Back to Journals » Research and Reports in Urology » Volume 13
Impact of Radical Nephrectomy and Partial Nephrectomy on Actual Estimated Overall Survival Compared to Life Expectancy in Patients with Renal Cell Carcinoma
Authors Hori S , Tanaka N , Iida K, Nakai Y, Miyake M , Anai S, Torimoto K , Fujimoto K
Received 16 January 2021
Accepted for publication 11 March 2021
Published 24 March 2021 Volume 2021:13 Pages 155—165
DOI https://doi.org/10.2147/RRU.S299801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Shunta Hori, Nobumichi Tanaka, Kota Iida, Yasushi Nakai, Makito Miyake, Satoshi Anai, Kazumasa Torimoto, Kiyohide Fujimoto
Department of Urology, Nara Medical University, Kashihara, Nara, 634-8522, Japan
Correspondence: Nobumichi Tanaka
Department of Urology, Nara Medical University, 840 Shijo-cho, Kashihara, Nara, 634-8522, Japan
Tel +81-744-22-3051
Fax +81-744-22-9282
Email [email protected]
Purpose: Reports suggest that partial nephrectomy provides no significant benefit in terms of cancer-specific and overall survival (OS) compared to radical nephrectomy. Here, we focused on survival in terms of life expectancy and investigated the significance of partial nephrectomy for localized renal cell carcinoma (RCC) patients.
Patients and Methods: Our retrospective study included 937 patients (median age 63 years) with localized RCC who underwent partial nephrectomy or radical nephrectomy. Various predictive factors were explored, and the association between actual OS and life expectancy was analyzed.
Results: Performance status (PS) ≥ 1 and tumor size ≥ 40 mm were identified as independent poor prognostic factors for cancer-specific survival. Age ≥ 60, male sex, PS ≥ 1, C-reactive protein elevation, pT1b stage, and radical nephrectomy were identified as independent poor prognostic factors for OS. OS and life expectancy did not differ in the partial nephrectomy group (P=0.11). OS was significantly shorter than life expectancy in the radical nephrectomy group (P< 0.0001). In PS0 or pT1a patients, there was a significant difference between actual OS and life expectancy in the radical nephrectomy group (P< 0.0001), but not in the partial nephrectomy group (P=0.15). In patients with a life expectancy ≥ 10 years, PS0, and pTa, OS and life expectancy differed in the radical nephrectomy group, but not in the partial nephrectomy group.
Conclusion: Partial nephrectomy can improve actual OS, and notably, PS and tumor size are crucial factors that determine the choice of surgical procedure. Further research is needed to establish appropriate treatment strategies and criteria for clinical practice.
Keywords: life expectancy, nephrectomy, performance status, renal cell carcinoma, tumor size
Introduction
Globally, renal cell carcinoma (RCC) accounts for approximately 3% of all cancers. By 2026, RCC is estimated to be the sixth and ninth most common cancer type in men and women, respectively.1 Recently, advancements in drug treatment, including immune-checkpoint inhibitors, have expanded treatment options, while surgical treatments, including partial nephrectomy (PN) and radical nephrectomy (RN), remain the gold standards for localized RCC. Improved surgical techniques, including robotic surgery, have enabled the treatment of challenging PN. Therefore, it may be possible to improve overall survival (OS) in RCC patients by making full use of advanced treatments.
In an analysis of 2459 patients with cT1 RCC, RN was associated with an increased risk of chronic kidney disease (CKD) compared to PN, whereas RN was not associated with increased risk of cancer-specific survival (CSS) or OS.2 An analysis from Italy showed no significant difference in metastasis-free survival, local recurrence-free survival, CSS, and OS between PN and RN patients.3 On the contrary, another report suggested that patients over 65 years who undergo PN have longer OS than those who undergo RN.4 In terms of surgical complications, the European Organization for the Research and Treatment of Cancer (EORTC) showed that PN was associated with slightly higher complication rates compared to RN, while PN was found to be an acceptable treatment for patients with small RCC.5 Although PN is expected to be associated with survival advantages, including prevention of CKD and cardiovascular disease, many studies have suggested that there are no statistical differences in CSS and OS between patients who underwent PN and RN.6–9 However, PN is recommended for patients with cT1 RCC and this is because surgeons believe in the potential survival benefits of PN. In a previous study that explored which patients would benefit from PN, patients with CKD stage-II demonstrated a decreased risk of developing significant renal impairment.10 Therefore, PN should be recommended to patients with poor renal function.
In the present study, we explored independent factors for CSS and OS, focusing on the association between OS and life expectancy calculated by an abridged life table published by the Ministry of Health, Labour and Welfare in Japan. Comparison of life expectancy may lead to novel findings and formulation of optimal strategies for RCC patients.
Patients and Methods
Ethics Approval and Consent to Participate
This study was conducted in compliance with the study’s protocol and following the provisions of the Declaration of Helsinki (2013) and was approved by the research ethics committee of Nara Medical University (project identification code: 685–4). The requirement for informed patient consent was waived because of the retrospective nature of the analysis. Personal information linked to research subjects and donors was anonymized (when necessary, the information was labeled with an identifying code to make it possible to distinguish between the individuals). Then, deidentified patient data were analyzed.
Patient Selection and Data Collection
The present study included 937 consecutive patients with localized RCC (pT1-2pN0cM0pV0) seen between the years 1980 and 2008, at our institute. We retrospectively reviewed medical charts and obtained clinical, pathological, and laboratory data of these patients.
Life Expectancy Calculation
Life expectancy was calculated according to an abridged life table published by the Ministry of Health, Labour and Welfare in Japan (Table S1). The date of birth of patients was obtained for calculating the life expectancy of each patient. This value was used for analysis in this study; however, if life expectancy was shorter than the actual OS, the latter was used.
Statistical Analysis
Statistical analyses were performed and figures were constructed using GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA, USA). The Student’s t-test or the Mann–Whitney U-test was applied for statistical analysis, as appropriate. The age cut-off was determined using a receiver-operating characteristic (ROC) curve analysis. A survival curve was obtained using the Kaplan–Meier method and compared using the Log-rank test. A multivariate Cox proportional hazards regression analysis was carried out using Statistical Package for the Social Sciences, version 21 (SPSS Inc., Chicago, IL, USA). Two-sided tests were used in all cases and a P value <0.05 was considered statistically significant.
Results
Patient Characteristics
Table 1 shows the clinicopathological characteristics of the cohort. The median age at surgery was 63 years (interquartile range [IQR] 54–70) and there were 646 men (68.9%) and 291 women (31.1%). Of 937 patients, 248 patients (26.5%) were diagnosed with RCC incidentally and 873 patients were categorized as performance status (PS) 0 according to the Eastern Cooperative Oncology Group. Preoperatively, 36 patients (3.8%) had fever at diagnosis and C-reactive protein (CRP) levels were increased in 117 patients (12.5%). The median tumor size was 39.5 mm (IQR, 25.0–51.0), and according to pT category, pT1a, pT1b, and pT2 occurred in 557 (59.4%), 292 (31.2%), and 88 (9.4%) patients, respectively. With regard to histological type, 712 patients (76.0%) were diagnosed with clear cell type. Of 937 patients, 45 patients (4.8%) died of RCC and 615 patients (34.4%) died of other causes during the median 95 months (IQR 38–150) of follow-up.
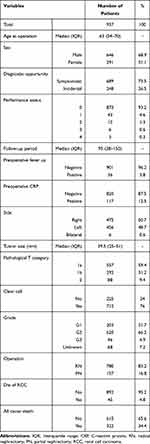 |
Table 1 Clinicopathological Information of Patients with Renal Cell Carcinoma |
Prognostic Factors for CSS and OS
Prognostic factors for CSS and OS were explored and a cut-off value for age (patients aged <60 or ≥60) was determined by ROC curve analysis. In the univariate analysis for CSS (Table 2), age ≥60, symptomatic disease, PS ≥1, fever, CRP elevation, tumor size ≥40 mm, and RN were identified as potential poor prognostic factors for CSS after RN or PN. In the multivariate analysis, PS ≥1 and tumor size ≥40 mm were identified as independent poor prognostic factors for CSS after RN or PN (PS ≥1: hazard ratio [HR] 3.31, 95% confidence interval [CI] 1.45–7.59; pT1b: HR 2.63, 95% CI 1.28–5.40; and pT2: HR 3.17, 95% CI 1.28–7.84, respectively). As for OS, in the univariate analysis (Table 2), age ≥60, male sex, PS ≥1, fever, CRP elevation, pT1b, and RN were identified as potential poor prognostic factors for OS after RN or PN. Multivariate analysis revealed that age ≥60, male sex, PS ≥1, CRP elevation, pT1b, and RN were independent poor prognostic factors for OS after RN or PN (age ≥60: HR 1.90, 95% CI 1.49–2.41; male sex: HR 1.45, 95% CI 1.13–1.85; PS ≥1: HR 2.44, 95% CI 1.72–3.48; CRP elevation: HR 1.89, 95% CI 1.37–2.63; pT1b: HR 1.32, 95% CI 1.03–1.68; and RN: HR 1.57, 95% CI 1.10–2.25). Notably, the type of nephrectomy was not significantly associated with CSS but was associated with OS. Patients who underwent RN for RCC ≤pT2N0M0 had a higher risk of mortality compared to those who underwent PN.
 |
Table 2 Cox Regression Analysis of Predictive Factors |
Comparison Between PN and RN Patients
We compared 157 patients who underwent PN with 780 patients who underwent RN. Table 3 shows the comparison of clinicopathological characteristics. The median tumor size was significantly smaller in the PN (22.0 mm [IQR, 18.0–30.0] vs 40.0 mm [IQR, 30.0–57.0]) (P<0.0001). There were significantly more patients with fever and CRP elevation at diagnosis in the RN (P=0.020 and P<0.0001, respectively).
 |
Table 3 Comparison of Patient’s Characteristics Between RN Group and PN Group |
Comparison of Actual Survival with Life Expectancy in the PN and RN Group
CSS was significantly longer in patients who underwent PN. The actual CSS was significantly longer than the life expectancy in both groups (Figure 1A; P<0.0001 and Figure 1B; P<0.0001, respectively), indicating that the control of RCC after surgery was acceptable. On the other hand, there was no significant difference between actual OS and life expectancy in the PN group (Figure 1C; P=0.11), whereas actual OS was significantly shorter than life expectancy in the RN group (Figure 1D; P<0.0001). In patients with PS0, actual OS and life expectancy did not differ in the PN group (Figure 1E; P=0.15), while there was a significant difference between actual OS and life expectancy in the RN group (Figure 1F; P<0.0001). In patients with T1a tumors, actual OS and life expectancy did not differ in the PN group (Figure 1G; P=0.28), whereas there was a significant difference between actual OS and life expectancy in the RN group (Figure 1H; P<0.0001).
Subgroup Analysis of OS According to Remaining Life Expectancy
In patients with life expectancy <10 years, there was a significant difference between actual OS and life expectancy in both groups (Figure 2A; P=0.033 and Figure 2B; P=0.031, respectively). When the analysis was limited to patients with life expectancy <10 years and PS0, there was a significant difference between actual OS and life expectancy in both groups (Figure 2C; P=0.033 and Figure 2D; P=0.0043, respectively). On the other hand, in patients with life expectancy ≥10 years, their actual OS and life expectancy differed significantly in both groups (Figure 2E; P=0.036 and Figure 2F; P<0.0001), whereas when the analysis was limited to patients with life expectancy ≥10 years and PS0, there was no significant difference between OS and life expectancy in the PN group (Figure 2G; P=0.053), while there was a significant difference between OS and life expectancy in the RN group (Figure 2H; P<0.0001). These results suggest that PN should be selected regardless of whether life expectancy is under or over 10 years. Furthermore, with regards to the association of tumor size, in patients with life expectancy ≥10 years and pT1a, there was no significant difference between OS and life expectancy in the PN group, but there was a significant difference in the RN group (Figure 3A; P=0.12 and Figure 3B; P<0.0001, respectively). On the contrary, in patients with life expectancy ≥10 years and pT1b/2, there was a significant difference between actual OS and life expectancy in both groups (Figure 3C; P=0.030 and Figure 3D; P<0.0001, respectively).
Discussion
The present study shows that PS and pT stage were associated with CSS and an age of ≥60 years, male sex, PS ≥1, CRP elevation, pT1b, and RN were independent predictive factors for OS. In terms of life expectancy, patients who underwent RN had a high rate of mortality, but those who underwent PN had similar mortality compared to life expectancy. PN in patients with PS0 or pT1a showed OS advantages, therefore PN should be recommended for patients with life expectancy <10 years. Moreover, in such patients with life expectancy ≥10 years, RN should be avoided because of a potential decrease in life expectancy.
The present study, which comprised a cohort of patients with pT1-2 RCC treated over three decades, suggested that PN might have an important role in improving actual OS, and notably, PS, tumor size, and life expectancy are crucial factors to consider when choosing surgical procedures.
Despite advancements in surgical tools and techniques, it remains difficult to eliminate surgical complications completely, and the EORTC reported that the risk of surgical complications in PN was higher than in RN.5 However, the preservation of remnant renal function (RRF) after PN is reported to be superior to that of RN, leading urologists to pose the question whether patients who undergo PN have a low risk of mortality.7,10 Contrary to logical expectations, many papers suggest no significant difference in OS between PN and RN.2,3,6,8,9 Moreover, although PN substantially reduces the incidence of eGFR <60, the incidence of eGFR <30 was similar in both PN and RN, and the incidence of eGFR <15 was nearly identical and therefore the advantage of PN in RRF did not contribute to improved survival.11 In this study, although PN patients had a low risk of mortality, the underlying reason is unclear. Renal function or comorbidities were not analyzed because of lack of data, but we found that patients with PS0 who underwent RN had a high risk of mortality (Figures S1 and S2). Our previous report showed that PN, which was carried out with a microwave tissue coagulator (MTC) without hilar clamping, was not associated with reduced RRF.12 In this study, all PNs were also carried out with MTC without hilar clamping regardless of laparoscopic or open surgery. It remains difficult to understand why temporal clamping affects long-term mortality in patients with localized RCC.
Considering that PS and comorbidities are obviously important in selecting surgical methods, there may be selection bias. Aziz et al suggested that young patients more frequently underwent PN than older patients.13 In this study, there was no significant difference in the age at surgery or life expectancy in the PN and RN groups and PN showed improved survival. In patients with PS0, PN had better survival compared to RN and there was no significant difference between actual OS and life expectancy in PN patients. Furthermore, even in patients with PS0 and life expectancy ≥10 years, PN showed better survival than RN and actual OS and life expectancy differed in the PN group, but it did not reach statistical significance. Therefore, the importance of considering PS is reaffirmed and PN should be considered for patients with PS0 and life expectancy ≥10 years when technically feasible.
In this study, we stratified patients by life expectancy because the implications of aging are different depending on sex. Daskivich et al showed that in patients with life expectancy <10 years, T1a RCC was treated with RN in 61% and PN in 24% of patients. They concluded that life expectancy should be incorporated into treatment decision-making in early-stage RCC.14 The present study suggested that PN patients with life expectancy <10 years tended to have OS as long as life expectancy, especially patients with life expectancy <10 years and PS0. Although our results do not justify the recommendation of PN in patients with life expectancy <10 years, PN may be recommended if the patients are PS0. On the other hand, PN had survival benefits for patients with life expectancy ≥10 years in this study as expected, especially in patients with life expectancy ≥10 years and PS0. In this study, renal function was not evaluated and the reason for the survival advantages of PN remains unclear. However, the present study suggested that PN plays a role in extending survival up to life expectancy.
With regard to the association between life expectancy and tumor size, PN had a low risk of mortality compared to RN in patients with life expectancy ≥10 years and pT1a, and patients who underwent PN tended to survive up to their life expectancy. On the other hand, PN was not associated with a survival advantage in patients with life expectancy ≥10 years and pT1b/2 compared to RN. Woldu et al showed that patients with CKD stage-II benefited from PN in terms of preserving renal function.10 Therefore, PN for localized RCC and tumor size >40 mm should be carried out preoperatively for patients with poor renal function and/or only one kidney. Advancement in surgical technologies, such as robotic surgery, should facilitate challenging PN, but further evidence is needed to aid surgical decision-making.
The present study had some limitations including its retrospective nature, intergroup differences in patient characteristics, potential differences in life expectancy in different countries, a lack of renal function data, and insufficient information on clinicopathological characteristics, such as comorbidities, perioperative complications, surgical techniques, and tumor grade. Furthermore, there was a big difference in the number of patients between RN group and PN group. Careful interpretation is necessary, and further research is warranted to support our findings. In the future, we plan to increase the number of patients, extend the follow-up period, and analyze again. Evidence-based recommendations for appropriate surgical decision-making are essential for improved management of patients with localized RCC.
Conclusion
Our findings suggested that PN has an important role for patients with PS0 or pT1a and that life expectancy should be considered in surgical decision-making. An understanding of this information could lead to improved treatment strategies and improved OS in patients with localized RCC. Further research is needed to establish appropriate treatment strategies and criteria to support current clinical practice.
Acknowledgments
The authors would like to thank all patients who participated in this study for their important contributions. We also wish to thank Mariko Yoshimura (Department of Urology, Nara Medical University, Nara, Japan) for invaluable help with obtaining and summarizing the data used in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.21349
2. Gershman B, Thompson RH, Boorjian SA, et al. Radical versus partial nephrectomy for cT1 renal cell carcinoma. Eur Urol. 2018;74(6):825–832. doi:10.1016/j.eururo.2018.08.028
3. Simone G, Tuderti G, Anceschi U, et al. Oncological outcomes of minimally invasive partial versus minimally invasive radical nephrectomy for cT1-2/N0/M0 clear cell renal cell carcinoma: a propensity score-matched analysis. World J Urol. 2017;35(5):789–794. doi:10.1007/s00345-016-1923-2
4. Takagi T, Kondo T, Iizuka J, et al. Comparison of survival rates in stage 1 renal cell carcinoma between partial nephrectomy and radical nephrectomy patients according to age distribution: a propensity score matching study. BJU Int. 2016;117(6B):E52–9. doi:10.1111/bju.13200
5. Van Poppel H, Da Pozzo L, Albrecht W, et al.; European Organization for Research and Treatment of Cancer (EORTC); National Cancer Institute of Canada Clinical Trials Group (NCIC CTG); Southwest Oncology Group (SWOG); Eastern Cooperative Oncology Group (ECOG). A prospective randomized EORTC intergroup Phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2007;51(6):1606–1615. doi:10.1016/j.eururo.2006.11.013
6. Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. 2012;188(1):51–57. doi:10.1016/j.juro.2012.03.006
7. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi:10.1056/NEJMoa041031
8. Shuch B, Hanley J, Lai J, et al.; Urologic Diseases in America Project. Overall survival advantage with partial nephrectomy: a bias of observational data? Cancer. 2013;119(16):2981–2989. doi:10.1002/cncr.28141
9. Wang Z, Wang G, Xia Q, et al. Partial nephrectomy vs. radical nephrectomy for renal tumors: a meta-analysis of renal function and cardiovascular outcomes. Urol Oncol. 2016;34(12):
10. Woldu SL, Weinberg AC, Korets R, et al. Who really benefits from nephron-sparing surgery? Urology. 2014;84(4):860–867. doi:10.1016/j.urology.2014.05.061
11. Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65(2):372–377. doi:10.1016/j.eururo.2013.06.044
12. Inoue T, Nakai Y, Miyake M, et al. Non-ischemic, non-renorrhaphy laparoscopic partial nephrectomy using a microwave tissue coagulator for small renal tumors. J Microw Surg. 2016;34:1–6. doi:10.3380/jmicrowavesurg.34.1
13. Aziz A, May M, Zigeuner R, et al.; Members of the CORONA Project and the Young Academic Urologists Renal Cancer Group. Do young patients with renal cell carcinoma feature a distinct outcome after surgery? A comparative analysis of patient age based on the multinational CORONA database. J Urol. 2014;191(2):310–315. doi:10.1016/j.juro.2013.08.021
14. Daskivich TJ, Tan HJ, Litwin MS, Hu JC. Life expectancy and variation in treatment for early stage kidney cancer. J Urol. 2016;196(3):672–677. doi:10.1016/j.juro.2016.03.133
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



