Back to Journals » Journal of Pain Research » Volume 16
Incidence and Predictive Factors of New Onset Postoperative Sacroiliac Joint Pain After Posterior Lumbar Fusion Surgery for Degenerative Lumbar Disease
Authors Yang P, Liang X, Xu X, Liu Q, Guo Z, Yuan H, Wang H, Ding W
Received 31 August 2023
Accepted for publication 3 November 2023
Published 14 December 2023 Volume 2023:16 Pages 4291—4299
DOI https://doi.org/10.2147/JPR.S431197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Puxin Yang,* Xiao Liang,* Xingzhu Xu, Qingtao Liu, Zhiyuan Guo, Hongru Yuan, Hui Wang, Wenyuan Ding
Department of Spine Surgery, HeBei Medical University Third Hospital, Shijiazhuang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenyuan Ding; Hui Wang, Department of Spine Surgery, HeBei Medical University Third Hospital, Shijiazhuang, The People’s Republic of China, Tel +86 311 88602317, Fax +86 311 87023626, Email [email protected]; [email protected]
Introduction: To explore the incidence and predictive factors of new onset postoperative sacroiliac joint pain (PSJP) after posterior lumbar fusion surgery for degenerative lumbar disease.
Methods: Three hundred and sixty-seven patient medical records from January 2020 to December 2021 were retrieved. The patients were divided into two groups: PSJP group and N-PSJP (non-postoperative sacroiliac joint pain group). To investigate potential risk factors for PSJP, HU value (Hounsfield unit value) was assessed on CT scans. ImageJ software was used to assess the fat and muscle of the lumbar multifidus muscle (LMM) in the axial MRI image, the red area was marked as fat and the rest were muscles to calculate the ratio of fatty infiltration. Patient characteristics, surgical variables and radiographic parameters were analyzed statistically.
Results: Twenty of 367 patients were diagnosed with PJSP at postoperative follow-up. Patients with PSJP presented with significantly higher HU value. For surgical variables, PSJP patients received more operations including distal fusion level at sacrum than the N-PSJP group. For radiographic parameters, most of the patients in the PSJP group had more severe fatty atrophic muscle in the LMM compared to the N-PSJP group. There was no statistically significant difference between the two groups in preoperative and postoperative lumbar lordosis (LL), angle of lumbar lordosis of fixed lumbar vertebrae (FV-LL), pelvic incidence (PI), sacrum slope (SS). The bivariate logistic regression model revealed preoperative fat infiltration rate of the LMM, and higher HU value were independently associated with PSJP.
Conclusion: PSJP for degenerative lumbar disease was 5.4%, the predictive factors included preoperative severe infiltration of LMM, distal fusion level at sacrum and higher HU value.
Keywords: sacroiliac joint pain, lumbar instrumented fusion surgery, bone mineral density, paraspinal muscles
Introduction
Posterior decompression and instrumented fusion surgery, including posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF), is the recommended routine surgical strategy for lumbar degenerative disease.1–3 Most of the patients gained neurological improvement and significant relief from low back and leg pain postoperatively, meanwhile some postoperative complications could occur and show a negative impact on the long-term efficacy of surgery. New onset postoperative sacroiliac joint pain (PSJP) is not rare.4–7 PSJP is a trigger of failed back syndrome, which may lead to decreased quality of daily life, increased use of analgesics and decreased patient satisfaction. Identifying potential risk factors and taking targeted preventive interventions are essential to reduce the occurrence of PSJP and improve the long-term effect after surgery.
Few studies exist about predictive factors of new-onset postoperative PSJP after posterior lumbar surgery. Kalidindi et al revealed that the cephalad migration of the lumbar apex is significantly associated with new-onset PSJP based on a retrospective case-control study including 354 patients.8 Several reasons including lumbosacral fusion, iliac graft harvesting, adjacent segment disease and fusion hardware-related complication could contribute to the PSJP after posterior lumbar surgery.3,9–11 The number of PSJP cases collected in previous studies was relatively insufficient, the risk factors may make interactions and are not independent factors of the disease. The purpose of this study was to further explore the incidence and predictive factors about new onset PSJP after posterior lumbar fusion surgery. Applying timely intervention toward risk factors could reduce occurrence of PSJP, and improve the postoperative efficacy for lumbar spine patients.
Materials and Methods
Subjects
This study of cases was collected by reviewing and following-up postoperative patients, and was approved by the Institutional Review Board of the Third Hospital of Hebei Medical University before data collection and analysis. The inclusion criteria were: (1) diagnosis of lumbar degenerative disease and surgical treatment of PLIF or TLIF; (2) regular follow-up in outpatient clinic at 3-month postoperative, the sacroiliac joint pain was evaluated at this time; (3) pain in the sacroiliac joint area was not developed before operation; (4) diagnosis of sacroiliac joint pain is based on the following provocation tests, with positive results in three out of five tests: pain caused by sacroiliac ligament compression includes the Patrick test, Gaenslen test, shear test, Yeoman action test, and standing extension test.12
Patients were excluded if they met any of the following: (1) spinal scoliosis or kyphosis as the primary indication for surgery; (2) secondary postoperative infection, pseudarthrosis, inflammation, trauma, tumor; (3) presence of schizophrenia, cognitive dysfunction or other psychotic disorder; (4) surgery under workman’s compensation claim or medical disputes.
By retrieving the clinical records from January 2020 to December 2021, 367 patients met both the inclusion and exclusion criteria were collected and all of them provided informed consent for the use of their data before enrollment. One hundred and eighty-seven females and 180 males, with mean age of 50.9±12.9 years (range from 22 to 82 years). 209 patients underwent PLIF (150 patients had single level in surgical treatment, 53 patients had double level in surgical treatment, and 6 patients had three or more levels in surgical treatment) and 158 patients undertook TLIF (97 patients received single level in surgical treatment, 52 patients received double levels in surgical treatment, and 9 patients received three or more levels in surgical treatment). This study complies with the Declaration of Helsinki.
Clinical and Radiological Evaluation
New postoperative pain in the sacroiliac joint region indicated PSJP. All provocative tests (Patrick test, Gaenslen test, shear test, Yeoman maneuver test, and standing extension test) were performed by two groups of physicians. Each group was guided by a senior surgeon and implemented by a resident surgeon. Two groups of surgeons independently tested the patients. Patients with three or more positive tests and reproducible results were included in the PSJP group. According to the occurrence of PSJP at three-month (98.13±5.17 days) follow-up, patients were divided into two groups: the PSJP group and the N-PSJP group. To investigate potential risk factors for PSJP after surgery, all patients data about general information, surgical variables and radiographic parameters were contrasted and analyzed statistically between the PSJP and N-PSJP groups.
The study collected general information including age, gender, body mass index (BMI), main diagnosis (lumbar disc herniation, lumbar spinal stenosis, spondylolisthesis), comorbidity (diabetes, rheumatism), smoking (yes vs no), drinking (yes vs no), osteoporosis (Hounsfield unit value of L1 vertebrae).
Surgical parameters are being documented in detail, surgical strategy (TLIF vs PLIF), surgical segment, number of fusion levels, fusion to sacrum (yes vs no), incision size, perioperative complications (cerebrospinal fluid leakage, poor wound healing, infection, screw loosening, cage displacement).
Radiographic parameters are included as important reference data, LL, FV-LL, PI, SS pre- and postoperatively (Figures 1 and 2). Lumbar multifidus muscle (LMM) atrophy can be identified in transverse planes of lumbar magnetic resonance imaging (MRI).13 PACS was used to calculate the L1 HU value by placing the region of interest (ROI) in an axial image in L1 (Figure 3), The ROI was traced as close as possible to 2 mm of the cortical bone of the vertebral body. Based on the studies of Parkkola et al14 and Kader et al,15 fat infiltration rate of the LMM was measured by ImageJ software (Figure 4). The LMM region was selected on the MRI image by ImageJ, and the fat in the LMM was marked in red (the threshold was set to 87% in our study), and the ratio of the red area to the unlabeled area was the fat infiltration rate. The above results were calculated by first and second authors independently, and the mean value was used for analysis.
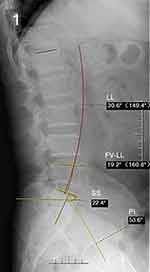 |
Figure 1 X-ray film showing the measuring method of preoperative LL, FV-LL, PI, SS. |
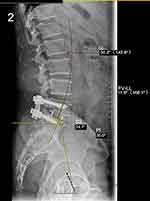 |
Figure 2 X-ray film showing the measuring method of postoperative LL, FV-LL, PI, SS. |
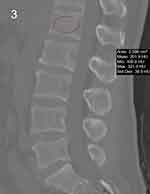 |
Figure 3 L1 vertebrae HU value in ROI area by CT. |
 |
Figure 4 Fatty infiltration of LMM on MRI by ImageJ software. |
Statistical Analysis
Statistical Package for Social Sciences software was used for analyzing all information and parameters of patient (SPSS v. 17, Chicago, IL). Continuous variables were measured as mean ±standard deviation, and categorical variables were expressed as frequency or percentages. The Mann–Whitney U-test and independent t-test was used to analyze the difference of continuous variables between two groups. An chi-squared analysis and Fisher’s exact test were used to examine the differences among categorical variables. Variables with p-values <0.05 in the univariate analyses, as well as a number of variables selected by experts, were entered into a logistic regression model. For each variable, we computed the odds ratio (OR) with its 95%CI.
Results
Twenty of 367 patients (5.4%) showed PJSP with at all postoperative follow-up times. For patient characteristics, no significant difference were detected between the two groups in age at operation, gender, BMI, comorbidity, smoke, drink (p>0.05) (Table 1).
 |
Table 1 The Relationship of Age at Operation, Gender, BMI, Comorbidity, Smoke, Drink Between PSJP Group (N=20) and N-PSJP Group (N=347) |
For surgical variables, distal fusion level at sacrum was statistically difference between the PSJP and N-PSJP groups (p<0.05). In addition, 13 patients showed PSJP performed distal fusion level at sacrum (65%), 7 patients showed PSJP performed distal fusion level above sacrum (35%), there was no difference between the two groups in surgical strategy (TLIF vs PLIF), number of fusion level, and the size of incision compared to the N-PSJP group (p>0.05). There was no significant difference in the number of levels accepted sacrum fusion surgery between the two groups (p>0.05) (Table 2).
 |
Table 2 The Relationship of Surgical Strategy (TLIF vs PLIF), Number of Fusion Level, and the Size of Incision Between PSJP Group (N=20) and N-PSJP Group (N=347) |
For radiographic parameters, patients with PSJP presented significantly higher HU value, most of the patients in PSJP group presented higher fat infiltration rate of the LMM compared to the N-PSJP group. There was no statistically significant difference between two groups in preoperative and postoperative LL, FV-LL, PI, SS (Table 3).
 |
Table 3 The Relationship of Imaging Parameters Between PSJP Group (N=20) and N-PSJP Group (N=347) |
Age, HU value, fat infiltration rate of the LMM, distal fusion level at sacrum were entered into the bivariate logistic regression model. The model revealed fat infiltration rate of the LMM were independently associated with PSJP (p<0.05) (Table 4).
 |
Table 4 Age, HU Value, Fatty Atrophic in LMM, Distal Fusion Level at Sacrum of Between PSJP Group (N=20) and N-PSJP Group (N=347) |
Discussion
The incidence of PSJP following posterior lumbar fusion surgery for degenerative lumbar disease was 5.4%, the predictive factors included distal fusion level at sacrum, higher HU value and preoperative fat infiltration rate of the LMM. These factors could be assessed before surgery, and were not confounded by other variables that potentially affect persistent postoperative sacroiliac joint pain. Meanwhile we found that there was no significant difference in age, body mass index, comorbidity, smoking, drinking, preoperative LL, postoperative LL, FV-LL, PI, SS which failed to present statistical difference between the two groups. New onset PSJP do not show special correlation with surgical strategy, number of fusion level, and the size of incision.
HU Value and Sacroiliac Joint Pain
Our study found that the HU value in the PSJP group was higher than in the N-PSJP group. BMD that was presented by HU value could reduce the influence of scoliosis, degenerative arthritis, osteophyte formation, bone sclerosis to increase measured accuracy.16 Meanwhile, BMD showed a negative correlation with age significantly.16 High HU value is more likely to be detected in younger age groups, this assumption could explain our results of PSJP patients presented higher HU value and younger age concurrently. We further explored the age difference and found that there was no statistical difference in age between the two groups (t=−1.748, p=0.081), which may due to fewer cases being included in the PSJP group.
Younger patients with PSJP would bear heavier labor load after surgery than older people in current social environment. Heavier labor load could cause PSJP by stretching of the ligament and soft tissue attached the sacroiliac joint. Schneider et al believed that injection on sacroiliac joint could stimulate the ligament and cause higher pressure in the joint cavity regarded as potential pain triggers.17 Meanwhile, sacroiliac joint mobility is closely related to ligaments in anatomy. Kiapour18 proposed that the movement of the sacroiliac joint may cause pain of sacroiliac joint region. The sacroiliac joint rotates about two degrees around three axial planes (flexion and extension, rotation and translation), which causes deformation of the posterior sacroiliac ligament. This could support our hypothesis that the ligament may be the trigger of sacroiliac joint pain. We suspected that heavier labor load mostly occurs in young adults with higher HU value (>165.40 HU), and excessive movement of sacroiliac joint caused by heavier labor load could be the trigger for PSJP.
Distal Fusion Level at Sacrum
We found that distal fusion level at sacrum was a risk factor for PSJP in our study. There are three possible explanations for this discovery. First, the surgical exposure and intraoperative use of muscle retractor may damage the paraspinal muscles inevitably, and the postoperative muscle scar could induce or increase the back pain. The lumbosacral joint is the most important region in the vertebral column in terms of weight bearing and mobility, it is widely accepted that mechanical disorders of this region could cause pain.19,20–23 Second, sacral pedicle screw placement is one of the necessary surgical steps for distal fusion level at sacrum. The sacral pedicle screw requires an outward-inclined than other vertebral bodies, this required a larger range of surgical exposure and increased difficulty and time of the procedure. The long incisions, extensive detachment of muscle from the spinal processes and subsequently prolonged wide retraction could cause ischemic necrosis and denervation of the paraspinal muscles.24,25 Third, PLIF and TLIF may increase intervertebral pressure and movement at the adjacent disc levels and cause adjacent segment degeneration,26 we believe distal fusion level at sacrum may increase stress concentration and accelerate adjacent segment to degenerate at sacroiliac joint. These may lead to pain at region of the sacroiliac joint.
Paraspinal Muscle Degeneration
We found preoperative fat infiltration rate of the LMM showed more serious in patients with PSJP (t=2.017, p<0.05). Previous studies found that paraspinal muscle degeneration is associated with lower back pain,27 but there are few studies devoted to the association between PSJP and paraspinal muscles. LMM as the risk factor was included in our study because LMM showed a trend of rapid degeneration in the process of lumbar paraspinal muscle degeneration.28 Generally degenerative grade of paraspinal muscle was evaluated by the high signal performance of paraspinal muscle on T2 MRI.29,30 Our study evaluated LMM based on MRI T2 images and found that PSJP had more severe fatty infiltration. There are several possible explanations for this. The blood supply and nerves of paraspinal muscles suffered irreversible damage in invasive surgery, which manifests as muscle atrophy, fibrosis, and fat deposition.31 In addition, overstretching paraspinal muscles during surgery can also aggravate paraspinal muscles with serious preoperative degeneration to further degenerate. We believed invasive surgery could cause further damage toward paraspinal muscle in patients with PSJP who already had poor paraspinal muscles. Preoperative serious fat infiltration of the LMM should be considered as a high risk factor for PSJP, surgeon should pay more attention to preoperative degenerated paraspinal muscles.
There are several potential limitations in this study. First, the number of patients is relatively small, and the study may be under powered to detect the significance of some risk factors. Second, the study was conducted retrospectively by case selection, and was not randomized or controlled.
Conclusion
Preoperative serious fat infiltration of the LMM, distal fusion level at sacrum and higher HU value are predictive factors of new onset postoperative PSJP. First we suggested that patients with preoperative degenerated paraspinal muscles should strictly consider whether to perform invasive surgery, it aims to reduce further damage to lumbar paraspinal muscles. Second, rehabilitation exercise with standard guidance in patients undergoing invasive lumbar surgery can reduce the incidence of PSJP.
Acknowledgments
We would like to thank Wenyuan Ding for advice on experimental design, Hui Wang for his guidance on the article. We thank the associate editor and the reviewers for their useful feedback that improved this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lequin MB, Verbaan D, Bouma GJ. Posterior lumbar interbody fusion with stand-alone Trabecular Metal cages for repeatedly recurrent lumbar disc herniation and back pain. J Neurosurg Spine. 2014;20(6):617–622. doi:10.3171/2014.2.SPINE13548
2. Cao P, Chen Z, Zheng Y, et al. Comparison of simple discectomy and instrumented posterior lumbar interbody fusion for treatment of lumbar disc herniation combined with Modic endplate changes. Chin Med J. 2014;127(15):2789–2794.
3. Satoh I, Yonenobu K, Hosono N, Ohwada T, Fuji T, Yoshikawa H. Indication of posterior lumbar interbody fusion for lumbar disc herniation. J Spinal Disord Tech. 2006;19(2):104–108. doi:10.1097/01.bsd.0000180991.98751.95
4. Frymoyer JW, Hanley E, Howe J, Kuhlmann D, Matteri R. Disc excision and spine fusion in the management of lumbar disc disease. A minimum ten-year followup. Spine. 1978;3(1):1–6. doi:10.1097/00007632-197803000-00001
5. Ross JS, Robertson JT, Frederickson RC, et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. Neurosurgery. 1996;38(4):855–863. doi:10.1227/00006123-199604000-00053
6. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine. 2016;41(12):999–1005. doi:10.1097/BRS.0000000000001409
7. Liliang PC, Lu K, Liang CL, Tsai YD, Wang KW, Chen HJ. Sacroiliac joint pain after lumbar and lumbosacral fusion: findings using dual sacroiliac joint blocks. Pain Med. 2011;12(4):565–570. doi:10.1111/j.1526-4637.2011.01087
8. Kalidindi KKV, Bansal K, Vishwakarma G, Chhabra HS. New onset sacroiliac joint pain after transforaminal interbody fusion: what are the culprits? Global Spine J. 2021;13(3):677–682. doi:10.1177/21925682211003852
9. Kim KH, Park JY, Chin DK. Fusion criteria for posterior lumbar interbody fusion with intervertebral cages: the significance of traction spur. J Korean Neurosurg Soc. 2009;46(4):328–332. doi:10.3340/jkns.2009.46.4.328
10. Fuji T, Oda T, Kato Y, Fujita S, Tanaka M. Posterior lumbar interbody fusion using titanium cylindrical threaded cages: is optimal interbody fusion possible without other instrumentation? J Orthop Sci. 2003;8(2):142–147. doi:10.1007/s007760300024
11. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623–629. doi:10.1016/j.spinee.2009.04.004
12. Telli H, Telli S, Topal M. The validity and reliability of provocation tests in the diagnosis of sacroiliac joint dysfunction. Pain Physician. 2018;21(4):E367–E376. doi:10.36076/ppj.2018.4.E367
13. Ekin EE, Yıldız HK, Mutlu H. Age and sex-based distribution of lumbar multifidus muscle atrophy and coexistence of disc hernia: an MRI study of 2028 patients. Diagn Interv Radiol. 2016;22(3):273–276. doi:10.5152/dir.2015.15307
14. Parkkola R, Rytökoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18(7):830–836. doi:10.1097/00007632-199306000-00004
15. Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55(2):145–149. doi:10.1053/crad.1999.0340
16. Zou D, Li W, Deng C, Du G, Xu N. The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. Eur Spine J. 2019;28(8):1758–1766. doi:10.1007/s00586-018-5776-9Kelsey
17. Schneider BJ, Rosati R, Zheng P, McCormick ZL. Challenges in diagnosing sacroiliac joint pain: a narrative review. PM R. 2019;11(Suppl 1):S40–S45. doi:10.1002/pmrj.12175
18. Kiapour A, Joukar A, Elgafy H, Erbulut DU, Agarwal AK, Goel VK. Biomechanics of the sacroiliac joint: anatomy, function, biomechanics, sexual dimorphism, and causes of pain. Int J Spine Surg. 2020;14(Suppl 1):3–13. doi:10.14444/6077
19. Papaioannou M, Skapinakis P, Damigos D, et al. The role of catastrophizing in the prediction of postoperative pain. Pain Med. 2009;10(8):1452–1459. doi:10.1111/j.1526-4637.2009.00730.x
20. Abbott AD, Tyni-Lenne R, Hedlund R. Leg pain and psychological variables predict outcome 2–3 years after lumbar fusion surgery. Eur Spine J. 2011;20(10):1626–1634. doi:10.1007/s00586-011-1709-6
21. Evcik D, Yücel A. Lumbar lordosis in acute and chronic low back pain patients. Rheumatol Int. 2003;23(4):163–165. doi:10.1007/s00296-002-0268-x
22. Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex and size. A prospective controlled clinical study. Spine. 1994;19(14):1611–1618. doi:10.1097/00007632-199407001-00010
23. Amonoo-Kuofi HS. Changes in the lumbosacral angle, sacral inclination and the curvature of the lumbar spine during aging. Acta Anat (Basel). 1992;145(4):373–377. doi:10.1159/000147392
24. Nakipoğlu GF, Karagöz A, Ozgirgin N. The biomechanics of the lumbosacral region in acute and chronic low back pain patients. Pain Physician. 2008;11(4):505–511. doi:10.36076/ppj.2008/11/505
25. Sihvonen T, Herno A, Palja¨rvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18(5):575–581. doi:10.1097/00007632-199304000-00009
26. Lee CS, Hwang CJ, Lee SW, et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18(11):1637–1643. doi:10.1007/s00586-009-1060-3
27. Crawford RJ, Volken T, Valentin S, Melloh M, Elliott JM. Rate of lumbar paravertebral muscle fat infiltration versus spinal degeneration in asymptomatic populations: an age-aggregated cross-sectional simulation study. Scoliosis Spinal Disord. 2016;11(1):21. doi:10.1186/s13013-016-0080-0
28. Zhu DC, Lin JH, Xu JJ, et al. An assessment of morphological and pathological changes in paravertebral muscle degeneration using imaging and histological analysis: a cross-sectional study. BMC Musculoskelet Disord. 2021;22(1):854. doi:10.1186/s12891-021-04734-3
29. Kang CH, Shin MJ, Kim SM, Lee SH, Lee CS. MRI of paraspinal muscles in lumbar degenerative kyphosis patients and control patients with chronic low back pain. Clin Radiol. 2007;62(5):479–486. doi:10.1016/j.crad.2006.12.002
30. Jin YM, Chen Q, Chen CY, et al. Clinical research and technique note of TLIF by Wiltse Approach for the treatment of degenerative lumbar. Orthop Surg. 2021;13(5):1628–1638. doi:10.1111/os.13055
31. Yoshihara K, Shirai Y, Nakayama Y, Uesaka S. Histochemical changes in the multifi dus muscle in patients with lumbar intervertebral disc herniation. Spine. 2001;26(6):622–626. doi:10.1097/00007632-200103150-00012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
