Back to Journals » OncoTargets and Therapy » Volume 14
Immunotherapy-Related Cystitis: Case Report and Review of the Literature
Authors Zhu L, Wang Z , Stebbing J , Wang Z, Peng L
Received 27 May 2021
Accepted for publication 19 July 2021
Published 31 July 2021 Volume 2021:14 Pages 4321—4328
DOI https://doi.org/10.2147/OTT.S321965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Liping Zhu,1,* Zhiqiang Wang,2,* Justin Stebbing,3 Zibing Wang,4 Ling Peng5
1Department of Medical Oncology, Shouguang Hospital of Traditional Chinese Medicine, Shouguang, Shandong Province, People’s Republic of China; 2Department of Urology, Shouguang Hospital of Traditional Chinese Medicine, Shouguang, Shandong Province, People’s Republic of China; 3Division of Cancer, Department of Surgery and Cancer, Imperial College London, London, UK; 4Department of Immunotherapy, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, Henan Province, People’s Republic of China; 5Department of Respiratory Disease, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ling Peng
Department of Respiratory Disease, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang, People’s Republic of China
Tel/Fax +86 571-85893509
Email [email protected]
Zibing Wang
Department of Immunotherapy, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, Henan Province, People’s Republic of China
Tel/Fax +86 37165587098
Email [email protected]
Abstract: Immune checkpoint inhibitors (ICIs) including anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4) and anti-programmed death cell protein 1 (anti-PD1) have extended patient survival benefit and revolutionized cancer treatment. As ICIs rely on immune regeneration to eliminate tumor cells, they can also lead to an imbalance of immune reactions often called immune-related adverse events (irAEs). Rare irAEs such as ocular or cardiac toxicity or vasculitis are seen in less than 1% of patients receiving ICIs. Immune-related cystitis remains a rare occurrence. Herein, we describe a patient with extensive-stage small cell lung cancer (SCLC) and a history of syphilis with a complete response to second-line treatment using nivolumab plus paclitaxel who complained of urinary irritation symptoms. At biopsy, we found infiltration of CD3+ and CD8+ lymphocytes in the urothelium. Although there are reports describing immune-related cystitis in cancer patients, our case has comprehensive pathological confirmation and a differentiation diagnosis. In this report, we review other cases to elucidate clinical characteristics and discuss suitable management of this rare irAE.
Keywords: immunotherapy, cystitis, lung cancer, syphilis, immune checkpoint inhibitor
Introduction
Immune evasion is one of the hallmarks of cancer cells.1 ICIs have improved the prognosis of several solid tumor types. Some of the drugs approved in this class are ipilimumab (CTLA-4 inhibitor), nivolumab and pembrolizumab (PD-1 inhibitors), atezolizumab, avelumab, and durvalumab (PD-L1 inhibitors). ICIs have a distinct toxicity profile usually manifesting with autoimmune-like symptoms. ICIs may be associated with diverse irAEs including skin, endocrine, pulmonary, gastrointestinal and other organs.2 All organs can be affected, although it is important to be aware of different patterns of irAE for patients treated with PD1/PD-L1 versus CTLA-4 inhibitors. Urinary irAEs are rare, and the diagnosis poses a challenge. The differential diagnosis of immune-related cystitis includes bacterial cystitis, radiation cystitis and cystitis caused by other drugs.
In this report, we describe an extensive-stage SCLC patient who achieved a complete response but developed non-infective cystitis during treatment of nivolumab plus paclitaxel as second-line therapy. The symptoms coincided with nivolumab administration and rapidly improved with steroids. Radiologic images, molecular sequencing, bladder biopsy and immunohistochemistry provided information regarding urinary immune-related toxicity management during immunotherapy. We review the literature to understand underlying mechanisms here and aim to highlight that early recognition and suitable management are crucial to improve patient outcomes.
Case History
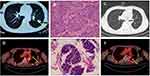
A 51-year-old man with a 50 pack-year history of smoking was referred to Shouguang Hospital of Traditional Chinese Medicine on March 2018 for further examination with a chronic cough. Chest CT scan identified a 3×3-cm space-occupying lesion (Figure 1A). Ten years prior to consultation, the patient was diagnosed as syphilis and received penicillin. He was positive for Treponema pallidum particle agglutination assay (TPPA) and negative for toluidine red unheated serum test (TRUST). Left lower lobectomy was performed on April 2nd, 2018, with histological analysis showing a left lung squamous carcinoma involving elastic fibers with the size of 3×3×2cm (Figure 1B), and the lymph nodes were negative. A post-operative CT scan is shown in Figure 1C. The tumor was staged as pT1cN0M0. Next-generation sequencing (NGS) of tumor sample revealed TP53 M246V mutation and FANCA Q133H mutation.
In July 2019, his cough persisted with intermittent hemoptysis. A PET-CT scan suggested a lesion of elevated uptake of FDG (SUV=9.5), 1.8×0.8cm (Figure 1D). Electronic bronchoscopy showed neoplasms in the left main bronchus, and histological analysis was SCLC (Figure 1E), with IHC positive for Syn and CGA and negative for Napsin A, TTF-1 (-), and P63 (-), with a Ki-67 index >50%. Brain MRI revealed no abnormalities. The diagnosis was a left small cell lung cancer, cT2aN0M0, Ib, limited stage. Routine blood tests and tumor markers were normal.
First-line treatment consisted of concurrent chemoradiotherapy (CCRT) (DT 60Gy/29 fractions) with etoposide/cisplatin with a good response (Figure 1F). However, after 5 cycles of chemotherapy, PET-CT scan revealed hepatic and bone metastases (Figure 2A and B). Bone biopsy of metastatic lesion was SCLC, positive for CK, TTF-1 and CD56. PD-L1 testing of tumor was <1% (clone 22C3; Dako, Glostrup, Denmark). NGS testing revealed an RB1 frameshift mutation, TP53 M246V missense mutation, MAP2K4 copy number loss and PIK3R1 copy number loss. Tumor mutational burden (TMB) was 7.98 mut/Mb, classified as microsatellite stable.
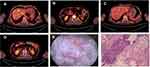
Second-line therapy was initiated with nivolumab 200mg d1 and paclitaxel 240mg d1, intravenously every 3 weeks. Bone radiotherapy of 30Gy/10f was also given (Figure 3). After 5 cycles of treatment, the patient complained of frequency of urination. Solifenacin was given with alleviation of the symptoms. Nivolumab plus paclitaxel were administered for 7 cycles, while his urinary symptoms worsened during the course, complaining of urgency of urination and accompanied by dysuria. During the therapy, the adverse events were grade 2 neutropenia. A further PET-CT confirmed a complete response using second-line nivolumab and paclitaxel (Figure 2C and D). Laboratory testing was generally normal at this time (Table 1) but urinalysis revealed neutrophils and lymphocytes; urine culture and cytology were negative. Cystoscopy showed that the entire bladder mucosa was edematous and red but there was no evidence of cancer (Figure 2E). Histologic analysis revealed no evidence of malignancy (Figure 2F) but infiltrates of CD3- and CD8-positive lymphocytes into the urothelium were present (Figure 4 and Table 2). Nivolumab and paclitaxel were discontinued, and methylprednisolone was administered intravenously, 80mg twice daily. Symptoms resolved after 3 days and steroids were tapered gradually over 6 weeks. The patient refused to take further chemotherapy or immunotherapy due to immune-related cystitis. After discontinuation of nivolumab and paclitaxel for 2 months, hepatic metastases regrew, and his performance status deteriorated rapidly. The clinical course of this patient is summarized in Figure 5.
 |
Table 1 Laboratory Testing Results |
 |
Table 2 IHC Staining for the Patient’s Tissue Samples |
Discussion
Increased T cell activation and proliferation can also provoke powerful immune-related reactions in other organ systems. As immunotherapy indications expand into the clinical setting, often with brief exposure and potentially sequenced with multimodality treatments, it will be necessary to recognize a diagnostic complex. Although ICIs have proven to be more effective and less toxic compared to chemotherapy, clinicians have reported the occurrence of adverse events.3 IrAEs including dermatologic, gastrointestinal/hepatic, pulmonary, and endocrine adverse events are well described in clinical trials and literature.4 As the use of ICIs becomes more prevalent, new and rare irAEs are coming to light. In this report, an extensive-stage SCLC patient receiving nivolumab and paclitaxel as second-line therapy was diagnosed with immune-related cystitis.
Up until now, there has been no standard to diagnose immune-related cystitis. Diagnosis of immune-related cystitis relies on a biopsy under cystoscopy to rule out other etiologies, such as bacterial infection, radiation-related cystitis and metastasis. Consultation with a specialized department when an irAE when suspected is also recommended. A bladder CT should be requested in case of symptoms or clinical suspicion of a secondary cause. In our case, the bladder was not involved in the target volume of radiation; therefore, radiation-induced cystitis was ruled out.
Previous reports of immune-related cystitis are listed in Table 3. Altogether, 4 cases were identified, among which 3 patients were given nivolumab and 1 was given pembrolizumab; all 4 patients had NSCLC. Regarding timing, there is no clearly defined timeframe for the development of immune-related cystitis. It can develop within 6 weeks of treatment, but some cases have been reported several years after starting treatment. In our case, immune-related cystitis developed after 15 weeks, which was within the range of previously reported cases.
 |
Table 3 Cases of Immune-Related Cystitis Following ICI: Clinical Characteristics and Malignancy Status |
We also queried the FDA adverse event database (FAERS) using web search tool (https://www.pharmapendium.com/home), using the drug names “atezolizumab, avelumab, cemiplimab, durvalumab, nivolumab, pembrolizumab and ipilimumab”, AND “non-bacterial cystitis” up to April 30th, 2020. Altogether, 19 reports were identified (Supplementary Table 1). Among them, 12 reports were associated with nivolumab, 3 with pembrolizumab, 3 with ipilimumab, and 1 with atezolizumab. Sixteen patients were male, and 3 patients were female.
Management depends on the clinical severity. Treatment involves with-holding therapy. Grading of immune-related cystitis is mainly based on Common Terminology Criteria for Adverse Events (CTC-AE, v5.0). According to the grading, this patient was categorized as grade 2 cystitis noninfective (moderate hematuria; moderate increase in frequency, urgency, dysuria, nocturia or incontinence; urinary catheter placement or bladder irrigation indicated; limiting instrumental ADL). Current guidelines rely heavily on the use of steroids for the management of irAEs.5,6 As indicated by guidelines, steroids were commenced to treat this irAE. Response to steroid differs depending on the type of irAEs. Most of irAEs respond within 2 months, as gastrointestinal, hepatic, and pulmonary toxicities; however, some irAEs resolved more slowly, such as skin and renal toxicities.7 Several immunosuppressive agents, such as intravenous immunoglobulin mycophenolate mofetil, calcineurin inhibitors, cyclophosphamide, and methotrexate, and biological immunosuppressive agents, such as infliximab, vedolizumab, and rituximab, have been used in the management of severe or steroid-refractory irAEs.8 Other strategies, including patient education, coordinating irAE management, prompt reporting of irAEs, and translational studies to better understand irAEs would improve the management of irAEs.9 For continuation of ICI administration, irAEs need to be stable at grade 1 or lower on prednisone at 10mg/day. Multi-omics prediction of irAEs using pharmacovigilance data and omics data is being developed to evaluate and early detect possible signals.10 Some classes of irAEs may be associated with efficacy; there is evidence that vitiligo might indicate activation of the immune system.11 The correlation between ICI-induced irAEs and clinical response has not been fully explored.12 Efficacy relating to irAEs reveals a complex picture, as in this patient, disease progression was accompanied by discontinuation of ICI-combination therapy.
A mechanism for irAE development due to enhanced T cell activity against shared antigens across normal and cancer cells is supported by several preclinical models.13 Other studies indicated that irAEs may also occur through a variety of mechanisms, including decreased immune tolerance and molecular mimicry.14 PD-L1 expression in bladder tissue was previously identified in patients with interstitial cystitis,15 which was more common in patient with severe bladder inflammation. Although this patient was negative for PD-L1 expression of his urothelium, the exact mechanism of immune-related cystitis remains undetermined. A previous case of immune-related cystitis raised the possibility that the unknown antigen in the urothelium is targeted by lymphocytes that are positive for TIA-1 and/or CD8.16 Predicting risks for irAEs will require insight into underlying mechanisms. Some clinical data suggest there are potential risk factors of developing irAE, such as a family history of autoimmune disease, previous viral infection, or drugs.17 In our case, the patient received chemotherapy in combination with immunotherapy. A meta-analysis indicated that ICI combined with conventional therapy such as chemotherapy have a higher incidence of irAE.18 The urinary microbiome may also exert a predictive role for the response to anticancer immunotherapy with ICIs.19 One proposed mechanism for irAE initiation involves a role for dysbiosis, in which exposure to microbiome-derived products can trigger an innate immune response, possibly leading to the activation of self-reactive immune cells.
Currently, the only FDA-approved second line of therapy for small cell lung cancer is topotecan and lurbinectedin. Other choices include paclitaxel, docetaxel, gemcitabine and vinorelbine. ICIs including nivolumab, pembrolizumab, durvalumab and atezolizumab were once approved by Chinese FDA but they all were withdrawn of their indication in second- and later-line of SCLC due to lack of benefit in this setting. The PASSION study demonstrated that caremlizumab plus apatinib have potential anti-tumor efficacy and manageable safety profile in chemotherapy-sensitive and chemotherapy-resistant ED-SCLC patients who are after platinum-based therapy.20 In our case, after MDT (multidisciplinary team) discussion, the combination of ICI and chemotherapy was proposed as second-line treatment regimen. Although combination therapy in the second-line setting for extensive-stage SCLC is not recommended in clinical practice, the strategies available currently confirmed the poor prognoses of these patients.21 Considering the patient was refractory to first-line chemotherapy of limited disease, he was treated with chemotherapy plus immunotherapy for the extensive disease.
Viruses such as HIV might influence the efficacy of ICI.22 However, cancer patients with a previous infection of syphilis receiving ICIs have not been reported. Based on the TRUST and TPPA test, his Treponema pallidum had been cleared.
There were several limitations of our report. First, the patient was treated with combination therapy in second-line for extensive stage SCLC, which was not the standard-of-care according to the guidelines. Second, although the biopsy of immune-related cystitis was performed to help make a diagnosis, the patient refused to have second checkup of cystoscopy to confirm the improvement of the bladder mucosa. There were no related imaging examinations or any examination results.
While commonly encountered irAEs are well described, rarer toxicities are not featured. These patients should be closely monitored by specialists and moving forward, collaborative effort between oncologists and specialists will be required to recognize and treat the irAEs to avoid long term debilitating complications to the patients. With expanding use of ICIs, healthcare providers must adequately evaluate rare urinary irAEs for the underlying pathology, besides bacterial cystitis induced by chemotherapy and other anti-cancer therapies. The safety and benefit of retreatment are unknown, and the decision to retreat should be considered on a case-by-case basis. The present case may help physicians diagnose and treat rare irAEs.
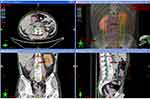 |
Figure 3 Radiotherapy planning to be delivered to bone metastases for this patient. |
 |
Figure 5 Clinical course of the patient. *means “times” (for 6 weeks). |
Data Sharing Statement
All relevant data and diagnostic results are contained. The raw data is not made available in consideration of confidentiality.
Ethics Statement
Institutional approval was waived to publish the case details due to local regulation. A patient’s relative provided informed consent for us to describe this case.
Acknowledgments
The authors would like to thank the patient’s family for giving consent and for providing the detail information of this case.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was partially supported by the Natural Science Foundation of Zhejiang Province, China (Grant number: LY19H160041).
Disclosure
Justin Stebbing reports being an Editor in Chief for Springer Nature, during the conduct of the study and outside the submitted work, and the following conflicts of interest from 2020 – present: Professor Stebbing, the Editor-in-Chief of Oncogene has sat on SABs for Vaccitech, Heat Biologics, Eli Lilly, Alveo Technologies, Pear Bio, Agenus, Equilibre Biopharmaceuticals, Graviton Bioscience Corporation, Celltrion, Volvox, Certis Oncology Solutions, Greenmantle, Zedsen, Bryologyx and Benevolent AI. He has consulted with Lansdowne partners and Vitruvian. He sits on the Board of Directors for Xerion and BB Biotech Healthcare Trust PLC. None is relevant here. The other authors declare no conflict for this work.
References
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
2. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi:10.3322/caac.21596
3. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362. doi:10.1093/annonc/mdw141
4. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi:10.1186/s40425-019-0805-8
5. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z
6. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264–iv266. doi:10.1093/annonc/mdy162
7. Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi:10.1016/j.ctrv.2016.02.003
8. Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer. 2021;9(1):e001731. doi:10.1136/jitc-2020-001731
9. Naing A, Hajjar J, Gulley JL, et al. Strategies for improving the management of immune-related adverse events. J Immunother Cancer. 2020;8(2):e001754. doi:10.1136/jitc-2020-001754
10. Jing Y, Liu J, Ye Y, et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat Commun. 2020;11(1):4946. doi:10.1038/s41467-020-18742-9
11. Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline summary. J Oncol Pract. 2018;14(4):247–249. doi:10.1200/JOP.18.00005
12. Hu W, Wang G, Wang Y, Riese MJ, You M. Uncoupling therapeutic efficacy from immune-related adverse events in immune checkpoint blockade. Iscience. 2020;23(10):101580. doi:10.1016/j.isci.2020.101580
13. Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi:10.1126/science.291.5502.319
14. Young A, Quandt Z, Bluestone JA. The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol Res. 2018;6(12):1445–1452. doi:10.1158/2326-6066.CIR-18-0487
15. Chen Y, Yu W, Yang Y, et al. Expression of programmed death ligand-1 on bladder tissues is detected in a clinically and histologically well-defined interstitial cystitis cohort. Neurourol Urodyn. 2018;37(4):1396–1404. doi:10.1002/nau.23459
16. Ueki Y, Matsuki M, Kubo T, et al. Non-bacterial cystitis with increased expression of programmed death-ligand 1 in the urothelium: an unusual immune-related adverse event during treatment with pembrolizumab for lung adenocarcinoma. IJU Case Rep. 2020;3(6):266–269. doi:10.1002/iju5.12211
17. Manson G, Norwood J, Marabelle A, Kohrt H, Houot R. Biomarkers associated with checkpoint inhibitors. Ann Oncol. 2016;27(7):1199–1206. doi:10.1093/annonc/mdw181
18. Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi:10.1136/bmj.k4226
19. Bersanelli M, Santoni M, Ticinesi A, Buti S. The urinary microbiome and anticancer immunotherapy: the potentially hidden role of unculturable microbes. Target Oncol. 2019;14(3):247–252. doi:10.1007/s11523-019-00643-7
20. Fan Y, Zhao J, Wang Q, et al. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): a multicenter, two-stage, phase 2 trial. J Thorac Oncol. 2021;16(2):299–309. doi:10.1016/j.jtho.2020.10.002
21. Chouaid C, Baize N, Monnet I. Second-line therapy for disseminated small-cell lung cancer: optimal management remains to be defined. Transl Lung Cancer Res. 2020;9(5):1732–1735. doi:10.21037/tlcr-20-362
22. Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. 2019;5(7):1049–1054. doi:10.1001/jamaoncol.2018.6737
23. Shimatani K, Yoshimoto T, Doi Y, Sonoda T, Yamamoto S, Kanematsu A. Two cases of nonbacterial cystitis associated with nivolumab, the anti-programmed-death-receptor-1 inhibitor. Urol Case Rep. 2018;17:97–99. doi:10.1016/j.eucr.2017.12.006
24. Ozaki K, Takahashi H, Murakami Y, Kiyoku H, Kanayama H. A case of cystitis after administration of nivolumab. Int Cancer Conf J. 2017;6:164–166. doi:10.1007/s13691-017-0298-6
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.