Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Impact of Oscillating Positive Expiratory Pressure Device Use on Post-Discharge Hospitalizations: A Retrospective Cohort Study Comparing Patients with COPD or Chronic Bronchitis Using the Aerobika® and Acapella® Devices
Authors Tse J, Wada K, Wang Y, Coppolo D, Kushnarev V , Suggett J
Received 8 May 2020
Accepted for publication 22 September 2020
Published 19 October 2020 Volume 2020:15 Pages 2527—2538
DOI https://doi.org/10.2147/COPD.S256866
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Jenny Tse,1 Keiko Wada,1 Yi Wang,1 Dominic Coppolo,2 Vladimir Kushnarev,3 Jason Suggett3
1IQVIA, Medical and Scientific Services, Real World Solutions, Cambridge, MA, USA; 2Monaghan Medical Corporation, Clinical Strategy and Development, Plattsburgh, NY, USA; 3Trudell Medical International, Science and Technology, London, ON, Canada
Correspondence: Jenny Tse
IQVIA, 201 Broadway, 5th Fl, Cambridge, MA 02139, USA
Tel +1 617-583-0627
Email [email protected]
Purpose: Managing and preventing disease exacerbations are key goals of COPD care. Oscillating positive expiratory pressure (OPEP) devices have been shown to improve clinical outcomes when added to COPD standard of care. This retrospective database study compared real-world resource use and disease exacerbation among patients with COPD or chronic bronchitis prescribed either of two commonly used OPEP devices.
Patients and methods: Patients using the Aerobika® (Trudell Medical International, London, ON, Canada) or Acapella® (Smiths Medical, Wampsville, New York, USA) OPEP device for COPD or chronic bronchitis were identified from hospital claims linked to medical and prescription claims between September 2013 and April 2018; the index date was the first hospital visit with an OPEP device. Severe disease exacerbation, defined as an inpatient visit with a COPD or chronic bronchitis diagnosis, and all-cause healthcare resource utilization over 30 days and 12 months post-discharge were compared in propensity score (PS)-matched Aerobika device and Acapella device users.
Results: In total, 619 Aerobika device and 1857 Acapella device users remained after PS matching. After discharge from the index visit, Aerobika device users were less likely to have ≥ 1 severe exacerbation within 30 days (12.0% vs 17.4%, p=0.01) and/or 12 months (39.6% vs 45.3%, p=0.01) and had fewer 12-month severe exacerbations (mean, 0.7 vs 0.9 per patient per year, p=0.01), with significantly longer time to first severe exacerbation than Acapella users (log-rank p=0.01). Aerobika device users were also less likely to have ≥ 1 all-cause inpatient visit within 30 days (13.9% vs 20.3%, p< 0.001) and 12 months (44.9% vs 51.8%, p=0.003) than Acapella users.
Conclusion: Patients receiving the Aerobika OPEP device, compared to the Acapella device, had lower rates of subsequent severe disease exacerbation and all-cause inpatient admission. This suggests that Aerobika OPEP device may be a beneficial add-on to usual care and that OPEP devices may vary in clinical effectiveness.
Keywords: database, exacerbations, re-hospitalization, sputum clearance, OPEP
Introduction
Chronic obstructive pulmonary disease (COPD) is a major source of morbidity and affects 6% of adults over the age of 40 and 10% of adults 65 years or older in the United States.1 The clinical course of COPD is punctuated by exacerbations, characterized by acute worsening of symptoms such as increased sputum production, airway inflammation, and breathlessness that require additional treatment.2,3 Severe cases require emergency department (ED) visits and/or hospitalizations.3 COPD exacerbations are common but often underreported, since as many as half of patients experiencing an exacerbation may not seek medical attention.4 One community-based study found that COPD patients had 1.5 disease exacerbations per year based on clinic visits, but 2.7 exacerbations per patient per year after including symptom patterns recorded in daily diary cards.4 COPD exacerbations are associated with long-term implications, including worsened quality of life,4,5 faster decline in lung function,6 and increased risk of all-cause mortality.7,8 The burden of COPD exacerbations becomes even greater among patients with longer recovery times9 and those with frequent exacerbations.6 Previous exacerbations are the most important predictor of future ones,10,11 and about one in five COPD patients hospitalized for an exacerbation will require re-hospitalization within 30 days of discharge.12 Thus, preventing disease exacerbations and appropriately treating exacerbations to minimize their consequences are key goals of COPD management.3,13,14
Current treatment guidelines recommend rescue inhalers, corticosteroids, and antibiotics for the treatment of severe exacerbations and maintenance medications for the management of COPD when stable.3,13,14 Oscillatory positive expiratory pressure (OPEP) devices, such as the Aerobika® device (Trudell Medical International, London, Ontario, Canada) and Acapella® (Smiths Medical, Wampsville, New York, USA), are non-pharmacological interventions that ease sputum clearance through positive pressure to hold airways open combined with airway oscillations that thin mucus15–17 and have low safety risks compared to pharmacologic therapies.18 Clinical evidence suggests that some OPEP devices not only help clear mucus but also improve short-term lung capacity, exercise capacity, and quality of life in patients with stable COPD17,19,20 and cystic fibrosis.21
While adjunct therapy with OPEP devices can potentially provide additional benefit to the management of COPD, evidence from large clinical trials or real-world studies on the benefit of these devices in relation to COPD disease exacerbations is limited. One database study reported that the Aerobika OPEP device reduces 30-day exacerbations by nearly 30% compared to no positive expiratory pressure (PEP) or OPEP device use following a hospital visit,22 and a clinical trial reported that patients who used an adjunct Acapella device had a 1-day reduction of hospital length of stay for acute exacerbation compared to those who received physician’s choice of standard COPD management.23 Moreover, there are functional differences among a few existing OPEP devices, as revealed by several laboratory studies using simulated lung models, which may impact clinical efficacy. In particular, the Aerobika OPEP device has been shown to have the most consistent pressure amplitude, highest mean pressure amplitude at higher resistance settings,24,25 and highest total pressure pulse impact26 compared to Acapella and other OPEP devices. However, clinical studies have been limited to comparisons of the efficacy of adjunct OPEP devices to usual care in COPD, rather than head-to-head comparisons.18 To that end, this real-world study aimed to compare healthcare resource use (HRU) and severe disease exacerbations 30 days and 12 months post-discharge in patients with COPD or chronic bronchitis, a subset of COPD characterized by mucus hypersecretion,27 treated with two of the most commonly used OPEP devices in the hospital setting.
Patients and Methods
Data Sources
This retrospective cohort study used IQVIA’s Hospital Charge Detail Master (CDM) hospital database from September 1, 2012 to April 30, 2019 (study period) linked to professional medical claims (Dx) and longitudinal prescription claims (LRx) databases (IQVIA, Inc., Durham, NC, USA). The CDM database includes service order records from over 650 hospitals, covering 7 million annual inpatient stays and 60 million annual outpatient visits. Patient-level data include healthcare services from hospital departments (inpatient, outpatient, ED, pharmacy), with detailed drug, procedure, and diagnosis codes from each encounter for the entire stay; each visit has both an admission date and discharge date. The Dx database captures over 1 billion pre-adjudicated claims and 3 billion records obtained annually from approximately 800,000 office-based physicians and specialists (75% of American Medical Association providers captured). Medical claims from ambulatory and general health care sites (ie, outpatient clinics associated with hospitals such as rehabilitation, same-day surgery, chemotherapy centers) are also included. The LRx database captures information on dispensed prescriptions with 92% coverage of prescriptions from the retail channel, 72% coverage of standard mail service, and 76% coverage of long-term care facilities. The study dataset was created based on Health Insurance Portability and Accountability Act (HIPAA)-compliant linking processes using IQVIA’s patented and proprietary encryption algorithm.28–30 As this retrospective cohort analysis was conducted using de-identified HIPAA-compliant data, Institutional Review Board (IRB) review was not required for this study.
Study Population
Figure 1 describes the patient selection criteria in detail. Patients with any evidence of OPEP device use (based on billing descriptions in CDM) during the patient selection window (September 1, 2013 to April 30, 2018) were identified; the index date was the admission date on the first CDM record with the OPEP device (index hospital visit). Adult patients were required to have at least one diagnosis of COPD or chronic bronchitis in any position (ie, not limited to diagnosis codes in the primary position) in CDM during the index visit (International Classification of Diseases Ninth Revision Clinical Modification [ICD-9-CM]: 491.XX and 496, ICD-10-CM: J41.X and J44.X, not including emphysema given that it is not characterized by sputum production3). As a proxy of continuous enrollment, patient eligibility as well as pharmacy stability in LRx and provider stability in Dx in the 12-month pre-index (ie, baseline) and post-index (ie, follow-up) periods were required. Patients were excluded if they had evidence of the index OPEP device in the baseline period, non-index OPEP devices in the follow-up period (including the index date), diagnosis of asthma on or before the index date, OPEP device use in a post-operative setting (identified with a surgical procedure of interest 30 days before the index visit admission date or during the index visit), or incomplete data.
 |
Figure 1 Patient Attrition.Notes: COPD and chronic bronchitis diagnoses were identified using ICD-9 codes 491.XX and 496 and ICD-10 codes J41.X and J44.X, not including emphysema given that it is not characterized by sputum production.3 Pharmacy and provider stability was used as a proxy for continuous enrollment, which was defined as stability of reporting claims in every month for at least one pharmacy in LRx or provider in Dx as well as at least one LRx claim for any medication and one Dx claim in the baseline period and the 12-month period after the index date. There were no patients excluded for incomplete data.Abbreviations: CDM, Hospital Charge Detail Master database; Dx, medical claims database; LRx, prescription claims database; OPEP, oscillating positive expiratory pressure. |
Patients who met all inclusion and exclusion criteria were stratified into two mutually exclusive study cohorts based on the OPEP device used on the index date: patients treated with Aerobika OPEP device (Aerobika device cohort) or Acapella OPEP device (Acapella cohort); patients using other OPEP devices were not included in the analysis due to a possibility of misclassification when using hospital billing descriptions. The cohorts were then matched using propensity score (PS) matching at a 1:3 ratio with a greedy nearest-neighbor matching algorithm without replacement. The logistic regression model to generate the PS included age, sex, payer type, comorbid conditions, COPD-related medication use, and history of exacerbations (Tables 1 and 2 list these variables in detail). Index year and geographic region were excluded from the PS model to limit misclassification of the index OPEP device and to maximize cohort sample sizes after matching.
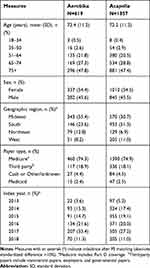 |
Table 1 Baseline Demographics in the Matched Cohorts |
 |
Table 2 Baseline Clinical Characteristics and HRU in the Matched Cohorts |
Study Measures
The baseline demographics and clinical characteristics, including history of severe and moderate disease exacerbations, were evaluated during the fixed 12-month baseline period. A severe disease exacerbation was defined as an inpatient visit with COPD or chronic bronchitis diagnosis in any position and moderate disease exacerbation was defined as an ED visit with COPD or chronic bronchitis diagnosis or prescription of an oral corticosteroid (OCS) within 14 days of an office visit with a COPD or chronic bronchitis diagnosis in any position (that did not lead to an inpatient visit).11,22,31
The follow-up measures included index visit characteristics and clinical and HRU outcomes among the matched cohorts. The index measures, evaluated from the admission date to the discharge date, were the initial care setting, length of stay, COPD-related medications and respiratory support. The follow-up measures, evaluated from the day after the discharge date to the end of 30-day post-discharge or 12-month follow-up, were severe exacerbations, moderate exacerbations, and all-cause HRU. Time following the index visit discharge date until the end of the 12-month follow-up period was also reported for each cohort as it varied depending on the duration of the index hospital visit (all patients had 12-month follow-up data following the index date [ie, the admission date on the index hospital record]); therefore, when reporting 12-month outcomes, counts for HRU categories were standardized and reported as per patient per year (PPPY).
Statistical Analyses
Baseline patient characteristics between the cohorts before and after PS matching were assessed using standardized difference, applying a commonly used threshold of >10% in absolute standardized difference to determine imbalance.32 Generalized estimating equations for generalized linear models without additional covariate adjustment were used to compare continuous and categorical outcome measures, respectively, between the PS-matched cohorts. Kaplan-Meier survival analysis with log-rank test was also used to compare time from index visit discharge to first severe disease exacerbation between the PS-matched cohorts. Generalized estimating equations for generalized linear models were used to estimate the association between index OPEP device and the number of severe disease exacerbations PPPY (with normal distribution and log link function) and between index OPEP device and the odds of experiencing at least one 12-month severe disease exacerbation (with binomial distribution and logit link function); the models additionally adjusted for the baseline and index patient characteristics not included in PS construction, such as patient geographic region, index visit type, and OCS use during the index visit. P-values <0.05 were considered statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Patient Characteristics
In total, 5029 patients met the inclusion criteria (662 Aerobika device and 4367 Acapella users; Figure 1). After 1:3 PS matching, the remaining study population consisted of 619 patients receiving the Aerobika device matched to 1857 patients receiving the Acapella device.
In the cohorts before PS matching, the baseline patient demographic and clinical characteristics were generally balanced. The cohorts were similar in terms of age (mean age, 72.5 years and 71.8 years for Aerobika device cohort and Acapella cohort, respectively), payer type (74.3% and 72.8% had Medicare), and Charlson Comorbidity Index (CCI) score (mean, 3.7 and 3.8; all absolute standardized differences ≤10%). Further details of the cohorts before matching are described in Supplemental Tables 1 and 2.
After matching, all baseline characteristics of the Aerobika device and Acapella users used in the PS model were well balanced. Patients in both cohorts were 72 years old on average, and more than half of the patients (54–55%) were female (Table 1). Geographic region and index year distribution (not included in the PS model) differed between the matched cohorts, with the majority of the Aerobika device cohort (55.4%) residing in Midwestern states and the majority of the Acapella cohort residing in Southern states (51.3%). All baseline clinical characteristics and HRU were well balanced after PS matching. The mean CCI score33 was 3.7, and the most common comorbidities of interest were hyperlipidemia (56.7% of the Aerobika device cohort and 57.3% of the Acapella cohort), cardiovascular disease (44.1% and 43.2%), and anxiety/depression (35.9% and 35.1%) (Table 2). During the baseline period, about half of patients in each cohort experienced at least one severe (45.4% of the Aerobika device cohort and 44.5% of the Acapella cohort) or moderate (46.0% and 45.7%) disease exacerbation. About one-fourth of patients also had oxygen therapy in the baseline period (29.4% of the Aerobika device cohort and 26.6% of the Acapella cohort).
Index Visit Characteristics
Most patients in both cohorts visited the ED first as part of the index visit (82.4% vs 72.5% of matched Aerobika device and Acapella users, respectively, p<0.001); the remaining patients were admitted to inpatient directly (13.2% vs 23.2%, p<0.001) or visited an outpatient department (4.4% and 4.3%, p=0.95) (Figure 2A). Nearly all patients, regardless of initial care setting during the index visit, eventually had an inpatient admission during the index visit (91.8% [n=568] of Aerobika device users and 94.9% [n=1762] of Acapella users; p=0.004) (Figure 2B). Among those with an inpatient stay, the length of stay was a week on average (mean days, 7.0 for the Aerobika device users vs 7.5 days for Acapella users, p=0.09).
There were some differences in disease- and exacerbation-related treatments received during patients’ inpatient stays. For example, the Aerobika device users had lower use of SABA (44.5% vs 53.5%), antibiotics (83.1% vs 88.1%), OCS (29.9% vs 50.8%), and LAMA (11.4% vs 20.4%), but higher use of LABA (10.9% vs 5.7%) and oxygen therapy (93.0% vs 74.0%; all p-values<0.05) (Figure 3).
Disease Exacerbations Post-Discharge
The duration of the “12-month post-discharge” period, from the day after the index visit discharge date to the end of the 12-month follow-up period, was on average 354.5 (standard deviation, 5.5) days for the Aerobika device users and 353.9 (5.2) days for the Acapella users (p=0.01). The proportion of patients who experienced at least one severe exacerbation post-discharge was lower for Aerobika device users compared to Acapella users (12.0% vs 17.4% for 30-day post-discharge [ie, early disease-related inpatient readmission], p=0.001; 39.6% vs 45.3% for 12-month post-discharge; p=0.01) (Table 3). Aerobika device users also had fewer 12-month severe exacerbations compared to Acapella users (0.7 vs 0.9 exacerbations PPPY, p=0.01) and shorter lengths of stay (mean, 6.5 vs 7.1 days, p=0.05). There was no significant difference in the proportion of patients with moderate exacerbations over 30 days post-discharge (12.3% for Aerobika device users vs 13.2% for Acapella users, p=0.58) or 12 months post-discharge (41.0% vs 41.0%, p=0.98), but Aerobika device users had fewer 12-month moderate exacerbations compared to Acapella users (1.0 vs 1.2 exacerbations PPPY, p=0.03) (Table 3).
 |
Table 3 Post-Discharge Severe Exacerbations and All-Cause Post-Discharge HRU in the 12-Month Follow-Up Period in the Matched Cohorts |
The significant findings for differences in severe exacerbations persisted in regression models which adjusted for a few additional baseline and index visit characteristics (see Table 4 for these variables). Compared to Acapella users, Aerobika device users had 17% fewer severe exacerbations PPPY (estimate, 0.83; 95% CI, 0.71–0.96) (Table 4) and had 20% lower odds of 12-month severe disease exacerbation (odds ratio, 0.80; 95% CI, 0.66–0.98) (Table 5). Index hospital visit being inpatient (whether admitted directly or from ED) compared to ED or outpatient hospital visit without inpatient admission and OCS use during the index visit were also significant predictors of increased number of 12-month severe disease exacerbations PPPY (Table 4) and having at least one post-discharge severe disease exacerbation (Table 5).
 |
Table 4 Adjusted Modela for the Association Between Index OPEP Device and Number of Post-Discharge Severe Exacerbations PPPY |
 |
Table 5 Adjusted Modela for the Association Between Index OPEP Device and the Odds of Post-Discharge Severe Exacerbations |
Furthermore, time from the index visit discharge date to the first severe exacerbation was similar between Aerobika device users and matched Acapella users (102.0 vs 96.6 days, p=0.44). When accounting for the duration of follow-up in Kaplan-Meier survival analysis, Aerobika device users had significantly longer times to severe disease exacerbation compared to Acapella users (log-rank p=0.01) (Figure 4).
 |
Figure 4 Kaplan-Meier analysis of time from index visit discharge date to first post-discharge severe exacerbation in the matched cohorts. |
All-Cause Post-Discharge HRU
Those who received the Aerobika device during the index visit were less likely to have at least one early (within 30 days post-discharge) all-cause inpatient readmission (13.9% for Aerobika device cohort vs 20.3% for Acapella cohort, p<0.001) and at least one all-cause inpatient admission 12 months post-discharge (44.9% vs 51.8%, p=0.003). Aerobika device users also had fewer all-cause 12-month inpatient visits (mean PPPY, 0.9 vs 1.1, p=0.003); the mean length of stay was 8 days for both cohorts (p=0.28). There was no significant difference in all-cause ED visits or outpatient visits between the cohorts (Table 3).
Discussion
To our knowledge, this is the first study to compare the impact of two commonly used OPEP devices on short- and long-term clinical and resource use outcomes in patients with COPD or chronic bronchitis. In the cohorts both before and after matching, patients with the Aerobika device or Acapella device were typically elderly (about half were 75 years of age or older), were predominantly female, had multiple Charlson comorbidities, and had a history of at least one severe or moderate disease exacerbation during the baseline period. After matching to ensure outcomes were compared in similar patient populations, we observed that during the index visit with OPEP device use and COPD or chronic bronchitis diagnosis, more than 90% of patients had an inpatient admission, consistent with the definition of a severe disease exacerbation used in this study. Following the index visit, those who received the Aerobika device had significantly lower rates of all-cause hospitalization and severe disease exacerbation within 30 days of the index visit discharge date, with sustained differences over 12 months of follow-up, and fewer moderate exacerbations over 12 months of follow-up. Our findings are supported by previous, albeit limited, research suggesting the clinical benefits of the Aerobika OPEP device in the treatment of COPD and differences in efficacy across different OPEP devices. In a small cross-over study, 27 COPD patients who used the Aerobika OPEP device daily for 4 weeks had subsequent improvements in Patient Evaluation Questionnaire (PEQ)-ease-bringing-up-sputum score and forced expiratory volume in 1 s (FEV1) related to improved ventilation.17 These improvements in airway clearance may also improve disease exacerbation outcomes demonstrated in a previous database study by Burudpakdee et al,22 where Aerobika device users had significantly lower rates of 30-day severe disease exacerbations (13.8%, consistent with the 12.0% reported in our study) compared to matched controls who did not receive an OPEP device (vs 19.0%). To our knowledge, the only study of Acapella effectiveness in treating acute COPD exacerbations was a clinical trial comparing patients who received an adjunct Acapella device (PEP or OPEP) to matched retrospective controls who received standard of care alone; this study found that patients who used Acapella had a 1-day reduction in the hospital length of stay.23
Although OPEP devices share the general objective of opening airways and using airway oscillations to loosen mucus, the device mechanisms and consequently the pressure pulse waveforms that they generate differ, and several studies have reported better performance characteristics related to airway clearance for the Aerobika device compared to Acapella.24–26 Therefore, the present study provides new evidence on how functional differences between the devices may translate to differences in preventing severe disease exacerbations, illustrated by the lower rate of subsequent inpatient admissions due to severe disease exacerbation in Aerobika device users compared to Acapella users in the PS-matched comparative analyses and further confirmed in adjusted analyses, as well as preventing moderate exacerbations and all-cause hospitalizations that may be due to worsening of overall quality of life4,5 and worsening comorbid conditions (eg, hospitalizations due to cardiac events34). Considering previous COPD exacerbations are the most important predictor of future events,10,11 this study demonstrates that the Aerobika device may mitigate the clinical and economic impact of exacerbations when used as an adjunct therapy in a population known to have a high economic burden.35 Aside from functional differences, high patient satisfaction with the Aerobika device and patient-reported ease of use, as revealed in a survey of Aerobika device users,36 may support real-world adherence to the Aerobika OPEP device and contribute to improved outcomes. However, considering the lack of published data, future research comparing adjunct OPEP device use to standard of care, to other commercially available OPEP devices, and to PEP devices is needed to provide guidance on how to incorporate OPEP devices into clinical practice.
The strengths of this study are the use of three linked databases to obtain a comprehensive view of HRU and treatments before and after the initial visit with OPEP device use and the statistical methodology to limit bias in the results. PS methods were used to mimic the selection process of clinical trials and create comparable cohorts of Aerobika device and Acapella device users for evaluating outcomes.37 Then, our findings that Aerobika device users were less likely to have a severe disease exacerbation over 12 months of follow-up compared to Acapella users persisted after adjusting for geographic region and characteristics of the index visit in regression models, which added to the internal validity of the findings.
With that said, a few limitations should be noted. First, patients with COPD and chronic bronchitis were identified using ICD diagnosis codes, and confirmation of diagnosis would require clinical measures not available in claims databases. To address this limitation, we required a diagnosis during the hospital visit with OPEP device therapy and excluded those with evidence of asthma or surgical procedure for which the OPEP device could also be used. Second, data on disease severity were not available, which would provide context on the generalizability of the study population. For example, some physicians may selectively prescribe OPEP devices to patients with a history of frequent severe exacerbations,38 but severe patients at risk of post-discharge mortality may be underrepresented, given our study requirement of 12 months of follow-up data. Thirdly, it is not known from the claims data who administered the OPEP device, whether patients used the OPEP devices with the proper techniques or with the appropriate frequency, if the devices were used with nebulizers, or what other physiotherapy treatments (eg, breathing programs) may have been administered, which could impact device efficacy and outcomes. Lastly, the claims data are not able to identify factors leading to OPEP device use such as potential regional and healthcare facility-level differences in practice patterns that may lead to residual confounding.
Conclusion
This study demonstrates that the Aerobika OPEP device significantly reduces all-cause inpatient visits and severe disease exacerbations, including 30-day inpatient readmissions and 12-month inpatient visits, compared to Acapella, when added to standard of care among patients with COPD and chronic bronchitis. Combined with previous clinical and real-world data, these findings further support the use of the Aerobika OPEP device as an add-on to usual care for the treatment of severe COPD exacerbations and highlights the benefit of the Aerobika device versus an alternative OPEP device.
Funding
This study was funded by Monaghan Medical Corporation and Trudell Medical International.
Disclosure
JT, KW, YW are employees of IQVIA, which received funding from Monaghan Medical Corporation and Trudell Medical International to conduct this study. DC is an employee of Monaghan Medical Corporation. VK and JS are employees of Trudell Medical International. DC, VK, JS are all employed within a Research/Medical capacity. The authors report no other conflicts of interest in this work.
References
1. Biener AI, Decker SL, Rohde F. Prevalence and treatment of Chronic Obstructive Pulmonary Disease (COPD) in the United States. JAMA. 2019;322(7):602. doi:10.1001/jama.2019.10241
2. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi:10.7326/0003-4819-106-2-196
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
4. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi:10.1164/ajrccm.157.5.9709032
5. Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387–395. doi:10.1136/thx.2003.008730
6. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847
7. Johannesdottir SA, Christiansen CF, Johansen MB, et al. Hospitalization with acute exacerbation of chronic obstructive pulmonary disease and associated health resource utilization: a population-based Danish cohort study. J Med Econ. 2013;16(7):897–906. doi:10.3111/13696998.2013.800525
8. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
9. Donaldson GC, Law M, Kowlessar B, et al. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):943–950. doi:10.1164/rccm.201412-2269OC
10. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
11. Abudagga A, Sun SX, Tan H, Solem CT. Exacerbations among chronic bronchitis patients treated with maintenance medications from a US managed care population: an administrative claims data analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:175–185. doi:10.2147/COPD.S40437
12. Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916–926. doi:10.1016/j.chest.2016.05.002
13. Wedzicha JAE-C-C, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791. doi:10.1183/13993003.00791-2016
14. Nice Guideline Updates Team. National institute for health and care excellence: clinical guidelines. In: Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management. London: National Institute for Health and Care Excellence (UK); 2018.
15. Osadnik CR, McDonald CF, Jones AP, Holland AE. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(3):Cd008328.
16. Volsko TA, DiFiore J, Chatburn RL. Performance comparison of two oscillating positive expiratory pressure devices: acapella versus flutter. Respir Care. 2003;48(2):124–130.
17. Svenningsen S, Paulin GA, Sheikh K, et al. Oscillatory positive expiratory pressure in chronic obstructive pulmonary disease. COPD. 2016;13(1):66–74. doi:10.3109/15412555.2015.1043523
18. Bourbeau J, McIvor RA, Devlin HM, Kaplan A. Oscillating positive expiratory pressure (OPEP) device therapy in Canadian respiratory disease management: review, care gaps and suggestion for use. Can Respir Crit Care Sleep Med. 2019;3(4):233–240. doi:10.1080/24745332.2018.1558426
19. Wolkove N, Kamel H, Rotaple M, Baltzan MA
20. Suggett J. Quality of life (QOL) responder rate analysis following use of an oscillating positive expiratory pressure (OPEP) device for chronic obstructive pulmonary disease (COPD): SGRQV CAT assessments. Chronic Obstr Pulm Dis. 2017;4(3):237.
21. West K, Wallen M, Follett J. Acapella vs. PEP mask therapy: a randomised trial in children with cystic fibrosis during respiratory exacerbation. Physiother Theory Pract. 2010;26(3):143–149. doi:10.3109/09593980903015268
22. Burudpakdee C, Seetasith A, Dunne P, et al. A real-world study of 30-day exacerbation outcomes in Chronic Obstructive Pulmonary Disease (COPD) patients managed with Aerobika OPEP. Pulm Ther. 2017;3(1):163–171. doi:10.1007/s41030-017-0027-5
23. Milan S, Bondalapati P, Megally M, et al. Positive expiratory pressure therapy with and without oscillation and hospital length of stay for acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2553–2561.
24. Van Fleet H, Dunn DK, McNinch NL, Volsko TA. Evaluation of functional characteristics of 4 oscillatory positive pressure devices in a simulated cystic fibrosis model. Respir Care. 2017;62(4):451–458. doi:10.4187/respcare.04570
25. Poncin W, Reychler G, Liistro M, Liistro G. Comparison of 6 oscillatory positive expiratory pressure devices during active expiratory flow. Respir Care. 2019;65(4):492–499.
26. Suggett J, Meyer A. A laboratory assessment into the efficiency and effectiveness of different oscillating positive expiratory pressure devices by means of patient simulated expiratory waveforms. Chest. 2017;152(4):A970. doi:10.1016/j.chest.2017.08.1005
27. Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228–237. doi:10.1164/rccm.201210-1843CI
28. Ober NS, Grubmuller J, Farrell M, et al. System and Method for Generating De-Identified Health Care Data. Google Patents; 2004.
29. Ober NS, Grubmuller J, Farrell M, et al. System and Method for Generating De-Identified Health Care Data. Google Patents; 2008.
30. Zubeldia K, Romney GW Anonymously Linking a Plurality of Data Records. Google Patents; 2002.
31. Nazir SA, Erbland ML. Chronic obstructive pulmonary disease: an update on diagnosis and management issues in older adults. Drugs Aging. 2009;26(10):813–831.
32. Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84–S90.e81. doi:10.1016/j.jclinepi.2013.01.013
33. Roos LL, Stranc L, James RC, Li J. Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32(2):229–238.
34. Reilev M, Pottegård A, Lykkegaard J, Søndergaard J, Ingebrigtsen TS, Hallas J. Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology. 2019;24(12):1183–1190. doi:10.1111/resp.13620
35. Menzin J, Boulanger L, Marton J, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in a U.S. Medicare population. Respir Med. 2008;102(9):1248–1256.
36. Harkness H, Patrick C, Lefebvre J Survey of patients using an oscillating positive expiratory pressure device indicates improvement in well-being and compliance to therapy.
37. Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices for retrospective database analysis task force report–part III. Value Health. 2009;12(8):1062–1073.
38. Barker R, Laverty AA, Hopkinson NS. Adjuncts for sputum clearance in COPD: clinical consensus versus actual use. BMJ Open Respir Res. 2017;4(1):e000226. doi:10.1136/bmjresp-2017-000226
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


