Back to Journals » Journal of Inflammation Research » Volume 15
Inflammation-Based Scores Predict Responses to PD-1 Inhibitor Treatment in Intrahepatic Cholangiocarcinoma
Authors Yang Z, Zhang D , Zeng H, Fu Y , Hu Z , Pan Y , Chen J , Wang J, Zhang Y , Zhou Z, Xu L , Hu D, Chen M
Received 11 August 2022
Accepted for publication 28 September 2022
Published 6 October 2022 Volume 2022:15 Pages 5721—5731
DOI https://doi.org/10.2147/JIR.S385921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Zhenyun Yang,1,2,* Deyao Zhang,1,2,* Huilan Zeng,1,2,* Yizhen Fu,1,2 Zili Hu,1,2 Yangxun Pan,1,2 Jinbin Chen,1,2 Juncheng Wang,1,2 Yaojun Zhang,1,2 Zhongguo Zhou,1,2 Li Xu,1,2 Dandan Hu,1,2 Minshan Chen1,2
1State Key Laboratory of Oncology in South China, Sun Yat-Sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, 510060, People’s Republic of China; 2Department of Liver Surgery, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dandan Hu; Minshan Chen, Tel +86-20-87343828 ; +86-20-87343117, Fax +86-20-87343585, Email [email protected]; [email protected]
Purpose: Inflammatory response is related to tumor progression and patient survival. We aimed to clarify the prognostic value of the inflammation-based scores in intrahepatic cholangiocarcinoma (ICC) patients receiving anti-PD1 therapy.
Patients and Methods: A total of 73 patients who received anti-PD-1 therapy from February 2019 to February 2021 were included in the study. Representative inflammation-based prognostic scores, including C-reactive protein (CRP), the platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-CRP ratio (LCR), lymphocyte-to-monocyte ratio (LMR), systemic immune inflammation index (SII), CRP-to-albumin ratio (CAR), prognostic nutritional index (PNI), Glasgow Prognostic Score (GPS), and prognostic index (PI), were assessed for prediction accuracy using Kaplan–Meier survival curves and time-dependent receiver operating characteristic (ROC). All the ten inflammation-based prognostic scores were measured before receiving anti-PD1 therapy.
Results: All the ten inflammation-based prognostic scores showed good discriminatory ability in terms of overall survival (OS) (all P < 0.01), the higher the score, the worse the prognosis, while the CRP score was a remarkable independent predictor for OS in multivariate analysis (hazard ratio, 6.032; confidence interval, 2.467– 14.752; P < 0.001). The area under the ROC curve at 6 months, 12 months, 18 months and 24 months consistently demonstrated that the predictive value of the CRP score was superior to other inflammation-based scores.
Conclusion: Inflammation-based scores predict the efficacy of anti-PD-1 therapy in patients with ICC and CRP score superior to the other inflammation-based prognostic scores in terms of predictive ability.
Keywords: inflammation-based score, intrahepatic cholangiocarcinoma, anti-PD-1 treatment, overall survival, C-reactive protein
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy with a high degree of malignancy and poor prognosis.1,2 The global incidence of ICC is expected to increase ten-fold over the next 20 to 30 years.3 However, the treatment modalities of ICC are still limited. Most patients are diagnosed at advanced stages, when the available systemic therapies are of limited benefit.4 In recent years, research on programmed cell death protein-1 (PD-1) inhibitors has continued to emerge,5 showing promising effects on patients with ICC. However, the efficacy of immunotherapy varies from person to person, based on results from completed studies of anti-PD1 monotherapy in ICC, objective response rates (ORRs) ranged from 3% to 21.4%.6,7 Meanwhile, the incidence of treatment-related adverse events was more than 90%, the most common all-grade adverse events were anaemia, fatigue, and dysphagia, respectively, and the most common grade 3 or higher adverse events were neutropenia, hypertension, lipase increased, and lymphopenia.8 Practical and reliable prognostic predictors are needed which can differentiate the ICC patients with the highest potential to benefit from PD-1 inhibitors. Also, this would help avoid unnecessary therapy and the occurrence of adverse events.
Cancer-related inflammation is recognized as a hallmark of cancer.9 In established cancers, there is increasing evidence for the roles that local immune response and systemic inflammation have in progression of tumors and survival of patients with cancer.10,11 For the past few years, many inflammation-based prognostic scores composed of systemic inflammatory response factors were proposed to exhibit good predictive ability in cancer prognosis, which included C-reactive protein (CRP),12,13 platelet-to-lymphocyte ratio (PLR),14 neutrophil-to-lymphocyte ratio (NLR),15,16 lymphocyte-to-CRP ratio (LCR),17,18 lymphocyte-to-monocyte ratio (LMR),19,20 (SII),21,22 CRP-to-albumin (ALB) ratio (CAR),23,24 prognostic nutritional index (PNI),25,26 Glasgow Prognostic Score (GPS),27,28 and prognostic Index (PI).29 These inflammation-based scores have been reported to be associated with the value in predicting survival after anti-PD-1 treatment in HCC patients.30 However, the function in predicting survival in ICC patients who received PD-1 inhibitors has not been clarified ever.
Herein, we aimed to evaluate the prognostic role of these inflammation-based scores and carry a direct comparison of various inflammation-based scores in ICC patients after anti-PD1 treatment.
Materials and Methods
Patients
A total of 73 patients diagnosed with ICC who received anti-PD-1 therapy between February 2019 and February 2021 at the Sun Yat-sen University Cancer Center were selected as the study population. Patients were included based on the following criteria: (a) aged from 18 to 75 years; (b) histological confirmation of ICC; (c) had confirmed records of receiving PD-1 inhibitors; (d) had a performance status (PS) score less than 2; (e) had no other malignant tumors; and (f) had complete medical and low-up data. The study used retrospective anonymous clinical data that were obtained after each patient agreed to treatment.
Treatment Procedure
PD-1 inhibitors were all administered according to the following regimens: Nivolumab at 1–3 mg/kg body weight per 2 weeks, Pembrolizumab at 200mg per 3 weeks, Toripalimab, Camrelizumab and Sintilimab at 240 mg, 200mg and 200mg per 2 weeks. PD-1 inhibitors could be combined with locoregional interventional therapies or tyrosine kinase inhibitors (TKIs) including sorafenib, lenvatinib or apatinib during treatment (Table S1).
Data Collection
The demographic and clinical characteristics included age, gender, hepatitis, ALT (U/L), AST (U/L), ALB (g/L), TBIL (umol/L), CA19-9 (U/mL), largest tumor size (cm), tumor number, macroscopic vascular invasion, lymph node metastasis, combination therapy, TNM stage, CRP, PLR, NLR, LCR, LMR, SII, CAR, PNI, GPS, PI. Demographic and clinical characteristics are summarized in Table 1.
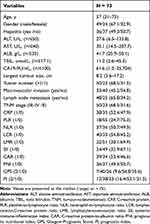 |
Table 1 Baseline Characteristics of the Enrolled Patients |
The counts of white blood cells, neutrophils, lymphocytes, monocytes, and platelets and the levels of CRP and ALB were measured within a period of 5 days prior to the onset of receiving anti-PD1 therapy. Serum samples were collected and clotted at room temperature, then centrifuged at 3500r/min for 10 min, which could be used to estimate the level of serum biomarkers. The concentrations of albumin and total proteins were measured using a Roche Cobas 702 Automatic Biochemical Analyzer. Albumin and total proteins were measured using colorimetry. The levels of neutrophil, lymphocyte, and platelet were derived from the blood count using a Sysmex XN-2000 automatic blood-cell counter. The coefficient of variation of the two tests in our laboratory is ≤5%. Radiological response was evaluated by computed tomography (CT) or magnetic resonance imaging (MRI) performed at a baseline, 6–12 weeks after treatment initiation, and approximately every 3 months thereafter according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
The CRP, PLR, NLR, LCR, LMR, SII, CAR, GPS, PI and PNI were calculated as described in Table 2. Overall survival (OS) was defined as the time interval from anti-PD-1 initiation to cancer-related death.
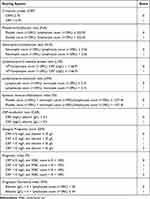 |
Table 2 Systemic Inflammation-Based Prognostic Scores |
Statistical Analysis
The results were described to use the median and range for nonnormally distributed values. Groups were compared by using Student’s t-test for continuous data and the χ2 test for categorical data. Independent predictive variables were determined with univariate and multivariate Cox regression analysis for OS by the method of Forward LR. The risk stratification survival curves were represented by Kaplan–Meier curves and analyzed using the Log rank test. For single value indicators, to avoid deviations from different criteria for the cutoff values of the prognostic scores in this cohort, the optimal cutoff point was calculated using R version 4.0.2 for CRP, PLR, NLR, LCR, LMR, SII, CAR, and PNI (Supplemental Figure 1A–H). For composite indicators, the GPS and PI scores were calculated as generally reported.28,29 Time-dependent receiver operating characteristic (ROC) curves at 6, 12, 18 and 24 months and the area under the curve (AUC) were calculated to compare the predictive ability of the ten inflammation-based scores. All data analyses were performed using SPSS 25.0 software (SPSS Inc., Chicago, IL) and R version 4.0.2.
Results
Patient Characteristics
A total of 73 patients with advanced ICC were treated with anti-PD-1 at the Sun Yat-sen University Cancer Center were included in the study. The cohort includes 49 (67.1%) males and 24 (32.9%) females. Ages ranged from 31 to 75 years with a median of 57 years. A majority of patients had multiple tumors (68.5%). A total of 33 (45.2%) patients had macrovascular invasion, and 48 (65.8%) patients had lymph node metastasis. The range of tumor size is from 3.6 to 21.5 cm, with a median of 8.2 cm. The clinical characteristics, including the ten inflammation-based scores of the patients, are summarized in Table 1. The average follow-up time after immunotherapy was 11.2 months. The median OS time was 10.5 months (95% CI: 8.9–12.1 months).
Univariate and Multivariate Cox Regression Analyses in the Cohorts
Univariate analysis involved prognostic factors related to ethnic characteristics, liver function, tumor burden and the ten inflammation-based scores. All ten inflammation scores were identified as significant prognostic factors for OS in addition to liver function and tumor burden. The details are described in Table 3. The multivariate Cox proportional analysis revealed that elevated serum CRP (P < 0.001), lymph node metastasis (P = 0.007), and PLR (P = 0.02) were significant and independent prognostic factors of OS (Table 3).
 |
Table 3 Univariate and Multivariate Cox Regression Analyses of Risk Factors for Overall Survival |
Overall Survival
All inflammation-based scores were associated with the OS of patients who received PD1 inhibitors (Figure 1). Low CRP PLR, NLR, LCR, LMR, SII, CAR, GPS, PI and PNI scores indicated good prognosis (all P < 0.01). The CRP score and PLR score remained as significant and independent predictors of OS in multivariate analysis. The CRP score differentiated the ICC patients into two groups with distinct prognoses. The median OS time was 20.4 months, 6.5 months.
 |
Figure 1 Kaplan–Meier curves of the overall survival of ICC patients after PD-1 inhibitors therapy. (A) CRP, (B) PLA, (C) NLR, (D) LCR, (E) LMR, (F) SII, (G) CAR, (H) PNI, (I) GPS and (J) PI. |
Comparison of the Performance of the Inflammation-Based Scores
Time-dependent ROC curves at 6, 12, 18 and 24 months of OS were formed to compare the performance of the ten inflammation-based scores (Supplemental Figure 2A–D), and the CRP score was better than the others. The AUC of the time-dependent ROC curve showed that the CRP score had a better ability to predict OS. Plots of the time-dependent AUC are shown in Figure 2. The area under the receiver operating characteristic curve (AUROC) was calculated, and the values are provided in Table 4. The CRP scores consistently had higher values than the other inflammation-based scores.
 |
Table 4 AUROC for the Comparison of Different Inflammatory-Based Scores |
 |
Figure 2 Time-dependent AUC plot for survival prediction of inflammation-based scores. |
Relationships Between the CRP Score and Clinical Characteristics and Efficacy
The correlations between the CRP score and clinical characteristics are shown in Table 5. A high CRP score is associated with lower albumin (P < 0.001), lymph node metastasis (P = 0.048), and worse TNM stage (P = 0.045). The survival curves of patients are stratified according to the presence of lymph node metastasis and TNM stage (Supplemental Figure 3A–D). Tumor response analysis indicated that the objective response rate (ORR) (22.9% vs 5.3%, P = 0.023) and disease control rate (DCR) (77.1% vs 52.6%, P = 0.002) were higher in the low CRP group.
 |
Table 5 Baseline Characteristics of the Patients Grouped by CRP Score |
Discussion
It is widely acknowledged that advanced ICC remains resistant to systemic treatments and therapy remains palliative. In recent years, immune checkpoint inhibitors (ICIs) targeting the programmed cell death 1 (PD-1) checkpoints have demonstrated the potential for relatively tumor-specific immune disinhibition across a range of tumor types. Immune checkpoint inhibitor (ICB), especially PD-1 inhibitors, has been demonstrated in several studies to have a definite effect on patients with ICC. However, due to different biological specificities of ICC, even patients have different prognoses for the same anti-PD1 treatment. Prognostic indicators for the use of PD-1 inhibitors are urgently needed. Emerging evidence shows that inflammation-based scores are associated with cancer-specific survival, but what components of the systemic inflammatory response best predict survival in ICC patients within anti-PD1 treatment remains unclear. This study is the first to comprehensively identify that inflammation scoring systems are associated with the OS of ICC patients who received PD-1 inhibitor therapy. Moreover, the CRP score was found to be superior to the other inflammation-based scores in predicting survival.
ICC is associated with primary sclerosing cholangitis, primary biliary cirrhosis and other diseases that cause biliary tract inflammation and fibrosis.31 Inflammation is crucial in the occurrence and development of ICC. Increasing evidence has shown that the inflammatory response is associated with the efficacy of PD-1 inhibitors in advanced cancers. The liver maintains a chronic state of immune tolerance that may be exploited by liver tumors, which further induce T cell exhaustion by upregulating immune checkpoints including TIM3, LAG3, PD-1, and CTLA-4,32,33 suggesting that ICIs such as PD-1 inhibitors could potentially restore antitumor immunity in liver tumors.
As an acute inflammatory response biomarker, serum CRP has been recognized as an indicator of progression for several cancers. CRP refers to acute proteins induced by IL-6, produced in the liver, which rises sharply in plasma when the body is infected or tissue damaged.34 CRP can activate complement and strengthen the phagocytosis of phagocytes to play an opsonizing role, thereby removing pathogenic microorganisms and damaged, necrotic, and apoptotic tissue cells that invade the body, and plays an important protective role in the body’s innate immune process.35,36 Our study demonstrated that compared with other inflammation-based scores, the presence of a systemic inflammatory response revealed by CRP is the best tool to assess survival in ICC patients receiving PD-1 inhibitor therapy. CRP is an acute-phase protein and a well-accepted marker of cancer-induced systemic inflammation, a condition which is clinically often reflected in cancer symptoms such as weight loss, fatigue, and anorexia.37–39 Therefore, the CRP level links with the clinical manifestations and may associate with the clinicopathological features of patients. The CRP level has been verified to be an effective prognostic predictor in many types of digestive system cancers, including ICC.17 The underlying mechanism has not been well elucidated. In our study, one possible explanation is that elevated CRP indicates sustained inflammation that may reflect a pro-angiogenic tumour microenvironment. Also, it has been reported that elevated CRP can help identify patients with impaired T lymphocytes, leading to poor prognosis in malignancy.40 On the other hand, CRP is associated with tumor immunosuppression. A previous study indicates that CRP can suppress proliferation and effector functions of activated CD4+ and CD8+ T cells from patients with melanoma.41 Thus, elevated CRP may play important roles in the poor prognosis observed for ICC patients with anti-PD-1 treatment.
This study has several limitations. Firstly, this was a retrospective study conducted on a single-center cohort in China. Secondly, the molecular mechanisms underlying inflammation and ICC are unknown and further basic research is needed. Thirdly, most of patients in this cohort received combination treatment and confounding factors were inevasible. As we can know, the ORR of anti-PD1 monotherapy for primary liver cancer is too low, combination therapy may be the mainstream treatment, we cannot change the clinical treatment strategy. Consequently, it is necessary to conduct more bench-scale study to figure out intrinsic mechanism that can guide the anti-PD1 treatment. Finally, CRP is a sensitive acute-phase protein, which could be affected by many factors, such as acute and chronic inflammation, undocumented infection, and trauma. Therefore, serum CRP levels should be detected in the stable phase to avoid the interference of other potential factors.
In conclusion, this study had demonstrated that CRP score predicts anti-PD-1 therapy response and survival in the cohort including a total of 73 patients with ICC. Meanwhile, CRP score is superior to the other inflammation-based prognostic scores in terms of predictive ability. This is easy to use for risk stratification and help patients receive appropriate treatment.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. This research was approved by the institutional review board of Sun Yat-sen University Cancer Center (Approval Number: B2020-250-01). The study used retrospective anonymous clinical data that were obtained after each patient agreed to treatment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is funded by the National Natural Science Foundation of China (No: 81874070).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289. doi:10.1016/j.jhep.2014.01.021
2. Sirica AE, Gores GJ, Groopman JD, et al. Intrahepatic cholangiocarcinoma: continuing challenges and translational advances. Hepatology. 2019;69(4):1803–1815. doi:10.1002/hep.30289
3. Rodriguez H, Pennington SR. Revolutionizing precision oncology through collaborative proteogenomics and data sharing. Cell. 2018;173(3):535–539. doi:10.1016/j.cell.2018.04.008
4. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi:10.1038/nrclinonc.2017.157
5. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi:10.1172/jci80011
6. Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, Phase 1 study. Lancet Gastroenterol Hepatol. 2019;4(8):611–621. doi:10.1016/s2468-1253(19)30086-x
7. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):353–363. doi:10.1016/j.jhep.2019.10.009
8. Zhou X, Yao Z, Bai H, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22(9):1265–1274. doi:10.1016/s1470-2045(21)00333-8
9. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
10. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi:10.1038/nrclinonc.2015.105
11. Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18(5):261–279. doi:10.1038/s41571-020-00459-9
12. Klümper N, Saal J, Berner F, et al. C reactive protein flare predicts response to checkpoint inhibitor treatment in non-small cell lung cancer. J Immunother Cancer. 2022;10(3):e004024. doi:10.1136/jitc-2021-004024
13. Zhang Y, Lu L, He Z, et al. C-reactive protein levels predict responses to PD-1 inhibitors in hepatocellular carcinoma patients. Front Immunol. 2022;13:808101. doi:10.3389/fimmu.2022.808101
14. Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3720 patients. Int J Cancer. 2016;139(1):164–170. doi:10.1002/ijc.30060
15. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
16. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360. doi:10.1186/s12916-020-01817-1
17. Lu LH, Zhong C, Wei W, et al. Lymphocyte-C-reactive protein ratio as a novel prognostic index in intrahepatic cholangiocarcinoma: a multicentre cohort study. Liver Int. 2021;41(2):378–387. doi:10.1111/liv.14567
18. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2020;272(2):342–351. doi:10.1097/sla.0000000000003239
19. Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: a meta-analysis. Int J Surg. 2018;55:128–138. doi:10.1016/j.ijsu.2018.05.030
20. Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: a meta-analysis. Int J Surg. 2018;50:67–71. doi:10.1016/j.ijsu.2018.01.002
21. Zhang K, Hua YQ, Wang D, et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med. 2019;17(1):30. doi:10.1186/s12967-019-1782-x
22. Nie D, Gong H, Mao X, Li Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: a retrospective study. Gynecol Oncol. 2019;152(2):259–264. doi:10.1016/j.ygyno.2018.11.034
23. Zhou J, Wei W, Hou H, et al. Prognostic value of C-reactive protein, Glasgow prognostic score, and c-reactive protein-to-albumin ratio in colorectal cancer. Front Cell Dev Biol. 2021;9:637650. doi:10.3389/fcell.2021.637650
24. Suzuki S, Akiyoshi T, Oba K, et al. Comprehensive comparative analysis of prognostic value of systemic inflammatory biomarkers for patients with stage II/III colon cancer. Ann Surg Oncol. 2020;27(3):844–852. doi:10.1245/s10434-019-07904-9
25. Kitahara H, Shoji F, Akamine T, et al. Preoperative prognostic nutritional index level is associated with tumour-infiltrating lymphocyte status in patients with surgically resected lung squamous cell carcinoma. EUR J Cardiothorac Surg. 2021;60(2):393–401. doi:10.1093/ejcts/ezab046
26. Wang X, Wang Y. The prognostic nutritional index is prognostic factor of gynecological cancer: a systematic review and meta-analysis. Int J Surg. 2019;67:79–86. doi:10.1016/j.ijsu.2019.05.018
27. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi:10.1016/j.ctrv.2012.08.003
28. Ando K, Sakamoto S, Saito S, et al. Prognostic value of high-sensitivity modified Glasgow prognostic score in castration-resistant prostate cancer patients who received docetaxel. Cancers. 2021;13(4):773. doi:10.3390/cancers13040773
29. Gruber ES, Jomrich G, Kaider A, Gnant M, Sahora K, Schindl M. The prognostic index independently predicts survival in patients with pancreatic ductal adenocarcinoma undergoing resection. Ann Surg Oncol. 2020;27(6):2017–2024. doi:10.1245/s10434-019-08161-6
30. Mei J, Sun XQ, Lin WP, et al. Comparison of the prognostic value of inflammation-based scores in patients with hepatocellular carcinoma after anti-PD-1 therapy. J Inflamm Res. 2021;14:3879–3890. doi:10.2147/jir.S325600
31. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi:10.1016/s0140-6736(13)61903-0
32. Kasper HU, Drebber U, Stippel DL, Dienes HP, Gillessen A. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol. 2009;15(40):5053–5057. doi:10.3748/wjg.15.5053
33. Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153(4):1107–1119.e10. doi:10.1053/j.gastro.2017.06.017
34. Del GM, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi:10.1016/j.bbi.2018.02.013
35. Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–2234. doi:10.1002/hep.26057
36. Toubi E, Vadasz Z. Innate immune-responses and their role in driving autoimmunity. Autoimmun Rev. 2019;18(3):306–311. doi:10.1016/j.autrev.2018.10.005
37. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi:10.1016/s1470-2045(14)70263-3
38. Sanghera C, Teh JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39(11):2008–2023. doi:10.1111/liv.14220
39. Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76(2):353–363. doi:10.1016/j.jhep.2021.09.035
40. Canna K, McArdle PA, McMillan DC, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92(4):651–654. doi:10.1038/sj.bjc.6602419
41. Yoshida T, Ichikawa J, Giuroiu I, et al. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. 2020;8(1):e000234. doi:10.1136/jitc-2019-000234
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
