Cox regression model was used to select the most influential factors among the eight inflammatory/nutritional indices to construct the INS. The INS cut-off values were determined by maximally selected rank statistics, which were then used to separate patients into high INS and low INS groups. High INS and low INS were scored as 1 and 0, respectively. The Kaplan–Meier method was used to evaluate the survival curves, which were compared using the Log rank test. The INS and other variables were analyzed using univariate Cox analysis. Variables (p<0.05) in the univariate Cox analysis were selected for multivariate Cox analysis. After multivariate Cox analysis, variables considered independent prognostic factors (p < 0.05) were integrated to develop a comprehensive nomogram. The performance of the model was assessed using the concordance index (C-index) and area under the curve (AUC) values at 1, 3, and 5 years. In addition, a calibration plot was used to assess the consistency between the predicted and actual observed survival probabilities. Comparisons between different staging systems were performed based on a time-dependent receiver operating characteristic (ROC) analysis. Bootstraps with 1000 resamples were used for internal validation.
All modeling and statistical analyses were performed using R version 4.0.3 (The R Foundation, Vienna, Austria). A two-sided p-value (<0.05) was deemed statistically significant.
Results
Baseline Characteristics
A total of 442 eligible patients with NDMM who visited our hospital between July 2009 and July 2021 were enrolled in this study. These patients were randomly separated into the training cohort (n = 354) and validation cohort (n = 88) at a ratio of 8:2. The baseline patient characteristics are presented in Table 1. The median age at diagnosis for all patients was 60 years (IQR: 53–67), and 366 patients (68.6%) were older than 50 years. A total of 264 patients (59.7%) were male. Advanced DS stage (stage III) was noted in 332 patients (75.1%), while stage II R-ISS was noted in 301 patients (68.1%). Most patients (394, 89.1%) exhibited good performance status (Eastern Cooperative Oncology Group [ECOG] score <2). The baseline characteristics were well-balanced and comparable between the two cohorts.
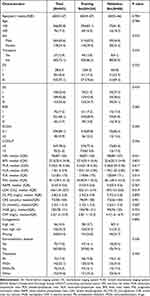 |
Table 1 The Baseline Characteristics of 442 NDMM Patients |
Building the INS
Figure 1 shows the process used to construct the INS. After examining eight inflammatory nutritional indices via LASSO Cox regression analysis, the six most valuable prognostic variables were sorted to build the INS, including the NRI, BMI, MLR, NLR, PLR, and AAPR (Figure 2A and B). Our results suggest that low NRI, low BMI, high MLR, high NLR, low PLR, and low AAPR were significantly associated with shorter OS.
High NRI (>89), high BMI (>21.4 kg/m2), high MLR (>0.53), high NLR (>2.57), high PLR (>55), and high AAPR (>0.4) were scored as 1, while other values were scored as 0. The INS was calculated based on the following formula: INS = 0.3158 × MLR - 0.3827 × NRI - 0.2800 × BMI - 0.4568 × PLR - 0.3094 × AAPR + 0.1238 × NLR. Using the INS cut-off value (−1) determined based on maximally selected rank statistics in the training cohort, patients were stratified into the low INS group (INS ≤ −1) and the high INS group (INS > −1). Thus, the training cohort included 185 patients with high INS and 169 patients with low INS, while the validation cohort included 49 patients with high INS and 39 with low INS. Therefore, the high and low INS groups included 234 and 208 patients overall, respectively. Significant differences in OS were observed between the high and low INS groups in the different cohorts (p < 0.001). Figure 3 shows the OS curves for all patients, the training cohort, and the validation cohort.
Selection of Independent Prognostic Factors
The results of the univariate and multivariate Cox analyses are shown in Table 2. The univariate analysis indicated that older age (>50 years), poor performance status (ECOG ≥ 2), high INS, high serum LDH, high serum β2_MG, low HGB, and high CRP levels were associated with a shorter OS. In the multivariate analysis, INS (HR = 2.172; 95% CI: 1.487–3.175, p < 0.001), age, ECOG, LDH, and CRP were retained as independent prognostic factors.
 |
Table 2 The Univariate and Multivariate Analysis for Overall Survival in the Training Cohort |
Construction and Evaluation of the Prognostic Nomogram
The above five independent prognostic factors (INS, age, ECOG, LDH, and CRP) were integrated to construct a prognostic nomogram for predicting OS at 1, 3, and 5 years (Figure 4). As shown in the nomogram, a higher total score implies a worse survival. The C-index for the nomogram was 0.708 and 0.749 in the training and validation cohorts, respectively. The AUCs for 1, 3, and 5 years OS in the training cohort were 0.740, 0.743, and 0.711, respectively (Figure 5A). In the validation cohort, the AUCs for 1, 3, and 5 years OS were 0.783, 0.689, and 0.777, respectively (Figure 5D). The calibration curves for 1, 3, and 5 years OS exhibited excellent consistency between the predicted OS and the actual observed OS in the training and validation cohorts (Figure 5B and E). Time-dependent ROC curves revealed that the prognostic nomogram exhibited better accuracy than other MM staging systems in both the training and validation cohorts (Figure 5C and F).
Discussion
In our study, we developed a new scoring system for NDMM based on six pretreatment inflammatory/nutritional indices, including NRI, BMI, MLR, NLR, PLR, and AAPR. Patients were divided into high INS and low INS groups based on the cut-off values determined via maximally selected rank statistics. According to the multivariate Cox analysis, INS was an independent prognostic factor for OS, and high INS was significantly associated with worse OS in patients with NDMM. Furthermore, the nomogram combining INS and the other four independent prognostic variables (age, ECOG, LDH, and CRP) exhibited robust predictive performance and good prognostic accuracy in both cohorts.
Although there are several widely utilized staging systems for MM (DS, ISS, and R-ISS), MM is a heterogeneous disease that cannot be adequately captured using a single staging system for all patients. The DS staging system mainly reflects tumor burden and includes some indicators that can be considered subjective. The ISS relies on two biomarkers (β2_MG and albumin). However, for patients with low-secretory or non-secretory MM exhibiting higher tumor burden with low serum β2_MG, ISS staging may be inaccurate. Furthermore, some patients with low serum β2_MG levels in the early stage of the disease have chromosome translocation t(4;14) and other cytogenetic abnormalities, which have been associated with poor prognosis. The mSMART staging system mainly relies on cytogenetic factors without including adequate clinical and laboratory information. Thus, further studies are required to develop a more accurate and clinically practical prognostic stratification system for NDMM.
As a recognized hallmark of malignancies, inflammation is important in the development and progression of cancers and has been strongly associated with cancer survival.24 Accordingly, some prognostic models based on multiple inflammatory indicators have demonstrated good predictive performance.25,26 Considering the close relationship between MM and inflammation, we believe that prognostic models should be based on multiple inflammatory variables rather than one or a few variables, as this can substantially improve their predictive accuracy. Several studies have also emphasized the role of nutritional status in survival for many cancer types.27–29 The disadvantage of single biomarkers is that they cannot completely reflect overall immune and nutritional status. Our model includes three nutritional indicators (BMI, AAPR, and NRI) as well as three inflammatory–immune indicators (MLR, NLR, and PLR). To reduce the impact of multicollinearity, the LASSO Cox model was used to effectively screen valuable biomarkers. In contrast, most previous researches simply integrated inflammatory variables with high collinearity and correlation into a multivariate Cox model to select independent prognostic factors, which can result in statistical issues.26
Some studies have provided evidence that high NLR and MLR are related to shorter OS,9–11 in accordance with our findings. Reactive thrombocytosis exhibits a known association with the systemic inflammatory response. Thus, many studies have explored the effect of PLR on hematologic and non-hematologic cancers, reporting that high PLR is relevant with poor prognosis and exerts a negative impact on survival.30–32 However, low PLR has been related to poor survival outcomes in MM, which is a hematologic malignancy involving inflammation-induced suppression of thrombopoiesis.33 Similarly, patients with a low PLR in our study exhibited poorer OS. A decreased PLR value implies a relatively reduced platelet level or an elevated lymphocyte count. Patients with low platelet counts are more likely to develop bleeding, which may lead to worse survival. Elevated lymphocyte levels indicate the development of inflammation, which may reflect MM progression. In addition to the above inflammatory indices, CRP is also a classic inflammatory biomarker that has been highlighted as a marker of tumor burden in patients with MM, and high serum CRP levels have been consistently associated with poor survival.34–36 Our results also demonstrate that elevated CRP is a strong prognostic biomarker for unfavorable survival outcomes.
The nutritional indicators included in our model (BMI, AAPR, and NRI) mainly depend on body weight and serum albumin levels. Involuntary weight loss is a negative prognostic factor for survival outcomes in many cancer types. One study reported that a loss of premorbid weight (>5%) before treatment predicts early mortality independent of performance status, tumor stage, and histology.37 However, most studies have focused on the effect of obesity rather than that of underweight on cancer prognosis. This may be because overweight and obesity are more common in Western countries than in Asian countries (33.8% vs 3.8%).17 In accordance with our findings, one study conducted in Korea reported that low BMI (<20 kg/m2) before treatment was related to unfavorable survival outcomes in patients with MM, highlighting the need for further studies to examine the effect of underweight on MM prognosis in Asian patients. Patients with a low BMI may be less likely to withstand the adverse effects associated with intense chemotherapy, which may lead to a delay or interruption in chemotherapy. Albumin, which is included in the ISS, is another important predictor of MM. The AAPR and NRI are also based on albumin, which has been verified as a prognostic factor for many cancers.21,38–41 In our study, low NRI and low AAPR were significantly related to poor OS. Moreover, as observed in previous studies, performance status, LDH, and age were significantly related to survival outcomes in our patients with MM.5,42–44
Recently, more studies on developing risk stratification or prognostic model using gene expression data from public databases for multiple myeloma are emerging, including The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), and so on. Still, most models are hard to put into clinical practice. Compared with our model, with an average of fewer than 200 dollars, it is hard to analyze above the genetic risk model because next-generation sequencing is always conducted in genetic sequencing companies instead of primary hospitals. In this study, we constructed a prognostic model combining INS with other independent variables, and the C-index of our model was 0.708 in the training cohort and 0.749 in the validation cohort. Moreover, compared with other staging systems of MM, our model exhibited a better predictive performance (higher AUC value) according to the time-dependent ROC curves in the training and validation cohorts. These findings implied that our model based on INS might be a better tool for MM, which might be helpful in individual prognosis predictions and personalized treatment guidance. For instance, patients with high total scores tended to have worse survival, so more intensive treatment and more frequent follow-up might be necessary for those patients to improve their prognosis.
Our study had some limitations, including its small sample size and single-center, retrospective design. Therefore, selection bias was inevitable, and our results must be validated in large-scale, prospective studies conducted across multiple centers. Second, we only investigated the prognostic value of pretreatment inflammatory nutritional variables. However, the dynamic changes in these variables that occur during subsequent antitumor treatment and their impact on the prognosis of patients remain poorly understood. Third, due to some restrictions, some patients did not undertake cytogenetic tests, and some other important prognostic factors were missing, which led to a small sample size of our study. Finally, due to the large time span and the retrospective nature of our study, the treatment regimen could be different in other areas, leading to different survival outcomes.
Conclusion
In this study, we constructed a comprehensive prognostic scoring system for MM (INS) based on pretreatment inflammatory/nutritional indices. Our subsequent analyses identified INS as an independent prognostic factor of OS in patients with NDMM. The nomogram based on the INS exhibited good predictive accuracy and discriminative ability, suggesting that it can aid in predicting individual survival probability in patients with NDMM.
Data Sharing Statement
The data of this study can be obtained from the corresponding authors upon reasonable request.
Ethics Approval and Informed Consent
This study got the approval of the Institutional Ethics Committee of Sun Yat-sen University Cancer Center. The requirement for informed consent was waived because it was a retrospective study. This retrospective study was performed in accordance with the Declaration of Helsinki. We covered patient data confidentially.
Funding
This study was supported in part by Sun Yat-sen University Start-up Funding (Grant No. 201603), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07S096), and the National Natural Science Foundation of China (Grant No. 81873428).
Disclosure
There are no conflicts of interest to declare.
References
1. van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397(10272):410–427. doi:10.1016/s0140-6736(21)00135-5
2. Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–2869. doi:10.1200/jco.2015.61.2267
3. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854. doi:10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u
4. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi:10.1200/jco.2005.04.242
5. Perrot A, Lauwers-Cances V, Tournay E, et al. Development and validation of a cytogenetic prognostic index predicting survival in multiple myeloma. J Clin Oncol. 2019;37(19):1657–1665. doi:10.1200/jco.18.00776
6. Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84(12):1095–1110. doi:10.4065/mcp.2009.0603
7. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–850. doi:10.1038/ni.3754
8. Caro J, Braunstein M, Williams L, et al. Inflammation and infection in plasma cell disorders: how pathogens shape the fate of patients. Leukemia. 2022;36(3):613–624. doi:10.1038/s41375-021-01506-9
9. Dosani T, Covut F, Beck R, Driscoll JJ, De lima M, Malek E. Significance of the absolute lymphocyte/monocyte ratio as a prognostic immune biomarker in newly diagnosed multiple myeloma. Blood Cancer J. 2017;7(6):e579. doi:10.1038/bcj.2017.60
10. Romano A, Laura Parrinello N, Cerchione C, et al. The NLR and LMR ratio in newly diagnosed MM patients treated upfront with novel agents. Blood Cancer J. 2017;7(12):649. doi:10.1038/s41408-017-0019-6
11. Romano A, Parrinello NL, Consoli ML, et al. Neutrophil to lymphocyte ratio (NLR) improves the risk assessment of ISS staging in newly diagnosed MM patients treated upfront with novel agents. Ann Hematol. 2015;94(11):1875–1883. doi:10.1007/s00277-015-2462-4
12. Binder M, Rajkumar SV, Lacy MQ, et al. Peripheral blood biomarkers of early immune reconstitution in newly diagnosed multiple myeloma. Am J Hematol. 2019;94(3):306–311. doi:10.1002/ajh.25365
13. Okamoto S, Ureshino H, Kidoguchi K, et al. Clinical impact of the CONUT score in patients with multiple myeloma. Ann Hematol. 2020;99(1):113–119. doi:10.1007/s00277-019-03844-2
14. Zhou X, Lu Y, Xia J, Mao J, Wang J, Guo H. Association between baseline Controlling Nutritional Status score and clinical outcomes of patients with multiple myeloma. Cancer Biomark. 2021;32(1):65–71. doi:10.3233/cbm-210073
15. Kamiya T, Ito C, Fujita Y, et al. The prognostic value of the controlling nutritional status score in patients with multiple myeloma. Leuk Lymphoma. 2020;61(8):1894–1900. doi:10.1080/10428194.2020.1749608
16. Garzón Herazo JR, Muñoz Velandia OM, Solano JC, Molina Pimienta L, Figueroa Lemus WJ. The nutrition risk index is associated with bacteremia within 30 days after autologous stem cell transplantation in patients with multiple myeloma. Transpl Infect Dis. 2020;22(4):e13302. doi:10.1111/tid.13302
17. Jung SH, Yang DH, Ahn JS, et al. Decreased body mass index is associated with poor prognosis in patients with multiple myeloma. Ann Hematol. 2014;93(5):835–840. doi:10.1007/s00277-013-1977-9
18. Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi:10.1016/0002-9610(80)90246-9
19. Liang F, Dong XY, Tang GF, et al. Influence of prognostic nutritional index and controlling nutritional status on the prognosis of patients with multiple myeloma. Zhonghua Xue Ye Xue Za Zhi. 2021;42(4):332–337. Chinese. doi:10.3760/cma.j.issn.0253-2727.2021.04.011
20. Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82(4):858–864. doi:10.1054/bjoc.1999.1012
21. Sandfeld-Paulsen B, Aggerholm-Pedersen N, Winther-Larsen A. Pretreatment albumin-to-alkaline phosphatase ratio is a prognostic marker in lung cancer patients: a registry-based study of 7077 lung cancer patients. Cancers. 2021;1(2):613. doi:10.3390/cancers1323613
22. Wang Y, Xiong F, Yang J, et al. Decreased albumin-to-alkaline phosphatase ratio predicted poor survival of resectable gastric cancer patients. J Gastrointest Oncol. 2021;12(4):1338–1350. doi:10.21037/jgo-21-430
23. Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
24. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi:10.1016/s1470-2045(14)70263-3
25. Li WZ, Hua X, Lv SH, et al. A scoring system based on nutritional and inflammatory parameters to predict the efficacy of first-line chemotherapy and survival outcomes for de novo metastatic nasopharyngeal carcinoma. J Inflamm Res. 2021;14:817–828. doi:10.2147/jir.S296710
26. Wang N, Xi W, Lu S, et al. A novel inflammatory-nutritional prognostic scoring system for stage III gastric cancer patients with radical gastrectomy followed by adjuvant chemotherapy. Front Oncol. 2021;11:650562. doi:10.3389/fonc.2021.650562
27. Mao YS, Hao SJ, Zou CF, Xie ZB, Fu DL. Controlling Nutritional Status score is superior to Prognostic Nutritional Index score in predicting survival and complications in pancreatic ductal adenocarcinoma: a Chinese propensity score matching study. Br J Nutr. 2020;124(11):1190–1197. doi:10.1017/s0007114520002299
28. Lee S, Fujita K, Morishita T, et al. Prognostic utility of a geriatric nutritional risk index in combination with a comorbidity index in elderly patients with diffuse large B cell lymphoma. Br J Haematol. 2021;192(1):100–109. doi:10.1111/bjh.16743
29. Chen L, Bai P, Kong X, et al. Prognostic Nutritional Index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front Cell Dev Biol. 2021;9:656741. doi:10.3389/fcell.2021.656741
30. Wang KF, Chang BY, Chen XQ, et al. A prognostic model based on pretreatment platelet lymphocyte ratio for stage IE/IIE upper aerodigestive tract extranodal NK/T cell lymphoma, nasal type. Med Oncol. 2014;31(12):318. doi:10.1007/s12032-014-0318-8
31. Reddy JP, Hernandez M, Gunther JR, et al. Pre-treatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio are prognostic of progression in early stage classical Hodgkin lymphoma. Br J Haematol. 2018;180(4):545–549. doi:10.1111/bjh.15054
32. Salman T, Kazaz SN, Varol U, et al. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for patients with neuroendocrine tumors: an Izmir oncology group study. Chemotherapy. 2016;61(6):281–286. doi:10.1159/000445045
33. Solmaz S, Uzun O, Acar C, et al. Is the platelet-to-lymphocyte ratio a new prognostic marker in multiple myeloma? J Lab Physicians. 2018;10(4):363–369. doi:10.4103/jlp.Jlp_36_18
34. Bataille R, Boccadoro M, Klein B, Durie B, Pileri A. C-reactive protein and beta-2 microglobulin produce a simple and powerful myeloma staging system. Blood. 1992;80(3):733–737. doi:10.1182/blood.V80.3.733.733
35. Offidani M, Corvatta L, Polloni C, et al. Serum C-reactive protein at diagnosis and response to therapy is the most powerful factor predicting outcome of multiple myeloma treated with thalidomide/ anthracycline-based therapy. Clin Lymphoma Myeloma. 2008;8(5):294–299. doi:10.3816/CLM.2008.n.041
36. Kim DS, Yu ES, Kang KW, et al. Myeloma prognostic index at diagnosis might be a prognostic marker in patients newly diagnosed with multiple myeloma. Korean J Intern Med. 2017;32(4):711–721. doi:10.3904/kjim.2016.054
37. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69(4):491–497. doi:10.1016/s0149-2918(05)80001-3
38. Tian G, Li G, Guan L, Yang Y, Li N. Pretreatment albumin-to-alkaline phosphatase ratio as a prognostic indicator in solid cancers: a meta-analysis with trial sequential analysis. Int J Surg. 2020;81:66–73. doi:10.1016/j.ijsu.2020.07.024
39. An L, Yin WT, Sun DW. Albumin-to-alkaline phosphatase ratio as a promising indicator of prognosis in human cancers: is it possible? BMC Cancer. 2021;21(1):247. doi:10.1186/s12885-021-07921-6
40. Riveros C, Jazayeri SB, Chalfant V, Ahmed F, Bandyk M, Balaji KC. The Geriatric nutritional risk index predicts postoperative outcomes in bladder cancer: a propensity score-matched analysis. J Urol. 2022;207(4):797–804. doi:10.1097/ju.0000000000002342
41. Ruan GT, Zhang Q, Zhang X, et al. Geriatric Nutrition Risk Index: prognostic factor related to inflammation in elderly patients with cancer cachexia. J Cachexia Sarcopenia Muscle. 2021;12(6):1969–1982. doi:10.1002/jcsm.12800
42. Ludwig H, Durie BG, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–4047. doi:10.1182/blood-2007-03-081018
43. Cejalvo MJ, Bustamante G, González E, et al. Treatment patterns and outcomes in real-world transplant-ineligible patients newly diagnosed with multiple myeloma. Ann Hematol. 2021;100(7):1769–1778. doi:10.1007/s00277-021-04529-5
44. Cook G, Royle KL, Pawlyn C, et al. A clinical prediction model for outcome and therapy delivery in transplant-ineligible patients with myeloma (UK Myeloma Research Alliance Risk Profile): a development and validation study. Lancet Haematol. 2019;6(3):e154–e166. doi:10.1016/s2352-3026(18)30220-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





