Back to Journals » International Journal of General Medicine » Volume 17
Increased PD-1+ NK Cell Subset in the Older Population
Authors Deng M, Zeng Y, Liu Y, Wang X, Chen N, Zhang M, Jiang M, Zhao H , Du J
Received 19 December 2023
Accepted for publication 13 February 2024
Published 26 February 2024 Volume 2024:17 Pages 651—661
DOI https://doi.org/10.2147/IJGM.S452476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Meiju Deng,1,2,* Yongqin Zeng,2,3,* Ying Liu,2 Xiaolei Wang,1,2 Na Chen,1,2 Mengyuan Zhang,1,4– 6 Meiqing Jiang,1,4– 6 Hongxin Zhao,2 Juan Du1,4– 6
1Beijing Key Laboratory of Emerging Infectious Diseases, Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 2Clinical Center for HIV/AIDS, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 3Department of Nephrology, The Affiliated Hospital Guizhou Medical University, Guiyang, Guizhou, 550004, People’s Republic of China; 4Beijing Institute of Infectious Diseases, Beijing, 100015, People’s Republic of China; 5National Center for Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 6National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, Beijing, 100015, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Juan Du; Hongxin Zhao, Email [email protected]; [email protected]
Background: The aging of the immune system is associated with various diseases. It is worth exploring the changes of the immune system in aging. Previous studies have shown that aged T cells have enhanced expression of co-inhibitory molecules. However, it remains unclear whether aged NK cells exhibit similar characteristics to aged T cells. The objective of our research was to clarify this aspect.
Patients and Methods: This study included 98 adults aged 24– 90 years (50 males and 48 females). We detected the subset of peripheral blood NK cells and the expression of various receptors on NK cells among donors of different age groups by flow cytometry. Immune subsets were initially defined by forward and side-scatter characteristics and then staining with the appropriate marker.
Results: The absolute number and subset distribution of NK cells were not associated with age. However, CD57 expression and CD69 expression were correlated with age. Furthermore, we found that PD-1 was up-regulated on NK cells in older people, associated with aging, while no such change was observed in other co-inhibitory molecules, including 2B4, CTLA-4, TIM-3, BTLA, CD70, CD39, CD160, and TIGIT. PD-1+ NK cells expressed high levels of CD57 and CD69, indicating PD-1+ NK cells displayed a phenotype of over-activation and aging.
Discussion: This study indicated that PD-1+ NK cells were one of the characteristics of NK cells in older people.
Conclusion: This study indicated that PD-1+ NK cells were one of the characteristics of NK cells in older people. Those findings provided new ideas to explore the underlying drivers of NK aging.
Keywords: PD-1, NK cell, aging, co-inhibitory molecules
Introduction
With increasing age, the susceptibility to pathogens and tumors increases with higher mortality and morbidity. The aging of the immune system is a critical cause of age-related diseases, including malignancies, autoimmune diseases, and infectious diseases.1 The aging immune system undergoes a gradual deterioration of immunity and an enhanced chronic inflammatory state.2–4
Studies have shown that co-inhibitory molecules are associated with T-cell aging. In humans as well as in murine models, PD-1, 2B4, CD160, TIM-3, TIGIT and CD70 were upregulated during the process of aging.5–10 For example, upregulated with age from donors, CD244+CD160+CD8+ T cells manifested by increased activity of β-GAL (aging marker), higher production of cytokines, and severe metabolic disorders, compared to CD244−CD160−CD8+ T cells.8 In addition, aged TIGIT+CD8+ T cells9 and CD70+ T cells10 from the elderly exhibit high levels of inhibitory receptors, high susceptibility to apoptosis and defects in cytokine production. These findings suggested that co-inhibitory molecules contribute to aging in terms of reduced proliferative capacity and defective T‐cell response.
As one of the major compartments of the innate immune system, NK cells mediate the anti-tumoral and anti-infection immune responses.11 However, it remains unclear whether aged NK cells have characteristics similar to aged T cells, such as increased expression of maturation markers, co-inhibitory molecules and hyperactivation phenotypes.
The phenotype and function of NK cells are modified throughout the aging process. Some studies indicated that the increased number and the redistribution of NK cell subset have been observed in the elderly, including a decrease in the CD56brightCD16− NK cells and an expansion of CD56dimCD16+ NK cells or CD56negCD16+ NK cells.12–15 However, other researchers found that there were no significant changes in the absolute number and percentage of NK cells.16,17 Age-related alterations for molecules have been characterized, including the decreased expression of activating receptors (NKp30, NKp46) and the increased expression of inhibitory receptors (NKG2A, KLRG1).12,18–20 Besides, an increase in CD57 expression (a hallmark for NK cell maturation) and a reduction in CD69 expression (an early activation antigen) have already been demonstrated during aging.21,22
About the effect of aging on NK cell function, there were some different views. Several studies supported that the cytotoxicity and cytokine production of NK cells declined with age,23–28 while others have characterized that the cytotoxic capacity of NK cells was preserved in the aging population of humans.12,17 The discrepant results that have been found may vary based on geographic regions, ethnicities, selected age ranges, status of the individuals and technical procedures.
The function of NK cells is tightly regulated by activating and inhibiting receptors.
However, comparatively little is known about the effect of aging on the expression patterns of co-inhibitory molecules in NK cells.
Here, we investigated the changes in NK cells related to age using blood samples from donors. We assessed the subset distribution and absolute number of NK cells. Then, we screened co-inhibitory molecules and identified a unique subset of PD-1+ NK cells only in the elderly. PD-1+ NK cells displayed a phenotype of over-activation and aging. Overall, this study suggested that PD-1 upregulation in NK cells was an important process associated with aging.
Materials and Methods
Participants
Inclusion criteria targeted volunteers aged 20–90 years (50 males and 48 females) recruited between September 2021 and 2022. A series of laboratory parameters including blood routine indexes, coagulation function, hepatic function, renal function, myocardial enzyme parameters, blood glucose, blood lipid, and tumor markers including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen (CA-199), CA-153, and CA-125 were considered in the exclusion criteria. The subjects who tested positive for human immunodeficiency virus (HIV) infection, hepatitis viral infections, systemic infection, connective tissue disease, cancer or abnormal laboratory indexes above were excluded from our study.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Peripheral blood samples were obtained from donors, and PBMCs were separated by standard Ficoll-Paque gradient centrifugation according to the instructions of the manufacturer (Amersham Pharmacia Biotech, Sweden). Cells were cryopreserved in fetal bovine serum (FBS) (GIBCO, Grand Island, NY, USA) supplemented with 10% dimethylsulfoxide (DMSO) and stored in liquid nitrogen.
Immunofluorescence Staining and Flow Cytometry Analysis
PBMCs from subjects were resuspended in PBS buffer and were incubated with directly conjugated antibodies for 30 min at 4°C. The cells were washed with 1× PBS before flow cytometry analysis. Antibodies used included anti-human CD3-BV786, CD19-BVAPC-H7, CD16-BV711, CD69 PE-CF594, CD57-BV421, PD-1-APC, CD70-PE, CD160-AF488, 2B4-AF700, CTLA-4-PE, TIM-3-FITC, 7-AAD (BD Biosciences, San Diego, CA, USA), CD56-BV510, BTLA-PE-CY7, CD39-APC (BioLegend, San Diego, CA, USA), TIGIT-PE-Cy7 (Ebioscience, San Diego, CA, USA), along with the corresponding isotype controls. NK cells were gated as CD3−CD14−CD19−CD56+CD16+, CD3−CD14−CD19−CD56+CD16−, or CD3−CD14−CD19−CD56−CD16+. Data were acquired with the LSR Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software version 10.5 (Tree Star, Ashland, OR, USA).
Statistical Analysis
The data are expressed as the mean ± standard deviation (SD). All data were analyzed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). The normality of each variable was assessed using the Kolmogorov–Smirnov test. In cases of two normally distributed data, the comparison of variables was analyzed using unpaired or paired where specified, two-tailed Student’s t-tests for unpaired and paired data, respectively. A one-way ANOVA or a repeated-measures ANOVA followed by Tukey’s multiple comparisons test was performed for comparing more than two unpaired and paired samples, respectively. When the data were not normally distributed, the comparison of variables was analyzed with a Mann–Whitney U-test or a Wilcoxon matched-pairs signed rank test for unpaired and paired data. For comparing more than two unpaired and paired samples, a Kruskal–Wallis test or a Friedman test with Dunn’s multiple comparisons test was used, respectively. The characteristics of the participants were compared using the Chi-square test (categorical variables) or the Kruskal–Wallis test (continuous variables). Pearson’s or Spearman correlation coefficients were performed to evaluate correlations for normally or non-normally distributed data, respectively. For all analyses, P < 0.05 was considered statistically significant.
Results
Age-Related Changes of Subset Distribution, Subset Distribution and Phenotype on NK Cells
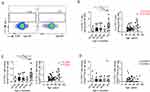
To explore the changes in NK cells related to age, we examined the subset of peripheral blood NK cells and the expression of various receptors from 98 donors by flow cytometry (characteristics of cohort in Table 1, flow cytometry gating strategy in Figure S1). The donors aged 30–39 years showed lower absolute number of total NK cells than the donors aged 50–59 years and older (≥60 years old) (Figure 1A). Based on the expression of the adhesion molecule CD56 and the activating receptor CD16, NK cells are generally divided into three subsets: CD56brightCD16− NK cells, CD56dimCD16+ NK cells, CD56negCD16+ NK cells. Compared to the donors aged 50–59 years and older group (≥60 years old), the donors aged 30–39 years also had a lower absolute number of CD56dimCD16+ NK cells and CD56negCD16+ NK cells. However, correlation analysis showed no correlation between age and absolute number among total NK cells or the NK subsets (Figure 1B–D). Moreover, the NK cell subset distribution among total NK cells exhibited no significant difference among age groups and was not correlated with age (Figure 1E–G). Compared with other age groups, the CD57 expression on NK cells was elevated in the elderly (≥60 years old). Meanwhile, the CD69 expression on NK cells presented a stepwise decline with age, and the decline was most severe in the elderly (≥60 years old) (Figure 1H and I). Correlation analysis showed a correlation of CD57 expression on NK cells with age and a strong correlation of CD69 expression with age (CD57: r = 0.3014, p = 0.0026; CD69: r = −0.6334, p < 0.0001). Due to the small sample size, we divided participants with a wider age span: young (people aged 20–39 years), middle age (people aged 40–59 years) and elderly (people aged 60 years or older). Changes in subset distribution, absolute number and phenotype on NK cells among three age groups were correspondingly shown in Figure S2.
 |
Table 1 Characteristics of Cohort |
Collectively, our findings were in line with previous studies, indicating a positive correlation of CD57 and a negative correlation of CD69 with age, rather than the absolute number and percentage of NK cells.
Co-Inhibitory Molecules (2B4, CTLA-4, TIM-3, BTLA, CD70, CD39, CD160, TIGIT) Associated with T Cell Senescence Were Not Elevated on NK Cells with Age
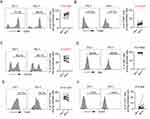
To determine whether co-inhibitory molecules, associated with T cell senescence, are age-related on NK cells, we detected the expression of 2B4, CTLA-4, TIM-3, BTLA, CD70, CD39, CD160, TIGIT on NK cells in donors. Higher frequencies of 2B4 and CTLA-4 were observed on NK cells from the donors aged 50–59 years compared with those young (20–29 and 30–39 years old) or the elderly (≥60 years old; Figure 2A and B). A weak correlation between 2B4 expression on NK cells with age was noted (r = 0.2007, p = 0.0464). Furthermore, the donors aged 50–59 years expressed higher frequencies of TIM-3 on NK cells than the donors aged 20–29 years and ≥60 years (Figure 2C). Additionally, the elderly (≥60 years old) showed higher expression of BTLA on NK cells than donors in young (20–29 and 30–39 years old) and aged 50–59 years, and weak positive correlation was also found in BTLA expression on NK cells with age (Figure 2D). Interestingly, the donors aged 50–59 years exhibited lower frequencies of CD70 than those in young (20–29 and 30–39 years old) and the elderly (≥60 years old; Figure 2E). However, the expression of CD39, CD160 and TIGIT showed no statistical difference between age groups (Figure 2F–H). Changes in co-inhibitory molecules on NK cells among three age groups were shown in Figure S3.
Totally, no significant correlation was observed between these co-inhibitory molecules (CTLA-4, TIM-3, CD70, CD39, CD160, TIGIT) and age (Figure 2A–H).
PD-1-Positive NK Cells Accumulated in Older People
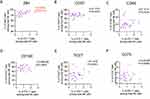
However, the level of PD-1 expression on NK cells was low among donors. The results in our data showed that the frequencies of PD-1+ NK cells from the elderly (≥60 years old) were significantly higher than other groups. Additionally, correlation analysis showed a positive correlation of PD-1 expression on NK cells with age (r = 0.3711, p = 0.0002; Figure 3A and B). This change also occurred in the major subset (CD56dimCD16+ NK cells), making up 90% of total NK cells (Figure 3C), whereas the PD-1 expression of other two subsets (CD56brightCD16− NK cells and CD56negCD16+ NK cells) was not up-regulated with age (Figures 3D and S4A). The expression of PD-1 on NK cells among three age groups was shown in the Figure S4B–E. Then, to determine whether gender had an impact on PD-1 expression among NK cells in aging, we compared PD-1 expression by sex group. There was no significant difference in frequencies of PD-1+ NK cells between males and females, regardless of the total or each age group of the donors (Figure S4F). Thus, the increased expression of PD-1 may be one of the characteristics of NK cell surface receptors in older people, independent of gender.
Aged PD-1+ NK Cells Displayed a Phenotype of Over-Activation and Aging
To explore the characteristics of PD-1+ NK cells, we compared the frequencies of other receptors on the PD-1+ NK cells and PD-1− NK cells among the elderly, including the maturation marker (CD57), activation marker (CD69), T cell exhaustion markers (CD160, 2B4, TIGIT, and CD70). The expression of CD57 and CD69 on PD-1+ NK cells was higher than on PD-1− NK cells (Figure 4A–B), indicating that PD-1 positive NK cells were more aging or activated. However, the expression of CD160 was lower on PD-1+ NK cells than on PD-1− NK cells (Figure 4C), and PD-1+ NK cells and PD-1− NK cells showed the same expression levels of other co-inhibitory receptors (2B4, TIGIT and CD70) (Figure 4D–F). Collectively, the results indicated that PD-1 expression on NK cells was associated with the phenotypic profile of over-activation and aging.
PD-1 Expression Was Associated with 2B4 Expression on NK Cells
Next, we determined the relationship between the expression of PD-1 and other receptors on NK cells in the elderly. PD-1 expression had a positive correlation with 2B4 expression (r = 0.4785, p = 0.0134) (Figure 5A). However, no significant correlation was observed between the expression of PD-1 and other receptors (CD57, CD69, CD160, TIGIT, CD70) (Figure 5B–F). Conclusively, the data indicated that PD-1 expression was associated with 2B4 expression, not with other receptors (CD57, CD69, CD160, TIGIT, CD70).
Discussion
Consistent with Kutza J’s16 and Le Garff-Tavernier M’s17 studies, the absolute numbers and subset distribution of NK cells were not associated with age in our study. In addition, Gayoso I’s21 and Solana R’s22 data supported our findings that the CD57 expression on NK cells elevated in the elderly and the CD69 expression on NK cells gradually declined with age. The aging indicators of NK cells in our cohort were consistent with other cohorts in the above study. Next, expression patterns of co-inhibitory molecules, associated with T cell aging, were studied on NK cells in aging. PD-1 was upregulated on NK cells in the elderly, suggesting that PD-1 is a potential biomarker of aging.
Few studies have focused on PD-1 among NK cells from donors, since the level of PD-1 on NK cells harvested from donors was extremely low. However, a recent study revealed the presence of a pool of PD-1 proteins and mRNA in the cytoplasm of donor NK cells, despite the absent/low PD-1 surface expression. Even resting NK cells were capable of a prompt externalization of PD-1.29 It provided a basis for our study that PD-1 could be expressed on NK cells in donors.
Under the tumor burden model, PD-1 was expressed on NK cells from peripheral blood.30–34 Vari indicated patients with Hodgkin lymphoma (cHL) or diffuse large B-cell lymphoma (DLBCL) presented a higher PD-1 expression on NK cells than donors.33 Beldi-Ferchiou A et al, showed PD-1+ NK cells were functionally hyporeactive in patients with Kaposi sarcoma. Following stimulation, PD-1+ NK cells responded much less to the degranulation and IFN-γ production than their PD-1− counterparts.34 Those evidence supported that in the case of tumors, expression of PD-1 induced functional exhaustion of NK cells.
There was also a significantly increased fraction of PD-1+ cells in infective diseases. In HIV, PD-1 expression was increased on NK cells from HIV-1-seropositive individuals, compared to seronegative controls.35 Moreover, patients in the low-CD4 group had higher expression of PD-1 on NK cells than patients in the high-CD4 group.36 In COVID-19, researchers evaluated that the expression level of PD-1 on NK cells is significantly elevated in patients with COVID-19 compared to volunteers.37,38 Mycobacterium tuberculosis stimulation significantly up-regulated PD-1 levels on NK cells. Furthermore, PD-1+ NK cells displayed a diminished IFN-γ mean fluorescence intensity, denoting the relevance of PD-1 on IFN-γ regulation.39 Thus, in the setting of infection, PD-1 was upregulated on NK cells and had a higher expression in severe infection.
Researches regarding PD-1 in aging mainly focused on T cells. In murine models, CD44HiCD8 T (memory CD8 T cells in mice) cells from aged mice had higher expression of PD-1 compared with CD8 T cells from young mice.6,40 Decman V compared IFN-g production with PD-1 expression. Generally, the PD1+ CD8 T cells from aged mice made less IFN-γ per cell compared with the PD-1− cells.6 Consistently, higher expression of PD-1 on CD4 T cells was presented in aged mice than in young mice.5,40 In Shimada Y’s study, PD-1+ CD4 T cells from old mice exhibited proliferative hyperactivation.41 In human studies, myeloma patients showed a higher percentage of co-expressed CD57 and PD-1 on CD8 T cells from the BM than donors. This enhanced co-expression of CD57 and PD-1 in myeloma suggested a distinct population of late-differentiated, senescent T cells.42 Totally, these findings indicated a pivotal role of PD-1 in T-cell aging. In NK cell research, Pesce S indicated that PD-1+ NK cells were confined to the terminally differentiated NK cells (CD57+ NK), with lower expression of NKp30 and NKp46 than PD-1− NK.43
There were some limitations in our work. Firstly, our study had a relatively small sample size. Secondly, there was no CMV test which could affect the phenotype of NK cells. Finally, we have not done a functional assay.
In summary, we found an increased frequency of PD-1+ NK cells in the elderly. PD-1+ NK cells have the phenotypic characteristics of aging NK cells. Our studies provided new insights into the co-inhibitory molecules on NK cells with aging, highlighting PD-1 may serve as a new potential marker of aging on NK cells.
Data Sharing Statement
All data are provided in this study, and raw data can be requested by the corresponding author.
Ethical Standards
This study was approved by the Committee of Ethics at Beijing Ditan Hospital, Capital Medical University, Beijing, China (No. 2021-22-01). All the human blood samples were collected with informed consent. The study complied with the Declaration of Helsinki.
Consent for Publication
Clinical and Research Center of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University approved the publication of data generated from this study.
Acknowledgments
We acknowledged Jialu Li, Xinyue Wang, Zhijiao He and Meng Zhao for helping to collect the data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (82371565), Beijing Natural Science Foundation (M21007), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20191802) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX202126).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chou JP, Effros RB. T cell replicative senescence in human aging.. Curr Pharm Des. 2013;19(9):1680–1698. doi:10.2174/138161213805219711
2. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi:10.1038/ni1033
3. Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi:10.1038/nri3547
4. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi:10.1111/j.1749-6632.2000.tb06651.x
5. Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130(10):709–712. doi:10.1016/j.mad.2009.08.006
6. Decman V, Laidlaw BJ, Doering TA, et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188(4):1933–1941. doi:10.4049/jimmunol.1101098
7. Lee KA, Shin KS, Kim GY, et al. Characterization of age-associated exhausted CD8+ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell. 2016;15(2):291–300. doi:10.1111/acel.12435
8. Wang X, Wang D, Du J, et al. High levels of CD244 rather than CD160 associate with CD8+ T-cell aging. Front Immunol. 2022;13:853522. doi:10.3389/fimmu.2022.853522
9. Song Y, Wang B, Song R, et al. T-cell immunoglobulin and ITIM domain contributes to CD8+ T-cell immunosenescence. Aging Cell. 2018;17(2):e12716. doi:10.1111/acel.12716
10. Wang D, Du J, Song Y, et al. CD70 contributes to age-associated T cell defects and overwhelming inflammatory responses. Aging. 2020;12(12):12032–12050. doi:10.18632/aging.103368
11. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi:10.1038/ni1582
12. Almeida-Oliveira A, Smith-Carvalho M, Porto LC, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72(4):319–329. doi:10.1016/j.humimm.2011.01.009
13. Sanchez-Correa B, Gayoso I, Bergua JM, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90(1):109–115. doi:10.1038/icb.2011.15
14. Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18(16):1613–1620. doi:10.1016/s0264-410x(99)00495-8
15. Gounder SS, Abdullah BJJ, Radzuanb NEIBM, et al. Effect of aging on NK cell population and their proliferation at ex vivo culture condition. Anal Cell Pathol. 2018;2018:7871814. doi:10.1155/2018/7871814
16. Kutza J, Murasko DM. Age-associated decline in IL-2 and IL-12 induction of LAK cell activity of human PBMC samples. Mech Ageing Dev. 1996;90(3):209–222. doi:10.1016/0047-6374(96)01772-1
17. Le Garff-Tavernier M, Béziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9(4):527–535. doi:10.1111/j.1474-9726.2010.00584.x
18. Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Hum Immunol. 2010;71(7):676–681. doi:10.1016/j.humimm.2010.03.014
19. Müller-Durovic B, Lanna A, Covre LP, Mills RS, Henson SM, Akbar AN. Killer cell lectin-like receptor G1 inhibits NK cell function through activation of adenosine 5’-monophosphate-activated protein kinase. J Immunol. 2016;197(7):2891–2899. doi:10.4049/jimmunol.1600590
20. Krishnaraj R, Svanborg A. Preferential accumulation of mature NK cells during human immunosenescence. J Cell Biochem. 1992;50(4):386–391. doi:10.1002/jcb.240500407
21. Gayoso I, Sanchez-Correa B, Campos C, et al. Immunosenescence of human natural killer cells. J Innate Immun. 2011;3(4):337–343. doi:10.1159/000328005
22. Solana R, Campos C, Pera A, Tarazona R. Shaping of NK cell subsets by aging. Curr Opin Immunol. 2014;29:56–61. doi:10.1016/j.coi.2014.04.002
23. Krishnaraj R, Bhooma T. Cytokine sensitivity of human NK cells during immunosenescence. 2. IL2-induced interferon gamma secretion. Immunol Lett. 1996;50(1–2):59–63. doi:10.1016/0165-2478(96)02519-9
24. Krishnaraj R. Senescence and cytokines modulate the NK cell expression. Mech Ageing Dev. 1997;96(1–3):89–101. doi:10.1016/s0047-6374(97)00045-6
25. Mariani E, Mariani AR, Meneghetti A, Tarozzi A, Cocco L, Facchini A. Age-dependent decreases of NK cell phosphoinositide turnover during spontaneous but not Fc-mediated cytolytic activity. Int Immunol. 1998;10(7):981–989. doi:10.1093/intimm/10.7.981
26. Mariani E, Pulsatelli L, Meneghetti A, et al. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech Ageing Dev. 2001;122(13):1383–1395. doi:10.1016/s0047-6374(01)00270-6
27. Mariani E, Pulsatelli L, Neri S, et al. RANTES and MIP-1alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002;37(2–3):219–226. doi:10.1016/s0531-5565(01)00187-5
28. Mariani E, Meneghetti A, Neri S, et al. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002;32(6):1524–1529. doi:10.1002/1521-4141(200206)32:6<1524::AID-IMMU1524>3.0.CO;2-E
29. Mariotti FR, Petrini S, Ingegnere T, et al. PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology. 2018;8(3):1557030. PMID: 30723590; PMCID: PMC6350684. doi:10.1080/2162402X.2018.1557030
30. Benson DM, Bakan CE, Mishra A, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi:10.1182/blood-2010-02-271874
31. Wiesmayr S, Webber SA, Macedo C, et al. Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur J Immunol. 2012;42(2):541–550. doi:10.1002/eji.201141832
32. MacFarlane AW, Jillab M, Plimack ER, et al. PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res. 2014;2(4):320–331. doi:10.1158/2326-6066.CIR-13-0133
33. Vari F, Arpon D, Keane C, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. 2018;131(16):1809–1819. PMID: 29449276; PMCID: PMC5922274. doi:10.1182/blood-2017-07-796342
34. Beldi-Ferchiou A, Lambert M, Dogniaux S, et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget. 2016;7(45):72961–72977. PMID: 27662664; PMCID: PMC5341956. doi:10.18632/oncotarget.12150
35. Norris S, Coleman A, Kuri-Cervantes L, Bower M, Nelson M, Goodier MR. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral Immunol. 2012;25(4):329–332. PMID: 22742708. doi:10.1089/vim.2011.0096
36. Sun S, Kong W, Cui X, et al. The abnormal distribution of NK cell subsets before HAART treatment may be related to the level of immune reconstitution in HIV patient. Int Immunopharmacol. 2021;96:107784. PMID: 34162148. doi:10.1016/j.intimp.2021.107784
37. Li M, Guo W, Dong Y, et al. Elevated exhaustion levels of NK and CD8+ T cells as indicators for progression and prognosis of COVID-19 disease. Front Immunol. 2020;11:580237. PMID: 33154753; PMCID: PMC7591707. doi:10.3389/fimmu.2020.580237
38. Varchetta S, Mele D, Oliviero B, et al. Unique immunological profile in patients with COVID-19. Cell Mol Immunol. 2021;18(3):604–612. PMID: 33060840; PMCID: PMC7557230. doi:10.1038/s41423-020-00557-9
39. Alvarez IB, Pasquinelli V, Jurado JO, et al. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis. 2010;202(4):524–532. doi:10.1086/654932
40. Mogilenko DA, Shpynov O, Andhey PS, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved hallmark of inflammaging. Immunity. 2021;54(1):99–115.e12. PMID: 33271118. doi:10.1016/j.immuni.2020.11.005
41. Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M. Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp Gerontol. 2009;44(8):517–522. PMID: 19457448. doi:10.1016/j.exger.2009.05.003
42. Zelle-Rieser C, Thangavadivel S, Biedermann R, et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol. 2016;9(1):116. PMID: 27809856; PMCID: PMC5093947. doi:10.1186/s13045-016-0345-3
43. Pesce S, Greppi M, Tabellini G, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J Allergy Clin Immunol. 2017;139(1):335–346.e3. PMID: 27372564. doi:10.1016/j.jaci.2016.04.025
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.