Back to Journals » Cancer Management and Research » Volume 11
Impact of tivozanib on patient outcomes in treatment of advanced renal cell carcinoma
Received 20 February 2019
Accepted for publication 13 June 2019
Published 16 August 2019 Volume 2019:11 Pages 7779—7785
DOI https://doi.org/10.2147/CMAR.S206105
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Suayib Yalcin,1 Sahin Lacin2
1Hacettepe University Institute of Cancer, Department of Medical Oncology, Ankara, Turkey; 2University of Health Sciences, Diyarbakir Gazi Yasargil Training and Research Hospital, Department of Medical Oncology, Diyarbakir, Turkey
Correspondence: Suayib Yalcin
Hacettepe University Institute of Cancer, Department Of Medical Oncology, Faculty of Medicine, Ankara 006100, Sihhiye, Turkey
Email [email protected]
Abstract: Renal cell carcinoma (RCC) is the most common type of kidney malignancy, and the clear-cell subtype represents the majority of RCCs. RCC is a heterogeneous disease in terms of genetic and histological features which determine the behavior of the disease. The von Hippel–Lindau (VHL) is a tumor suppressor gene and mutations of this gene are seen in 95% of clear-cell RCCs. Inactivation of VHL causes the accumulation of hypoxia-inducible factor-1 (HIF-1), and in turn, accumulation of HIF-1 induces overexpression of vascular endothelial growth factor (VEGF); the increase in VEGF expression makes RCC a highly vascularized tumor, and forms the rationale for antiVEGF treatment. In the past decade, improvement in the survival of RCC patients has been observed due to new effective therapies, such as antiVEGF and mammalian target of rapamycin (mTOR) targeting agents and immune checkpoint inhibitors. The majority of VEGF targeted agents are not just selective to VEGF receptors, but usually also have inhibitory effects on other kinases, such as c-KIT and FLT3. Tivozanib is an extremely potent and selective tyrosine kinase inhibitor (TKI) of VEGFR-1, 2, and 3, with a relatively long half-life, that is approved by the European Commission for the treatment of advanced/metastatic RCC. Tivozanib, at very low serum concentration can inhibit phosphorylation of VEGFR −1, −2, and −3 tyrosine kinase activity. This article summarizes the clinical data on tivozanib in the treatment of advanced/metastatic RCC.
Keywords: renal cell carcinoma, vascular endothelial growth factor, tyrosine kinase inhibitors, tivozanib, clear-cell carcinoma
Introduction
Comprising 2–3% of all malignancies, kidney cancers are one of the most prevalent cancers in the world.1 Renal cell carcinoma (RCC) is the most common type (85%) among them, and clear-cell subtype tumors represent the majority of RCC.2 RCC is a heterogeneous disease in terms of genetic and histological characteristics. The von Hippel–Lindau (VHL) gene is a tumor suppressor gene and its inactivation is seen as one of the most common oncogenic events that play a pivotal role in 95% of clear-cell RCCs. Inactivation of VHL causes accumulation of hypoxia-inducible factor-1 (HIF-1) which induce overexpression of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF) that make RCC a highly vascularized tumor.3,4 This is why RCC is susceptible to anti-VEGF treatment. The prognosis of the patients with early, localized RCC is very good, and the cure rate is high with approximately 90% of 5-year survival.5 However, about 16% of RCC patients are diagnosed at the metastatic stage and, the 5-year survival rate of these patients with metastatic RCC is only around 12%. Currently, several treatment options are available and also some more are being developed in the management of the advanced disease.6 RCC is highly resistant to conventional chemotherapy, therefore in the past, systemic treatment of mRCC was limited to cytokine treatment with interferon α (IFNα) and interleukin-2.7,8 The advancements in the antiVEGF, and mammalian target of rapamycin (mTOR) targeting therapies and immune checkpoint inhibitors in the last years have obviously improved treatment outcomes and success rate of treatment in patients with advanced or metastatic RCC.9,10 Development of the new therapies coupled with improved knowledge of disease biology have led to increased survival such that the median survival of the patients now is more than 2 years.11,12
Targeting VEGF pathway for the treatment of mRCC
In recent years, many new molecules that target VEGF signaling pathways have been tested and some of them already approved for the treatment of patients with metastatic renal cell carcinoma worldwide.13 These VEGF targeted therapies include sunitinib, bevacizumab, tivozanib, sorafenib, pazopanib, lenvatinib, axitinib, cabozantinib, and nintedanib. Sorafenib and sunitinib are the first tyrosine kinase inhibitors (TKIs) that gained regulatory approval in the treatment for metastatic RCC. Their antitumor activity has been reported in phase III trials by improved progression-free survival (PFS) compared with interferon alfa or placebo.14,15 Both agents are not selective to VEGF receptors, they have also inhibitory effects on other kinases, such as c-KIT and FLT3.16 These less selective targeted agents are also associated with some adverse events which include skin rash, stomatitis, hand-foot skin reaction, diarrhea, fatigue, myelosuppression, therefore, in order to improve patient outcomes, a more potent and more selective inhibitor for VEGFRs may demonstrate improved antitumor activity and tolerability in metastatic RCC and may reduce the off-target toxicities of less selected anti VEGF agents.
Tivozanib; mechanism of action
Tivozanib is designed to target the vascular endothelial growth factor (VEGF) pathway, a clinically validated target in RCC. Tivozanib is a TKI of VEGFR-1, 2, and 3 (Figure 1). It is an extremely potent and selective tyrosine kinase inhibitor with a relatively long half-life. At very low serum concentration tivozanib can inhibit phosphorylation of VEGFR1, −2, and −3, however, for the inhibition of other kinases such as c-KIT and PDGFR much higher concentrations are needed, which makes tivozanib more potent and specific to VEGFR tyrosine kinases. The serum concentration of tivozanib to inhibit each of the three VEGFRs, is 0.21 nM for VEGFR-1, 0.16nM for VEGFR-2, and 0.24 nM for VEGFR-3 (Table 1).16–18 The European Commission (EC) has approved tivozanib for the treatment of adult patients with advanced RCC in the European Union and also approved in Norway and Iceland in 2017. This approval based on a phase 3 trial that compared tivozanib with sorafenib as first-line treatment.19,20
 |
Table 1 Cross trial comparison of VEGF targeted agents, their minimal inhibitory concentrations for VEGFRs, and their adverse events rates (grades 3-4) |
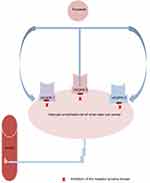 |
Figure 1 Mechanism of action of tivozanib on vascular endothelial cell in renal clear cell cancer. |
Clinical data
In the initial phase I study that demonstrated safety and efficacy of tivozanib in patients with advanced RCC, the dose of tivozanib was reported as 1.5 mg/day in a 4-week-on, 2-week off schedule.21 Subsequently, in order to determine antitumor activity and safety of tivozanib in metastatic RCC population, a phase II randomized study was conducted in a heterogeneous group of patients with metastatic RCC. In this study, adult patients with non-resectable primary metastatic RCC, measurable recurrent or metastatic RCC were enrolled. The patients were allowed to receive one previous systemic treatment that not contains an inhibitor of VEGF pathway for RCC. Additionally, patients with Karnofsky performance status ≥70% and sufficient hepatic, renal, and bone marrow functions were eligible and included for the phase II study as well. As expected patients with central nervous system (CNS) involvement were excluded. Other exclusion criteria were a symptomatic cardiovascular disease (CVS) that contains uncontrolled hypertension, active clinically symptomatic left ventricular heart failure, and myocardial infarction within 3 months previously. In the study 46% of the patients had been previously treated, majority of the patients had clear-cell subtype (83%), and most of the patients (73%) of had prior nephrectomy for the primary tumor removal. The patient was scheduled to have tivozanib at the dose of 1.5 mg/d orally as a three week on, one week off schedule for 4 months. Tumoral lesions were evaluated radiologically and the response was determined according to Response Evaluation Criteria in Solid Tumors (RECIST) in every 2 cycles of treatment. The diameters of target lesions were compared with their baseline, if a patient had ≥25% tumor diameter shrinkage then continued to take open-label tivozanib, on the contrary to this, patients who had radiological progressive disease did stop to take treatment. Patients who could not be classified as progressive or responsive with a less than 25% change in tumor size (decrease or increase) were randomly assigned to for the next 12 weeks in a double-blind tivozanib or placebo. Finally, assessment of the patients in sixteen weeks open-label, the objective response rate (ORR) of patients treated with tivozanib was 18% (95%CI, 14–23%), and median progression-free survival (PFS) was significantly higher in patients who treated with tivozanib than patients receiving placebo, 10.3 and 3.3 months, respectively (P=0.01). Nephrectomy is known as a positive contributor factor on the prognosis of metastatic RCC that has been reported.22 Therefore, the lower rate of nephrectomy in this study may have decreased the activity of tivozanib. A subgroup analysis of the study for patients with clear-cell RCC and nephrectomy showed higher median PFS and ORR than reported. PFS was 14.8 months and the objective response rate was 30% for the patients who had nephrectomy. In conclusion of the study, tivozanib obtained promising PFS rate and proved its activity in patients with advanced/metastatic RCC. The obtained PFS rate of the study was higher than reported rates with other tyrosine kinase inhibitors for VEGFRs. Entire PFS for tivozanib in this study was 11.7 months, however, it was shorter with other TKI agents that reported in their phase II trials.23–25 This phase II trial showed the efficacy and safety of tivozanib in advanced RCC and supported its usage in patients with advanced or metastatic clear-cell RCC in phase III trials.
Sorafenib and sunitinib are the first TKIs that gained approval for advanced RCC treatment, and they showed substantial antitumor activity as first-line treatment in phase III trials in terms of improved PFS compared with interferon alfa or placebo.14,26 The data achieved in the phase II trial of tivozanib provided the rationale for a phase III trial comparing tivozanib with sorafenib as first-line targeted therapy for patients with metastatic RCC.
In the randomized phase III trial that compared tivozanib and sorafenib, patients were randomly assigned to receive either tivozanib or sorafenib treatment. Patients with age ≥18 years, with measurable disease and histologically, confirmed recurrent or metastatic RCC with a dominantly clear cell component were enrolled in this study. Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1, with adequate hepatic, hematologic, and renal functions were the main eligibility criteria. The patients could be treatment-naive or could have received one or fewer prior systemic treatments (chemotherapy, hormonal, or immunotherapy therapy) for metastatic RCC, however prior VEGF targeted therapies or mTOR inhibitors were not permitted. Patients who had unstable brain metastases or significant CVS including uncontrolled hypertension, myocardial infarction, or thromboembolic disorders were also excluded.
Tivozanib was administered orally at the dose of 1.5 mg/day every day for 3 weeks followed by 1 week off. Sorafenib was administered orally at a dose of 400 mg twice per day continuously. Patients continued to receive drugs until disease progression, unacceptable toxicity, or death. Tivozanib significantly improved PFS compared with sorafenib such that the median PFS was 11.9 months in the tivozanib arm while only 9.1 months in sorafenib arm (HR, 0.797; 95% CI, 0.639–0.993; P=0.042). The tumor response was also improved. According to the blinded independent radiological assessment ORR was 33.1% (95% CI, 27.4–39.2%) in tivozanib arm and 23.3% (95% CI, 18.3–29.0%) in sorafenib arm, which was statistically significant superiority (P=0.014). Median PFS was also improved; 12.7 months for tivozanib and 9.1 months for sorafenib: this difference was also statistically meaningful (HR, 0.756; 95% CI, 0.580–0.985; P=0.037) among patients who were treatment naive for metastatic RCC.
At the time of the final OS analysis, a total of 219 patients (42%) died in the ITT population with 118 deaths among tivozanib-treated patients and 101 deaths were observed in the sorafenib arm. At the final analyses, a trend toward longer survival rate was seen in patients treated with sorafenib in the first line compared to the patients in tivozanib arm (median, 29.3 v 28.8 months; HR, 1.245; 95% CI, 0.954–1.624; P=0.105). This was due to probably the availability of second-line therapies; the OS outcome of the patients in the study seems to be confounded by differential use of next-line targeted cancer therapies because the crossover allowed patients who had progressed on sorafenib to switch to tivozanib. The final OS analysis of the study reported that 156 patients (61%) randomly assigned to sorafenib had crossed over to tivozanib, but vice versa was not possible. Such that the majority of patients in the sorafenib arm received a next-line targeted therapy for RCC (63% in the sorafenib arm vs 13% in the tivozanib arm), this is one of the major confounding factors that may have affected the OS rate in this study. However the study still confirmed that tivozanib prolonged PFS compared with sorafenib in patients with metastatic RCC, and besides tivozanib was better tolerated and had lower AEs rates that included hand-foot skin reaction and diarrhea. Tivozanib required fewer dose reductions and interruptions compared with sorafenib, however, higher rates of hypertension and dysphonia were reported in tivozanib arm.
In this study, the median duration of treatment was 12 months for tivozanib, and 9.5 months for sorafenib. The rate of patients who experienced at least one treatment-emergent AE was 91% in the tivozanib arm versus 97% in the sorafenib arm. The rate of reported grade 3–4 AEs was 61% of patients in tivozanib arm and 70% of patients in the sorafenib arm. The adverse events which were more common in tivozanib arm were hypertension and dysphonia, whereas hand-foot syndrome and diarrhea were reported more commonly in the sorafenib arm. The rate of treatment discontinuation due to treatment-related AEs was reported in 4% patients for tivozanib and 5% sorafenib, and also treatment interruptions due to AEs occurred in 36% of patients treated with sorafenib versus 19% of patients treated with tivozanib. Dose reductions due to AEs occurred more common in sorafenib treatment arm 43% versus 14% in tivozanib treatment arm, the most common adverse events that cause dose reduction were hand-foot syndrome (tivozanib v sorafenib, 2% vs 18%), diarrhea (1% vs 5%), and hypertension (2% vs 4%). Health-related quality of life (HRQoL) questionnaires were completed by >99% of patients in both arms at baseline, but completion rates decreased over time. Statistical analysis of score changes from baseline between the two treatment arms with well-balanced baseline HRQoL scores was not significantly different.
Recently, the efficacy of tivozanib treatment after sorafenib in patients with advanced renal cell carcinoma was evaluated by a phase 3 crossover study. The crossover of the patients in the study that compared sorafenib with tivozanib who progressed on sorafenib was subsequently treated with tivozanib. A total 74% of patients randomized to sorafenib in phase III trial were treated with a next-line therapy after progression, however, only 35% of tivozanib-treated patients received a next-line therapy.27 The majority of next-line treatment in the study was tivozanib which was due to the lack of available second-line therapies in the Eastern European countries at that time, whereas the patients who randomized to tivozanib had no salvage therapy. That is why the results of overall survival in the phase 3 tivozanib trial were considered to be confounded by the differential use of subsequent RCC therapy in the two treatment arms. Therefore, this crossover study designed to determine the efficacy of tivozanib in the patients who progressed on sorafenib treatment.
A total 161 of the patients with ECOG PS ≤2 were included to the analysis who initially treated with sorafenib, these patients were two groups which including patients who crossed over to tivozanib after the first documented disease progression and patients who continued on sorafenib on entering this study and subsequently progressed and started tivozanib. Exclusion criteria were the patients who progressed during sorafenib treatment ≥4 weeks since the last dose of sorafenib, newly detected CNS mmetastasis or documented progression of CNS metastases, inadequate hepatic, renal, or hematologic functions, unhealed wounds, uncontrolled hypertension, active infections or infections requiring parenteral antibiotics, current receipt of treatment with another oncology therapy. Tivozanib was administered orally 1.5 mg/day continuously with a 3 week on 1 week off schedule. Treatment was continued until disease progression or unacceptable toxicity.
A prolongation of median PFS which showed the anti-tumor activity of tivozanib was demonstrated in the study, and it was 11 months. The median overall survival that started from the first tivozanib dose was 22 months. The objective response rate all of which was PR, was 18%, without a CR, stable disease was observed in further 52% of the patients, while the progressive disease was 21%. A majority of crossover patients had measurable disease post baseline, 92.6% of them had a reduction in target lesion diameter. Hypertension was the most common tivozanib treatment-related adverse event observed and that was 26% in the crossover population. This study provided evidence of tivozanib treatment activity in patients with recurrent disease after sorafenib treatment.28
As ongoing trials, tivozanib is currently being evaluated in third-line treatment of patients with RCC who have failed 2–3 prior systemic regimens (one of which is a VEGF inhibitor other than sorafenib or tivozanib) in a comparative study to sorafenib, and immune checkpoint inhibitor-targeted therapy combination in the ongoing TiNivo phase 1/2 trial (nivolumab, tivozanib). The immune-targeted combination treatment trial has passed successfully from phase I to phase II.29,30 The characteristics of the trials with tivozanib in RCC are given in Table 2.
 |
Table 2 Characteristics of phase I-III trials of tivozanib |
As a more selective antiVEGFR therapy, tivozanib appeared to exhibit greater clinical benefit in terms of response rate and significantly longer PFS than sorafenib in a phase 3 comparison trial. The objective response rate of tivozanib was greater than sorafenib, also sorafenib was associated with a higher incidence of diarrhea and hand-foot syndrome compared with tivozanib. The approval of tivozanib in the European Union plus Norway and Iceland in 2017 after the completed phase 3 comparison trial and its possible effective combinations with emerging immunotherapies seem to reopen for tivozanib the way to the future therapeutic field for patients with mRCC.
Conclusion
The treatment paradigm in mRCC is rapidly changing and evolving. Newer TKI’s and immune check points inhibitors alone or in combination have been proven to be effective.31,33 More recently pembrolizumab in combination with axitinib has been approved, after phase 3 study showing superiority of this combination over sunitinib monotherapy as a frontline treatment irrespective of PDL-1 expression or risk status.31 As an effective TKI, further studies are needed to better define the place of tivozinib as a monotherapy or in combination with immune check point inhibitors, in the first and second line treatment and according to risk group status and/or PDL-1 expression in patients with RCC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li P, Znaor A, Holcatova I, et al. Regional geographic variations in kidney cancer incidence rates in European countries. Eur Urol. 2015;67:1134–1141. doi:10.1016/j.eururo.2014.11.001
2. Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis. 2016;9:45–52. doi:10.2147/IJNRD.S75916
3. Brugarolas J. Molecular genetics of clear-cell renal cell carcinoma. J Clin Oncol. 2014;32:1968–1976. doi:10.1200/JCO.2012.45.2003
4. Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23:1028–1043. doi:10.1200/JCO.2005.01.186
5. Colombo JR
6. DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi:10.3322/caac.21340
7. Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med. 1998;338:1272–1278. doi:10.1056/NEJM199804303381805
8. Rohrmann K, Staehler M, Haseke N, et al. Immunotherapy in metastatic renal cell carcinoma. World J Urol. 2005;23:196–201. doi:10.1007/s00345-004-0470-4
9. Rodriguez-Vida A, Hutson TE, Bellmunt J, et al. New treatment options for metastatic renal cell carcinoma. ESMO Open. 2017;2:e000185. doi:10.1136/esmoopen-2017-000185
10. Grunwald V, Karakiewicz PI, Bavbek SE, et al. An international expanded-access programme of everolimus: addressing safety and efficacy in patients with metastatic renal cell carcinoma who progress after initial vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. Eur J Cancer. 2012;48:324–332. doi:10.1016/j.ejca.2011.06.054
11. Cella D, Cappelleri JC, Bushmakin A, et al. Quality of life predicts progression-free survival in patients with metastatic renal cell carcinoma treated with sunitinib versus interferon alfa. J Oncol Pract. 2009;5:66–70. doi:10.1200/JOP.0922004
12. Yalcin S, Yildiz R, Dane F, et al. A national, multicenter, non-interventional, observational study on treatment patterns in patients with metastatic renal cell carcinoma in Turkey - NOTES study. Onco Targets Ther. 2018;11:1223–1228. doi:10.2147/OTT.S148917
13. Erman M, Benekli M, Basaran M, et al. Renal cell cancer: overview of the current therapeutic landscape. Expert Rev Anticancer Ther. 2016;16:955–968. doi:10.1080/14737140.2016.1222908
14. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi:10.1056/NEJMoa060655
15. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi:10.1056/NEJMoa065044
16. Kumar R, Crouthamel MC, Rominger DH, et al. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer. 2009;101:1717–1723. doi:10.1038/sj.bjc.6605366
17. Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–984. doi:10.1016/S1470-2045(07)70285-1
18. Gross-Goupil M, Francois L, Quivy A, et al. Axitinib: a review of its safety and efficacy in the treatment of adults with advanced renal cell carcinoma. Clin Med Insights Oncol. 2013;7:269–277. doi:10.4137/CMO.S10594
19. Nakamura K, Taguchi E, Miura T, et al. KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res. 2006;66:9134–9142. doi:10.1158/0008-5472.CAN-05-4290
20. Hutson TE, Bukowski RM, Rini BI, et al. Efficacy and safety of sunitinib in elderly patients with metastatic renal cell carcinoma. Br J Cancer. 2014;110:1125–1132. doi:10.1038/bjc.2013.832
21. Eskens FA, de Jonge MJ, Bhargava P, et al. Biologic and clinical activity of tivozanib (AV-951, KRN-951), a selective inhibitor of VEGF receptor-1, −2, and −3 tyrosine kinases, in a 4-week-on, 2-week-off schedule in patients with advanced solid tumors. Clin Cancer Res. 2011;17:7156–7163. doi:10.1158/1078-0432.CCR-11-0411
22. Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27:3225–3234. doi:10.1200/JCO.2008.19.9836
23. Nosov DA, Esteves B, Lipatov ON, et al. Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Clin Oncol. 2012;30:1678–1685. doi:10.1200/JCO.2011.35.3524
24. Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi:10.1200/JCO.2005.03.6723
25. Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. doi:10.1200/JCO.2008.21.6994
26. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi:10.1200/JCO.2008.20.1293
27. Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol. 2013;31:3791–3799. doi:10.1200/JCO.2012.47.4940
28. Molina AM, Hutson TE, Nosov D, et al. Efficacy of tivozanib treatment after sorafenib in patients with advanced renal cell carcinoma: crossover of a phase 3 study. Eur J Cancer. 2018;94:87–94. doi:10.1016/j.ejca.2018.02.009
29. Kim ES. Tivozanib: first global approval. Drugs. 2017;77:1917–1923. doi:10.1007/s40265-017-0825-y
30. Rini BI, Atkins MB, Escudier BJ, et al. Tivo-3: a phase 3, randomized, controlled, multi-center, open-label study to compare tivozanib hydrochloride to sorafenib in subjects with refractory advanced renal cell carcinoma (RCC). Am Soc Clin Oncol. 2017;35:TPS4600–TPS4600. doi:10.1200/JCO.2017.35.15_suppl.TPS4600
31. Powles T, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for locally advanced or metastatic renal cell carcinoma (mRCC): phase III KEYNOTE-426 study. Am Soc Clin Oncol. 2019;37:543. doi:10.1200/JCO.2019.37.7_suppl.543
32. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi:10.1200/JCO.2009.23.9764
33. Powles T, Larkin JMG, Patel P, et al. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol. 2019;37(suppl 7; abstr 545):545. doi:10.1200/JCO.2019.37.7_suppl.545
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
