Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Impaired Sensitivity to Thyroid Hormone and Risk of Carotid Plaque Development in a Chinese Health Check-Up Population: A Large Sample Cross-Sectional Study
Authors Wang J, Yang L, Liu W, Wei C, Shen J
Received 8 December 2023
Accepted for publication 23 February 2024
Published 29 February 2024 Volume 2024:17 Pages 1013—1024
DOI https://doi.org/10.2147/DMSO.S454023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Jiangling Wang,1,2,* Lijuan Yang,3,* Wei Liu,1 Chaogang Wei,1 Junkang Shen1
1Department of Radiology, The Second Affiliated Hospital of Soochow University, Soochow, Jiangsu, People’s Republic of China; 2Department of Physical Diagnosis, Huadong Sanatorium, Wuxi, Jiangsu, People’s Republic of China; 3Department of Geriatric, Huadong Sanatorium, Wuxi, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junkang Shen; Chaogang Wei, Department of radiology, The Second Affiliated Hospital of Soochow University, No. 1055 Sanxiang Road, Soochow, 215004, People’s Republic of China, Email [email protected]; [email protected]
Objective: Previous research on the correlation between thyroid function and carotid plaque has revealed conflicting results, possibly attributable to the sensitivity of thyroid hormone indices. In this study, we aimed to analyze the association between thyroid hormone sensitivity indices and the risk of carotid plaque development in a Chinese health check-up population.
Methods: A total of 19,388 health check-up subjects were included in this study (mean age: 50.78± 10.17 years). Central sensitivity to thyroid hormone was evaluated using the thyroid feedback quantile-based index (TFQI), the Chinese-referenced parametric TFQI (PTFQI), the TSH index (TSHI), and the thyrotropin thyroxine resistance index (TT4RI), while peripheral sensitivity to thyroid hormone was assessed by free triiodothyronine/free thyroxine (FT3/FT4) ratio. Multivariable logistic regression analyses were performed to detect the association between thyroid hormone sensitivity indices and carotid plaque risk, and subgroup analysis was also conducted to explore this association stratified by sex, age, obesity, and the status of smoking, drinking, diabetes, hypertension and dyslipidemia.
Results: Among the 19,388 participants, 3753 (19.4%) had carotid plaque. In multivariable adjustment models, the risk of carotid plaque was positively associated with TSHI (odds ratio [OR]: 1.23; 95% confidence interval [CI]: 1.18~1.28), TT4RI (OR: 1.28; 95% CI: 1.23~1.33), TFQI (OR: 1.06; 95% CI: 1.02~1.10), and PTFQI (OR: 1.11; 95% CI: 1.07~1.16), respectively. Conversely, the risk of carotid plaque was negatively correlated with FT3/FT4 (OR: 0.94; 95% CI: 0.90~0.98). In stratified analyses, all thyroid hormone sensitivity indices significantly increased the risk of carotid plaque especially in females, subjects< 65 years, non-obese individuals, and those without current smoking, drinking, diabetes, hypertension and dyslipidemia.
Conclusion: In Chinese health check-up populations, a considerable connection between reduced sensitivity to thyroid hormones and carotid plaque has been observed, especially in females, those younger than 65 years, non-obese individuals, and those without any current smoking, drinking, diabetes, hypertension, or dyslipidemia.
Keywords: carotid plaque, thyroid hormone sensitivity, euthyroid, resistance to thyroid hormone, Chinese population
Introduction
Cardiovascular disease is a major global health concern, significantly impacting a person’s quality of life and life expectancy.1,2 It has been demonstrated that carotid plaque burden is a reliable indicator of cardiovascular or cerebrovascular disease, and is particularly relevant in patients with overt thyroid diseases compared to the general population.3–5 Research has shown that thyroid hormones have a significant effect on the cardiovascular system, with studies indicating that abnormalities in thyroid function can influence carotid intima-media thickness (CIMT) in those with overt thyroid dysfunctions. According to international standards, particular treatments should be prescribed for overt thyroid disorders, as chronic exposure to clinically relevant abnormal concentrations of thyroid hormone could aggravate some cardiovascular risk factors.6,7
Individuals with subclinical hypothyroidism, characterized by normal thyroid hormones and increased thyroid-stimulating hormone (TSH), are usually free of symptoms.8 Nevertheless, hypothyroidism has been linked to cardiovascular disease,9 and overt hypothyroidism can cause dyslipidemia and atherosclerosis.10,11 Mildly impaired thyroid activity has been associated with dyslipidemia, enhanced low-grade inflammation, and impaired endothelial function.12–14 In a double-blind, controlled trial, it was found that individuals with overt hypothyroidism had larger carotid intima-media thickness (CIMT) compared to those with normal thyroid function, which decreased after levothyroxine treatment.15 Dullaart et al conducted a cross-sectional study of euthyroid subjects, discovering that CIMT was linked to free thyroxine (fT4) levels, but not to thyroid-stimulating hormone (TSH) or thyroid antibodies. This suggests that even within the normal ranges, low thyroid function may be detrimental to cardiovascular health.16 However, Delitala et al conducted a population-based survey and did not find any association between thyroid hormones and the presence of carotid artery plaque.17
Previous analyses have mainly focused on TSH, FT3, and FT4 levels to assess the risk of carotid plaque. Indices of thyroid hormone sensitivity, which take into account FT3, FT4, and TSH, can also be used to evaluate the complex interactions between these hormones and serve as a new reference marker for thyroid function. Thyroid hormones sensitivity is a recently proposed functional entity that comprises both FT4 and TSH levels. Both high FT4 and high TSH are present in the resistance to thyroid hormones syndrome, indicating energy balance problems.17
The main feature of this condition is an increase in FT4 and FT3, along with a normal or slightly elevated thyrotropin level.18 Subsequent research has proposed indices to measure reduced sensitivity to central thyroid hormones, such as the thyroid feedback quantile-based index [TFQI], parametric thyroid feedback quantile-based index (PTFQI), thyrotrophic thyroxine resistance index (TT4RI) and thyroid-stimulating hormone index (TSHI).18–20 Several studies confirm that decreased thyroid hormone sensitivity is associated with hyperglycemia, cardiovascular disease and metabolic syndrome.21–23 However, no studies have yet explored the relationship between thyroid hormone sensitivity indices and carotid plaque in Chinese health check-up participants, regardless of their thyroid function status.
In the present study, we aimed to investigate the relationship between central and peripheral thyroid hormones sensitivity and carotid plaque risk in a relatively large general population.
Materials and Methods
Study Population and Design
This cross-sectional study retrospectively recruited 67,801 participants who underwent the annual health check-up in health examination center of Huadong sanatorium from March to December 2021. A total of 20,112 participants who had undergone carotid ultrasonography and thyroid function measurements were originally included in this study, participants were excluded if they meet the following criteria: 1) age less than 18 years; 2) oncology, severe infection, Severe liver and kidney dysfunction, or a history of thyroid surgery; 3) treated with drugs directly or potentially altering thyroid hormone concentrations; Therefore, a total of 19,388 participants aged 18–91 years (mean age: 50.78±10.17 years) were finally enrolled in current analyses. A flowchart of the participants recruitment process is shown in Figure 1.
 |
Figure 1 Flowchart of the study population. |
This research was conducted with the principles of the Declaration of Helsinki in mind and was approved by the Ethics and Research Committee of the Huadong Sanatorium Health Examination Center. Private details were anonymized to secure confidentiality; analyses were conducted with strict security and the data was only used for scientific purposes. As such, the need for informed consent was waived.
Data Collection
Our personnel used a standard questionnaire to acquire demographic characteristics such as age and sex, in addition to lifestyle elements like current smoking and drinking. Furthermore, they collected health-related information, including whether the person had a previous diagnosis of hypertension, diabetes or thyroid dysfunction, and if they were taking any medications. Diabetes was defined as FBG levels≥7.0 mmol/L, currently treated with insulin or oral hypoglycemic agents, or a self-reported history.24 Hypertension was defined as systolic blood pressure (SBP)≥140 mmHg or diastolic blood pressure (DBP)≥90mmHg, currently taking antihypertensive agents.25 Dyslipidemia was defined as TC≥5.2 mmol/L or LDL-C≥3.4 mmol/L or HDL-C<1.0 mmol/L or TG≥1.7 mmol/L as previously described.26 Physical examination included measurements of height, weight and blood pressure. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as a BMI≥28.0 kg/m2 for the Chinese population.27 Systolic blood pressure and diastolic blood pressure were measured on the right arm using a sphygmomanometer after at least 5 min of rest and were calculated as the average of two measurements. For the Chinese population, obesity was characterized as a BMI of 28.0 kg/m2 or higher. After resting for at least 5 minutes, systolic and diastolic blood pressure were taken on the right arm with a sphygmomanometer, and the average of the two measurements was calculated.
Fasting venous blood samples were obtained from all participants vein after 12 hours of overnight fasting. Fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), neutrophil (NE), and lymphocyte (LY) counts levels were measured using an automatic hematology analyzer. Standard laboratory procedure for quality control were strictly followed.
An automated immunochemiluminescent assay kit from Abbott Diagnostics (Abbott Park, IL, USA) was used to measure the concentrations of TSH, FT3, and FT4 with an Abbott Architect i2000. The reference ranges of FT3, FT4, and TSH were 3.10~6.80 pmol/L, 12.00~22.00 pmol/L and 0.27~4.20mIU/L, respectively.
Indices of Thyroid Hormone Sensitivity
Thyroid Feedback Quartile-Based index (TFQI), parametric thyroid feedback quantile-based index (PTFQI), TSH index (TSHI), and Thyrotroph T4 Resistance Index (TT4RI) were allocated to evaluate the participants’ central sensitivity to thyroid hormones. FT3 to FT4 ratio (FT3/FT4) was used to assess peripheral thyroid sensitivity. For TFQI, PTFQI, TSHI, and TT4RI, the higher the values, the lower the central sensitivity to thyroid hormones. For FT3/FT4, higher values indicate higher peripheral sensitivity to thyroid hormones. The equations used for calculation are as follows:
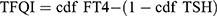 ; cdf: cumulative distribution function.28
; cdf: cumulative distribution function.28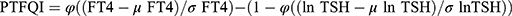 , where μfT4=15.60, σfT4=2.17, μln TSH=0.69, and σln TSH=0.62 for the Chinese population.28
, where μfT4=15.60, σfT4=2.17, μln TSH=0.69, and σln TSH=0.62 for the Chinese population.28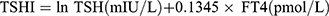 .20
.20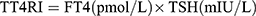 .19
.19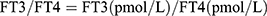 .
.
Carotid Artery Ultrasonography
Using a LOGIQ E9 ultrasound diagnostic system (GE, USA) with a 10-MHz linear transducer, certified physicians performed carotid artery ultrasounds in the supine position. The carotid arteries were scanned in multiple directions with B-mode imaging, and the CIMT was evaluated at three points on the far wall of the middle and distal carotid arteries and 1–1.5 cm proximal to the dilatation of the carotid bulb. The mean CIMT value of six measurements from both the right and left carotid arteries were used for analysis. Quality control procedures were implemented for image acquisition and analysis, and inter-laboratory quality evaluations were conducted by certified personnel. Carotid plaque was defined as a focal wall thickness of a common carotid artery of ≥1.5 mm or a focal thickening structure of the surrounding wall of >50%.29
Statistical Analyses
All analyses were performed using SPSS 26.0 (Chicago, IL, USA) and GraphPad Prism 9.0 (Inc, CA, USA). Normality of the variables was assessed using the Kolmogorov–Smirnov test. Normally distributed variables are expressed as the mean (standard deviation), skewed variables are expressed as the median [interquartile range], and categorical variables are expressed as frequencies (proportions). Continuous variables were compared using Student’s t-test or the Mann–Whitney U-test. Categorical variables were compared using the chi-square test. P for trend was calculated to present the linear trend of the carotid plaque incidence with the increasing quartiles of thyroid hormones sensitivity indices. Multivariable logistic regression analysis was performed to evaluate the associations of carotid plaque risk with per SD increase or quartiles of thyroid hormones sensitivity indices using three models adjusting for possible confounding factors. Model 1 was adjusted for sex, age and BMI.; Model 2 was adjusted for variables in model 1 plus smoking and drinking; Model 3 was adjusted for variables in model 2 plus dyslipidemia, diabetes, hypertension and NLR. Subgroup analysis, adjusted for sex, age, WC, BMI, smoking, drinking, dyslipidemia, diabetes and NLR, was used to examine the correlation between thyroid hormones sensitivity indices and risk of carotid plaque among sex (males/females), age (≥65 years/<65 years), obesity/non-obesity, smokers/non-smokers, drinkers/non-drinkers, diabetes/non-diabetes, hypertension/non-hypertension, dyslipidemia/non-dyslipidemia. P < 0.05 (2-tailed) was considered statistically significant.
Results
Baseline Characteristics
Baseline characteristics of all participants are presented in Table 1. A total of 19,388 participants were analyzed, with 11,438 males (59.0%) and 7950 females (41.0%). 3753 of those had carotid plaque (19.4%). Compared to those without carotid plaque, those with it were more likely to be male, older, smokers, drinkers, and had higher rates of hypertension, diabetes, and dyslipidemia (P-value < 0.001). Additionally, these participants had higher levels of TFQI, PTFQI, TSHI, TT4RI, and NLR, while FT3/FT4 levels were lower (P-value < 0.001). Furthermore, comparisons of carotid plaque incidence among different quartiles of thyroid hormones sensitivity indices stratified by sex and age were also described in Figure 2. In males, subjects less than 65 years tended to have a higher carotid plaque incidence with the increasing quartiles of TFQI, TSHI and TT4RI, and decreasing quartile of FT3/FT4 (all P for trend<0.05); In females, subjects less than 65 years tended to have a higher carotid plaque incidence with the increasing quartiles of TFQI, PTFQI, TSHI and TT4RI, and decreasing quartile of FT3/FT4 (all P for trend<0.001). Besides, increasing trends of carotid plaque incidence were not statistically significant among quartiles of almost all thyroid hormones sensitivity indices in participants over 65 years old (P for trend>0.05).
 |
Table 1 Comparison of Baseline Characteristics Between Participants with and without Carotid Plaque |
 |
Figure 2 Carotid plaque incidence based on quartiles of thyroid hormones sensitivity indexes by gender and age. *P for trend<0.05; **P for trend<0.01; ***P for trend<0.001; ns, no significance. |
Association Between Thyroid Hormone Sensitivity Indices and Carotid Plaque Risk
After adjusting for potential confounding factors, with regard to continuous variables in multivariate logistic regression analysis, TSHI, TT4RI, TFQI, and PTFQI levels were positively associated with carotid plaque risk, and FT3/FT4 level was negatively associated with carotid plaque risk. With per SD increase of TSHI, TT4RI, TFQI, and PTFQI, the ORs of carotid plaque were 1.23 (95% CI:1.18~1.28), 1.28 (95% CI: 1.23~1.33), 1.06 (95% CI:1.02~1.10), and 1.11 (95% CI:1.07~1.16), respectively. FT3/FT4 (per SD increase) was also correlated with the risk of carotid plaque (OR=0.94, 95% CI:0.90~0.98). All associations were significant (all P < 0.01) (Table 2). Multivariate logistic regression analysis also showed that when Q1 was used as a reference, the fourth quartile (Q4) of TFQI, PTFQI, TSHI, TT4RI, and FT3/FT4 had the highest OR for carotid plaque compared with Q2 and Q3 (all P < 0.01).
 |
Table 2 Association Between Thyroid Hormone Sensitivity Indices and the Risk of Carotid Plaque |
Subgroup Analysis for Association Between Thyroid Hormone Sensitivity Indices and Carotid Plaque Risk
As shown in Figure 3, after multivariable adjustment for confounders, for every 1 SD increase of TSHI, TT4RI, FT3/FT4, TFQI and PTFQI, the ORs (95% CI) for carotid plaque risk were 1.42(1.31~1.53), 1.44(1.35~1.53) 0.85(0.78~0.93),1.07(1.00~1.15) and 1.22(1.14~1.32) in females, 1.25(1.20~1.31), 1.31(1.25~1.36), 0.94(0.90~0.99), 1.06(1.02~1.11) and 1.12(1.08~1.17) among participants aged less than 65 years, 1.25(1.20~1.31), 1.33(1.28~1.39), 0.93(0.89~0.98), 1.06(1.02~1.11) and 1.12(1.07~1.17) among participants without obesity, 1.31(1.24~1.38), 1.35(1.29~1.41), 0.95(0.90~0.99), 1.10(1.04~1.16) and 1.15(1.10~1.21) among non-smokers, 1.23(1.18~1.30), 1.28(1.22~1.22), 0.94(0.90~0.99), 1.06(1.01~1.11) and 1.11(1.06~1.16) among non-drinkers, 1.25(1.20~1.31), 1.30(1.25~1.35), 0.94(0.90~0.98), 1.07(1.02~1.11) and 1.13(1.08~1.18) among non-diabetic subjects, 1.28(1.22~1.34), 1.32(1.27~1.38), 0.92(0.88~0.97), 1.08(1.03~1.13) and 1.15(1.11~1.21) among non-hypertensive subjects, and 1.24(1.18~1.32), 1.34(1.27~1.42), 0.92(0.87~0.98), 1.05(1.01~1.11) and 1.12(1.06~1.18) among subjects without dyslipidemia (all P-value<0.001), respectively. Moreover, almost central sensitivity to thyroid hormone indices had a positive association with carotid plaque risk in all subgroups except those with diabetes or hypertension, however, no significant associations were observed between FT3/FT4 and carotid plaque risk especially in males, subjects>65 years, obese individuals, and those with current drinking, diabetes, hypertension and dyslipidemia.
Discussion
To the best of our knowledge, this is the first study to assess and confirm the association between impaired sensitivity to thyroid hormones indices and carotid plaque risk in a large sample of Chinese health check-up populations. In this cross-sectional study, we found that decreased central thyroid hormone sensitivity (increased TFQI, PTFQI, TSHI and TT4RI) were associated with a high risk of carotid plaque, Moreover, reduced peripheral sensitivity, indicated by a decreased FT3/FT4 ratio, was associated with an increased risk of carotid plaque. With gradual increase in TFQI, PTFQI, TSHI and TT4RI, and decrease in FT3/FT4 ratio, the OR value of carotid plaque also gradually increased. Besides, subgroup analysis also proved that all thyroid hormone sensitivity indices significantly increased the risk of carotid plaque especially in females, subjects<65 years, non-obese individuals, and those without current smoking, drinking, diabetes, hypertension and dyslipidemia. This study provides a new insight into the analysis of thyroid hormone sensitivity indices and their connection to carotid plaque risk, surpassing the absolute circulating values of TSH, FT3 and FT4. It establishes a relationship between thyroid hormone resistance and carotid artery abnormalities, thus contributing to the existing knowledge.
Previous research has suggested a link between thyroid hormone levels and the formation of carotid plaque.30,31 A cross-sectional study found that TSH was independently associated with carotid plaque, particularly in individuals with increased TSH levels.32 A prospective cohort study in a Chinese population revealed that higher mean levels and higher changes in FT3 and FT4 were associated with a greater risk of carotid atherosclerosis in middle-aged and older euthyroid participants.5 More recently, even small alterations in thyroid function within the normal range were associated with atherosclerosis in both the general population and patients with angina pectoris.33 However, the inconsistent findings from previous studies suggest that TSH or thyroid hormone levels may not be enough to explain the relationship between the thyroid system and carotid plaque. In 2019, Laclaustra et al proposed two new resistance indices of central thyroid hormones,28 TFQI and PTFQI, to address the inconsistencies in previously proposed central thyroid hormone sensitivity indices (TSHI, TT4RI) and peripheral thyroid hormone sensitivity indices FT3/FT4. TFQI is based on the empirical joint distribution of FT4 and TSH and has the advantage of not producing extreme values in cases of thyroid dysfunction. PTFQI can be adapted to apply to new values or different populations, with the same range and interpretation as an approximation. This enables the index to have smaller discrepancies, and consequently, give a more detailed explanation of the association between changes in thyroid hormones and carotid plaque than a single index.
This study calculated the association between central and peripheral thyroid hormone sensitivity indices and carotid plaque risk, which was not done in previous researches. Our results showed that higher TFQI, PTFQI, TSHI, and TT4RI were correlated with increased carotid plaque risk, whereas FT3/FT4 had a significantly negative association with carotid plaque risk. Similarly, A recent cross-sectional study examined the relationship between thyroid hormone sensitivity and the risk of carotid plaque in patients with coronary heart disease (CHD). The study found a significant association between thyroid hormone sensitivity and carotid plaque in CHD patients, particularly among smokers and drinkers.34 This finding differed from the results of our study, which did not show the same level of significance in these subgroups. The presence of dyslipidemia in most CHD patients, combined with smoking or drinking, may have contributed to the stronger association observed in the study. These factors were not as prevalent in the health check-up population included in our study. Additionally, other studies have revealed conflicting opinions. Dörr et al conducted a cross-sectional analysis that revealed a correlation between subclinical hyperthyroidism and carotid plaque (OR= 1.67,95% CI:1.11–2.51), this suggests that individuals with lower TSH levels should be monitored for atherosclerotic risk factors and treated promptly.35 Additionally, a recent study on euthyroid type 2 diabetes participants demonstrated that low-normal FT3 levels significantly increased the risk of atherosclerosis.36 Besides, Delitala et al determined that TSH and free thyroxine (FT4) were not significantly linked to carotid plaque.17 The discrepancies in the methodologies used in various investigations might explain the contrasting findings.
In addition, our study also conducted the subgroup analysis stratified by sex, age, obesity, and the status of smoking, drinking, diabetes, hypertension and dyslipidemia, respectively. All thyroid hormone sensitivity indices significantly increased the risk of carotid plaque especially in females, subjects<65 years, non-obese individuals, and those without current smoking, drinking, diabetes, hypertension and dyslipidemia. Moreover, almost central sensitivity to thyroid hormone indices had a positive association with carotid plaque risk in all subgroups except those with diabetes or hypertension. Studies have demonstrated that individuals with thyroid issues are affected by glucose–lipid metabolism disturbances to some degree.37 Prolonged poor glucose control can lead to metabolic dysfunction, which may have an indirect effect on the hypothalamus-pituitary-thyroid axis system.38 Insensitivity causes a reduction in TSH secretion, which reduces the activity of 5’-deiodinase and FT3 levels.39 This disruption in FT3 also influences the antilipolytic effect of insulin, leading to a rise in free fatty acid concentration and triglyceride synthesis.40 Moreover, high T3 levels can cause metabolic and hemodynamic alterations, as well as a decrease in systemic vascular resistance, resulting in augmented cardiac output and hypertension.41 It appears that impaired sensitivity to thyroid hormone may be more closely connected to diabetes or hypertension than to carotid plaque. Additionally, the FT3/FT4 ratio, which indicates peripheral sensitivity to thyroid hormone, does not appear to have any correlation with carotid plaque risk in males, elderly people, obese people, or individuals with current drinking, diabetes, hypertension, or dyslipidemia. These individuals often have lipid abnormalities, which may influence the direct effect of sensitivity to thyroid hormone on carotid plaque formation.
Previous research has examined the pathways through which the thyroid hormone influences carotid atherosclerosis. A study observed that type 2 iodothyronine deiodinase (D2), a thyroid hormone-activating enzyme that transforms T4 to T3, is present in arterial smooth muscle cells (hCASMCs).42 The intracellular thyroid hormone, which is triggered by D2, has been found to repress the DNA synthesis and migration ability of hCASMCs,43 indicating that thyroid hormone has a direct inhibitory effect on atherosclerosis. An abnormally low thyroid hormone level can have a negative impact on endothelial cells, reducing the production of reactive oxygen species and the degree of vasodilation.44,45 Additionally, thyroid hormone has been found to activate the PI3K/Akt/endothelial nitric oxide synthase pathway in endothelial cells, which is thought to be involved in the process of vascular repair and angiogenesis. Thyroid hormone has direct anti-atherosclerotic effects, including blood vessel dilatation, the production of vasodilatory molecules, and the inhibition of angiotensin II receptor expression and its signal transduction. These findings indicate that thyroid hormone can inhibit the development of atherosclerosis by directly affecting the vasculature and modifying risk factors for the condition.46 Therefore, it is recommended that individuals undergo regular screenings for thyroid hormone levels to lower the risk of carotid plaque.
However, this study has several limitations. First, although our sample size was large, this retrospective study could not confirm a causal association between impaired sensitivity to thyroid hormones and carotid plaque risk, future large prospective studies are needed to confirm these results. Second, despite adjusting for multiple confounding factors, we cannot fully rule out the likelihood that carotid plaque might be impacted by other factors such as regular physical activity, diet pattern, and the use of lipid-lowering medication, which we were not able to include in our statistical analysis as covariates. Finally, as the data of this study was from a Chinese population, it is uncertain whether the conclusions can be extended to other ethnic backgrounds.
Conclusion
This study demonstrated that impaired thyroid hormone sensitivity is significantly associated with carotid plaque risk in Chinese health check-up populations. This association is more significant in females, subjects<65 years, non-obese individuals, and those without current smoking, drinking, diabetes, hypertension and dyslipidemia. Evaluating resistance to thyroid hormone is essential for the stratification of risk and the personalization of treatment for those with atherosclerosis.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the first author (Jiangling Wang, email: [email protected]) on reasonable request.
Acknowledgments
We also thank all staff involved in this study for their painstaking efforts in conducting the data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by the Suzhou Science and Technology Bureau Development Plan (Grant No. SYS2020147), the National Natural Science Foundation of China (Grant No. 81801754).
Disclosure
The authors declare no potential competing interests in this work.
References
1. Virani S, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi:10.1161/CIR.0000000000000757
2. Doan LN, Takata Y, Mendez-Luck CA, et al. Cardiovascular disease and health-related quality of life among asian American, native Hawaiian and pacific islander older adults. J Aging Health. 2022;34(9–10):1254–1268. doi:10.1177/08982643221118440
3. Bos D, Arshi B, van den Bouwhuijsen Q, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol. 2021;77(11):1426–1435. doi:10.1016/j.jacc.2021.01.038
4. Gepner AD, Young R, Delaney JA, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8(1). doi:10.1161/CIRCIMAGING.114.002262
5. Gu Y, Meng G, Zhang Q, et al. Association of longitudinal trends in thyroid function with incident carotid atherosclerosis in middle-aged and older euthyroid subjects: the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) cohort study. Age Ageing. 2022;51(1). doi:10.1093/ageing/afab276
6. Bahn CR, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21(6):593–646. doi:10.1089/thy.2010.0417
7. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. doi:10.1089/thy.2014.0028
8. Huang WH, Sung KT, Kuo JY, et al. Atrioventricular longitudinal mechanics using novel speckle-tracking improved risk stratification beyond baseline thyroid hormone in asymptomatic subclinical hypothyroidism. Circ Cardiovasc Imaging. 2021;14(11):e12433. doi:10.1161/CIRCIMAGING.121.012433
9. Gluvic ZM, Zafirovic SS, Obradovic MM, et al. Hypothyroidism and Risk of Cardiovascular Disease. Curr Pharm Des. 2022;28(25):2065–2072. doi:10.2174/1381612828666220620160516
10. Liu H, Peng D. Update on dyslipidemia in hypothyroidism: the mechanism of dyslipidemia in hypothyroidism. Endocr Connect. 2022;11(2). doi:10.1530/EC-21-0002
11. Saif A, Mousa S, Assem M, et al. Endothelial dysfunction and the risk of atherosclerosis in overt and subclinical hypothyroidism. Endocr Connect. 2018;7(10):1075–1080. doi:10.1530/EC-18-0194
12. Gu Y, Meng G, Zhang Q, et al. Thyroid function and lipid profile in euthyroid adults: the TCLSIH cohort study. Endocrine. 2020;70(1):107–114. doi:10.1007/s12020-020-02312-6
13. Kvetny J, Heldgaard PE, Bladbjerg EM, et al. Subclinical hypothyroidism is associated with a low-grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin Endocrinol. 2004;61(2):232–238. doi:10.1111/j.1365-2265.2004.02088.x
14. Dagre AG, Lekakis JP, Protogerou AD, et al. Abnormal endothelial function in female patients with hypothyroidism and borderline thyroid function. Int J Cardiol. 2007;114(3):332–338. doi:10.1016/j.ijcard.2005.12.013
15. Kim SK, Kim SH, Park KS, et al. Regression of the increased common carotid artery-intima media thickness in subclinical hypothyroidism after thyroid hormone replacement. Endocr J. 2009;56(6):753–758. doi:10.1507/endocrj.k09e-049
16. Dullaart RP, de Vries R, Roozendaal C, et al. Carotid artery intima media thickness is inversely related to serum free thyroxine in euthyroid subjects. Clin Endocrinol. 2007;67(5):668–673. doi:10.1111/j.1365-2265.2007.02943.x
17. Delitala AP, Filigheddu F, Orru M, et al. No evidence of association between subclinical thyroid disorders and common carotid intima medial thickness or atherosclerotic plaque[J]. Nutr, Metab Cardiovasc Dis. 2015;25(12):1104–1110. doi:10.1016/j.numecd.2015.09.001
18. Pappa T, Refetoff S. Resistance to thyroid hormone beta: a focused review. Front Endocrinol. 2021;12:656551. doi:10.3389/fendo.2021.656551
19. Yagi H, Pohlenz J, Hayashi Y, et al. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3’-triiodothyroinine binding affinity. J Clin Endocrinol Metab. 1997;82(5):1608–1614. doi:10.1210/jcem.82.5.3945
20. Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin Endocrinol. 2009;71(4):529–534. doi:10.1111/j.1365-2265.2009.03534.x
21. Liu B, Wang Z, Fu J, et al. Sensitivity to thyroid hormones and risk of prediabetes: a cross-sectional study. Front Endocrinol. 2021;12:657114. doi:10.3389/fendo.2021.657114
22. Mehran L, Delbari N, Amouzegar A, et al. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab. 2022;107(1):167–176. doi:10.1210/clinem/dgab646
23. Sun Y, Teng D, Zhao L, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia, obesity, and cardiovascular disease risk in subjects with subclinical hypothyroidism. Thyroid. 2022;32(4):376–384. doi:10.1089/thy.2021.0500
24. ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–S40. doi:10.2337/dc23-S002
25. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160–164. doi:10.1016/j.tcm.2019.05.003
26. Wang J, Wang Y, Li Y, et al. High normal urinary albumin-creatinine ratio is associated with hypertension, type 2 diabetes mellitus, HTN with T2DM, dyslipidemia, and cardiovascular diseases in the Chinese population: a report from the REACTION study. Front Endocrinol. 2022;13:864562. doi:10.3389/fendo.2022.864562
27. Tian Y, Liu D, Wang D, et al. Obesity in Chinese patients with chronic schizophrenia: prevalence, clinical correlates and relationship with cognitive deficits. Schizophr Res. 2020;215:270–276. doi:10.1016/j.schres.2019.10.017
28. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. 2019;42(2):303–310. doi:10.2337/dc18-1410
29. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290–296. doi:10.1159/000343145
30. Shimizu Y, Kawashiri SY, Noguchi Y, et al. Association between thyroid cysts and hypertension by atherosclerosis status: a cross-sectional study. Sci Rep. 2021;11(1):13922. doi:10.1038/s41598-021-92970-x
31. Manolis AA, Manolis TA, Melita H, et al. Subclinical thyroid dysfunction and cardiovascular consequences: an alarming wake-up call? Trends Cardiovasc Med. 2020;30(2):57–69. doi:10.1016/j.tcm.2019.02.011
32. Sakamaki K, Tsunekawa K, Ishiyama N, et al. Association between high normal-range thyrotropin concentration and carotid intima-media thickness in euthyroid premenopausal, perimenopausal and postmenopausal women. Maturitas. 2021;144:29–36. doi:10.1016/j.maturitas.2020.10.022
33. Cappola AR, Arnold AM, Wulczyn K, et al. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. 2015;100(3):1088–1096. doi:10.1210/jc.2014-3586
34. Liu Y, Li Z, Yang T, et al. Impaired sensitivity to thyroid hormones and carotid plaque in patients with coronary heart disease: a RCSCD-TCM study in China. Front Endocrinol. 2022;13:940633. doi:10.3389/fendo.2022.940633
35. Dorr M, Empen K, Robinson DM, et al. The association of thyroid function with carotid artery plaque burden and strokes in a population-based sample from a previously iodine-deficient area. Eur J Endocrinol. 2008;159(2):145–152. doi:10.1530/EJE-08-0140
36. Wang L, Chen T, Yu J, et al. Clinical associations of thyroid hormone levels with the risk of atherosclerosis in euthyroid type 2 diabetic patients in central China. Int J Endocrinol. 2020;2020:2172781. doi:10.1155/2020/2172781
37. Salter AM, Hayashi R, Al-Seeni M, et al. Effects of hypothyroidism and high-fat feeding on mRNA concentrations for the low-density-lipoprotein receptor and on acyl-CoA:cholesterol acyltransferase activities in rat liver. Biochem J. 1991;276(P3):825–832. doi:10.1042/bj2760825
38. Yan F, Wang Q, Lu M, et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J Hepatol. 2014;61(6):1358–1364. doi:10.1016/j.jhep.2014.06.037
39. Damiano F, Rochira A, Gnoni A, et al. Action of thyroid hormones, T3 and T2, on hepatic fatty acids: differences in metabolic effects and molecular mechanisms. Int J Mol Sci. 2017;18(4). doi:10.3390/ijms18040744
40. Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet. 2007;41:401–427. doi:10.1146/annurev.genet.41.110306.130315
41. Yamashita S, Sakai N, Hirano K, et al. Roles of plasma lipid transfer proteins in reverse cholesterol transport. Front Biosci. 2001;6:D366–D387. doi:10.2741/yamashita
42. Mizuma H, Murakami M, Mori M. Thyroid hormone activation in human vascular smooth muscle cells: expression of type II iodothyronine deiodinase. Circ Res. 2001;88(3):313–318. doi:10.1161/01.res.88.3.313
43. Kasahara T, Tsunekawa K, Seki K, et al. Regulation of iodothyronine deiodinase and roles of thyroid hormones in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):207–214. doi:10.1016/j.atherosclerosis.2005.07.018
44. Lekakis J, Papamichael C, Alevizaki M, et al. Flow-mediated, endothelium-dependent vasodilation is impaired in subjects with hypothyroidism, borderline hypothyroidism, and high-normal serum thyrotropin (TSH) values. Thyroid. 1997;7(3):411–414. doi:10.1089/thy.1997.7.411
45. De Sibio MT, Luvizotto RA, Olimpio RM, et al. A comparative genotoxicity study of a supraphysiological dose of triiodothyronine (T3) in obese rats subjected to either calorie-restricted diet or hyperthyroidism. PLoS One. 2013;8(2):e56913. doi:10.1371/journal.pone.0056913
46. Ichiki T. Thyroid hormone and atherosclerosis. Vascul Pharmacol. 2010;52(3–4):151–156. doi:10.1016/j.vph.2009.09.004
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

