Back to Journals » Infection and Drug Resistance » Volume 15
Immunosuppressed Patients with Clinically Diagnosed Invasive Fungal Infections: The Fungal Species Distribution, Antifungal Sensitivity and Associated Risk Factors in a Tertiary Hospital of Anhui Province
Authors Xia J, Wang Z, Li T, Lu F, Sheng D, Huang W
Received 25 November 2021
Accepted for publication 14 January 2022
Published 2 February 2022 Volume 2022:15 Pages 321—333
DOI https://doi.org/10.2147/IDR.S351260
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jinxing Xia,1 Zhongxin Wang,1 Tingting Li,1 Fanbo Lu,1 Daping Sheng,1 Wei Huang2
1Department of Clinical Laboratory, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China; 2Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China
Correspondence: Jinxing Xia
Department of Clinical Laboratory, the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China
, Email [email protected]; Wei Huang
Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China
, Email [email protected]
Objective: Since the nosocomial fungal infections increasingly emerge, we extensively investigated the fungal species stratification and antifungal sensitivity profiles, clinical characteristics and associated risk factors of immunosuppressed patients with clinically diagnosed invasive fungal infections (IFIs) in a tertiary hospital of Anhui province.
Methods: In total, 112 subjects with immunosuppressive state were enrolled from a comprehensive tertiary hospital in Central China between July 2019 and December 2021. Eight-one fungal isolates were clinically recovered by fungus-culturing approaches. The identifications were conducted through a mass spectrometry detecting platform. The susceptibilities to antifungals were tested using the broth micro-dilution method, and the possible antifungal azole-resistance mechanism in specific Candida species was availably explored by sequencing. Patient medical profiles were accessed via the digitized retrieval system of hospital, from which clinical outcomes and multiple risk factors for immunosuppressed patients with clinically diagnosed IFIs were explicitly documented for evaluation.
Results: Candida species predominated in clinically diagnosed IFIs of immunosuppressed patients (accounting for 88.88%), followed by Trichosporon and Aspergillus species (6.17% and 4.94%, respectively). The source types of specimen were primarily comprised of urine (41.98%), respiratory samples (33.33%) and peripheral blood (9.88%). Frequently isolated Candida and Trichosporon species exhibited a high level of in vitro sensitivity for amphotericin B and 5-fluorocytosine, whereas a substantial portion of Candida species including C. glabrata, C. parapsilosis complex and C. tropicalis, and Trichosporon species showed lowered sensitivity patterns toward itraconazole, fluconazole and voriconazole at different levels. Specifically, gene mutations of ERG11 were identified in azole-resistant C. tropicalis. Distinct risk factors were analyzed to be highly associated with the clinically diagnosed IFI incidence, mainly including hospitalization duration, surgical procedures, immunosuppressive treatments, underlying diseases and other conditions.
Conclusion: Candida, Trichosporon and Aspergillus species were the top three pathogenic fungal agents causing clinically diagnosed IFIs in immunosuppressed patients. The attenuated sensitivity to azoles in Candida and Trichosporon species needs close surveillance, and ERG11 polymorphism might contribute to azole resistance in specific Candida species. Multiple featured risk factors for immunosuppressed patients developing clinically diagnosed IFIs require further consideration during clinical practice.
Keywords: invasive fungal infections, immunosuppressed patients, species distribution, antifungal sensitivity, risk factors, treatment outcome
Introduction
Invasive fungal infections (IFIs) are increasingly recognized as a major threat in immunocompromised and critically-ill patients with a high morbidity and mortality.1,2 It is estimated that approximately 1.5 million invasive fungal deaths occur worldwide annually,3,4 more than 80% of which is attributable to infections of Candida, Aspergillus or Cryptococcus.5 Though advanced approaches of diagnoses and treatments allow patients to survive life-threatening diseases in clinical settings, many of them are gradually involved in immunosuppressive state and subsequently vulnerable to a variety of infectious disorders including clinically diagnosed IFIs during hospitalization. These vulnerable or susceptible hosts are mainly comprised of cancer patients and recipients with solid organ or blood cell/bone marrow transplantations.2,6–8 In particular, clinically diagnosed IFIs are more likely associated with invasive diagnoses and treatments, and immuno-modulating interventions during clinical practice.2,9 It is considered that extensive applications of broad-spectrum antibiotics, more potent immunosuppressant drugs for transplantation, and more aggressive anti-cancer chemo-/radio-therapies readily leading to mucositis and neutropenia, would inevitably increase the risk of clinically diagnosed IFIs.7,10,11 The incidence and severity of these infections also highly correlate with other risk factors, including host underlying diseases, hospitalization time, age, etc.
With the improvement of effective prophylactic strategies and the refinement of immunosuppressive regimens, the occurrence of infectious complications in immunocompromised patients tends to be reduced; however, the clinically diagnosed IFIs are still able to keep hospitalized patients in danger occasionally.3,11,12 Furthermore, emerging multidrug-resistant fungi of clinical origin would be a fatal threat for the immunosuppressed inpatients and especially for those with transplantations or chemo-/radio-therapies if novel and strong antifungal agents are unavailable.13–15 Recent studies on new potent antimicrobial drugs against multidrug-resistant Candida and filamentous fungi hold a promising perspective to overcome refractory and invasive fungal infections.13–18 Given the widespread administration of clinically commonly-used antifungals including azoles to a large number of patients experiencing malignancy and transplantation, it is of significant importance to understand the changing trends of clinically diagnosed IFIs in terms of the fungal species stratifications, antifungal sensitivities and possible underlying azole-resistance mechanisms related to sterolΔ5,6-desaturase (ERG3) and 14-ɑ-sterol demethylase (ERG11) genes,19,20 as well as clinical characteristics and distinctive risk factors in combination with relevant clinical profiles.
In the present study, therefore, we conducted these integrated analyses for clinically diagnosed IFI patients with immunosuppressed conditions, especially focusing on cancer and/or transplantation cases in regional China. In addition, the antifungal treatment effects and clinical outcomes of the subjects (particularly with azole resistance) were also documented for assessment.
Materials and Methods
Study Design
This study was carried out within a 2800-bed comprehensive tertiary teaching hospital of a provincial capital city in Central China, and collected the cases in which all the subjects were under immunosuppressive state (mainly with cancers or transplantations) and were admitted to hospital between July 2019 and December 2021. Specimens from both sterile and non-sterile body sites were carefully screened out for the study. Specifically, samples from the conventionally non-sterile body sites (primarily including urine, sputum and pus materials) were harvested/aspirated aseptically by the paracentesis of bladders or abscesses, or from deep lower respiratory tracts of patients with fungal pneumonia (≥50 colonies of fungi) by sterile procedures including ventilating devices or bronchofiberscopes, for direct microscopic examinations and mycological cultures. The criteria of diagnosis for clinically diagnosed IFIs were referenced to the updated Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group,2 and/or the local Chinese Medical Association (CMA) Expert Consensus guidelines,21 based on host factors, clinical features and mycological evidence (Supplementary Figure S1). Sample cultures with same isolates from same subjects were excluded in the present study. Patient medical profiles were accessed through digitized retrieval system, and laboratory testing data were available in patient charts in hospital. Multiple potential risk factors which are highly related to clinically diagnosed IFIs were comprehensively assessed, eg, immunosuppressive state (with cancers or transplantations), underlying diseases, minimally/aggressively invasive clinical interventions, hospitalization time and other infectious or featured factors. To those repeatedly admitted to hospital, the very first episode of hospitalization was accepted for analysis within the study period.
Organism Identification
As per the study protocol, all the specimens were timely harvested from the inpatients, mainly including urine, peripheral blood, ascitic fluid, pus, drainage fluid, as well as sputum and bronchoalveolar lavage fluid (BALF) selectively from deep lower respiratory tracts. Peripheral blood received rapid culture and expansion processes in a BacT/AlerT 3D automatic microbe detector (bioMérieux, Marcy l’Étoile, France). The rest specimens were aseptically inoculated directly onto Chromogenic Candida Agar and/or Sabouraud Dextrose Agar medium plates, and cultured at 35°C for 48-hour or more time period. The isolated fungal colonies were then identified on a VITEK mass spectrometry platform using a matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS, bioMérieux, Marcy l’Étoile, France) according to the manufacturer’s instructions.
Antifungal Susceptibility
The common antifungal susceptibility assays in clinical laboratories were conducted for the isolated fungal species if applicable using an ATB FUNGUS 3 strip product (bioMérieux, La Balme-Les Grottes, France) following the producer’s specifications. Briefly, the mean minimal inhibitory concentrations (MICs) of the antifungal drugs, primarily including amphotericin B (AMB), 5-fluorocytosine (5-FC), and commonly used azoles in clinic, were determined visually and automatically on an Automatic Testing Bacteriology (ATB) Expression Bacteriology Analyzer with expert system (bioMérieux, La Balme-Les Grottes, France). The interpretative criteria of sensitivities and breakpoints for these antifungals complied with the reference method for broth micro-dilution of the CLSI M27-A3.22 The reference strains of C. parapsilosis (ATCC22019) and C. krusei (ATCC6258) were set for quality control.
Sequencing of Azole-Resistant Genes
The azole-resistance related genes of ERG3 and ERG11 were identified through polymerase chain reaction (PCR) and sequencing with primers of ERG3-Forward: 5’-ATGGATATCGTACTAGAAATTTGTG-3’, ERG3-Reverse: 5’-TCATTGTTCAACATATTCTCTATCC-3’, and ERG11-Forward: 5’-GTTTTCTACTGGATCCCATG-3’, ERG11-Reverse: 5’-TACATCTGTGTCTACCACC-3’.23,24 The PCR programme included: 95°C for 5 min, 35 cycles of 94°C for 30s, 50°C for 90s, and 72°C for 90s, then a final extension of 72°C for 10 min. Genomic DNA was extracted and PCR products were quantified for sequencing (Sangon Biotech Co., Ltd., Shanghai, China). The obtained sequences were then strictly aligned with the corresponding reference sequences of ERG3 (GenBank accession no. XM002550136.1) and ERG11 (XM_002550939.1) in C. tropicalis (ATCC750).
Statistical Analysis
All analyses were performed using SPSS software (SPSS version 21, Chicago, USA). The comparison of categorical data was determined by the Chi-squared tests. Statistical significance was determined using two-tailed tests. A P value of <0.05 was considered significant in all analyses.
Results
Clinical Distribution Characteristics of Clinically Diagnosed IFIs
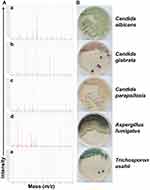
In total, 112 subjects with immunosuppressed state primarily due to cancers and/or transplantations were screened during the study period, and in these cases a total of 81 patients were with clinically diagnosed IFIs. As shown in Figure 1, the corresponding fungal isolates (n = 81) from the patients were recovered and identified to a species level by a MALDI-TOF MS platform (a VITEK MS system), along with microbial morphological examinations macroscopically and microscopically. For fungal species stratification in the present study, as summarized in Table 1, the most frequently isolated strains belonged to Candida spp. (88.89%, n = 72), Trichosporon spp. (6.17%, n = 5) and Aspergillus spp. (4.94%, n = 4). Specifically, C. albicans predominated in Candida spp. isolated from clinically diagnosed IFI patients, accounting for 32.10%, followed by C. glabrata (22.22%), C. parapsilosis (9.88%), C. orthopsilosis (9.88%), C. tropicalis (8.64%) and C. krusei (2.47%). These inocula originated from different specimen sources. The major parts of the fungal species (41.98%) were obtained from urine, then sputum (28.40%), peripheral blood (9.88%), BALF (4.94%), ascitic fluid (3.70%), pus (3.70%), drainage fluid (2.47%), and others including bile (1.23%), catheter (1.23%), and secretion (1.23%), respectively.
 |
Table 1 The Fungal Species Distribution and Characteristics of Specimen Source Types from Clinically Diagnosed IFI Patients |
Antifungal Susceptibility Profiles and Drug-Resistant Host Clinical Features
The clinical commonly administered antifungals were tested for Candida and Trichosporon species in terms of the in vitro sensitivities, including AMB, 5-FC, fluconazole (FLU), itraconazole (ITR) and voriconazole (VOR). As listed in Table 2, the assay results illustrated that both AMB and 5-FC exerted a potent influence on all the Candida spp. at the in vitro level. One hundred percent of the Candida isolates were fully susceptible to AMB, and nearly each member presented in this study showed 100% of sensitivities to 5-FC except C. glabrata (94.40%). VOR was also observed 100% sensitive for most Candida isolates, apart from C. parapsilosis and C. orthopsilosis (87.50% and 75.00%, respectively). However, the Candida species primarily exhibited relatively lowered susceptibilities to both FLU (ranging from 96.20% to 62.50%) and ITR (from 96.20% to 42.90%). This study found several rare Candida species whose susceptibility rates for the five drugs were all able to reach 100% if intrinsic resistance was not taken into account. In addition, for Trichosporon spp., their susceptibilities for FLU were the highest and up to 100%, whereas all these isolates attenuated sensitivities to 5-FC, ITR, FLU and VOR (50.00%, 50.00%, 75.00% and 75.00%, respectively). Incidentally, among all the isolated fungal species confirmed definitely resistant to azoles (Table 3), the C. tropicalis was spotted out to harbor altered drug resistant genes of ERG3 and ERG11 through sequencing, trying to hold a clue to possible reasons for the in vitro resistance to azole antifungals. Briefly, after aligned with the reference sequences in GenBank, our sequencing analysis proved the presence of one silent mutation of G366A in ERG3, two silent mutations of T225C and G264A in ERG11, but two homozygous missense mutations of A395T and C461T in ERG11 which were able to consequently produce changes of Y132F and S154F, respectively, at the amino acid level. In particular, of all the seven azole-resistant patients, five underwent prior treatment of VOR, one of them even received sequential administration of VOR and then caspofungin (CAS) separately. Four patients receiving final antifungal monotherapy of FLU, VOR or CAS turned out to be unsatisfactory (with infection persistence/progression, or moribund); whereas the other two eventually did very well post the switch of VOR or CAS monotherapy into a combined antifungal strategy of VOR plus CAS. Notably, half of the patients with azole-resistant Candida spp. presented infections at more than one site, without desirable treatment effects compared to those at a single one (Table 3).
 |
Table 2 In vitro Drug Susceptibility Profile for Candida and Trichosporon Species |
 |
Table 3 Characteristics of All the Immunosuppressed Patients with Azole-Resistant Fungal Species in This Study |
Clinical Characteristics, Outcomes and Risk Factors of Clinically Diagnosed IFIs
The clinical characteristics of the cohort for fungal culture-positive subjects under immunosuppressive state are described in Table 4. Among the clinically diagnosed IFI patients, males accounted for 59.26% (48/81) and females for 40.74% (33/81) who appeared more susceptible compared to the control cohort without IFIs. The most commonly encountered invasive Candida spp. in males were C. albicans (31.25%, 15/48) and C. glabrata (22.92%, 11/48), which in females were C. albicans (33.33%, 11/33) and C. parapsilosis (24.24%, 8/33) instead. In particular, all the rare Candida spp. including C. guilliermondii, C. pseudotropicalis and C. rugosa, as well as all the Trichosporon spp., were exclusively derived from male cases. And of Aspergillus spp., A. fumigatus was isolated from males and A. flavus from the opposite sex. Moreover, it’s believed that patients’ age may play a role in the infections. Of the clinically diagnosed IFI patients, those over 65 years of age were the most vulnerable to the infections, accounting for 40.74% (33/81), in which C. albicans infections were prominent with 51.52% (17/33, P = 0.002 compared to the other age groups), followed by the infections of C. glabrata (15.15%, 5/33), C. parapsilosis (9.09%, 3/33) and C. tropicalis (9.09%, 3/33). While those with <15 years old presented barely 2.47% (2/81) in the incidence of clinically diagnosed IFIs. All the Trichosporon and Aspergillus strains were found in the subjects with >14 years of age. Our analyses unveiled that with the stay length of hospitalization prolonged, the percentage of clinically diagnosed IFI events subsequently occurring in these inpatients grew significantly from 3.70% (3/81, with <7 days, P = 0.006) to 74.07% (60/81, with >14 days, P = 0.023). As expected, the prominent infectious agent was invariably C. albicans for each hospitalization duration group. Interestingly, all of the Trichosporon and Aspergillus isolates were gained from the patients with >7 days of hospitalization. Next, when compared with the counterparts without IFIs, the clinical outcomes of clinically diagnosed IFI patients were significantly unsatisfactory with infection deteriorations or being moribund (P < 0.001), especially to those with azole resistance as described above.
 |
Table 4 Clinical Distribution, Characteristics and Outcomes of Clinically Diagnosed IFI Patients |
The demographic characteristics of each invasive fungal species along with additional distinctive risk factors associated with clinically diagnosed IFIs were comprehensively investigated. As summarized in Table 5, and a vast majority of the patients developed one or more comorbidities, and the common ones prior to the IFIs as documented were cancers (70.37%, especially accompanied with C. albicans), severe renal failure or dysfunction (28.40%, many received renal transplantations later, chiefly with C. orthopsilosis), and diabetes (18.52% and P = 0.039, usually with C. albicans or C. glabrata) (Table 6). Invasive interventions or surgical procedures as well as host immunosuppressive state were generally considered as highly relevant risk factors for invasive fungal infections. In the present study, the frequently encountered invasive procedures prior to the IFIs were as follows: surgery (41.98% and P = 0.025, mainly with C. albicans), urinary catheters (34.57% and P = 0.023, mainly with C. parapsilosis complex), arteriovenous cannulation (34.57%, P = 0.023, mainly with C. parapsilosis), ventilator support (33.33%), tracheal intubation (29.63%), endoscope (16.05%), and hemodialysis/peritoneal dialysis (12.35%); and those directly affecting host immune systems were involved with immuno-modulations (41.98% and P = 0.01, primarily with C. albicans, C. glabrata or C. orthopsilosis) including immunosuppressant tacrolimus/FK506 or cyclosporine A treatment, and chemotherapy and/or radiotherapy (23.46%) (Table 6). Other potentially associated risk factors were also explicitly analyzed when available, and a large portion of these factors were immune-related and relevant to C. albicans-mediated clinically diagnosed IFIs in this study: (1) aberrant tumor biomarker expressions (51.85%), such as alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 19–9 (CA19-9), CA12-5, cytokeratin 19 fragment (CYFRA 21–1), neuron-specific enolase, tumor abnormal protein (TAP), ferritin, etc.; (2) virus-mediated infections (20.99%), primarily involving hepatitis caused by hepatitis B virus (HBV) rather than HAV or HCV, and infections by Epstein-Barr virus, BK virus or human cytomegalovirus (but without the coronavirus), and interestingly one patient was found to be infected with influenza B virus who received kidney transplantation and suffered candidemia with C. orthopsilosis; (3) abnormal cytokines release (12.35%), basically including interleukin-1β (IL-1β), IL-2R, IL-6, IL-8, IL-10, and tumor necrosis factor-ɑ; (4) Mycoplasma pneumoniae infection (3.70%); and (5) Treponema pallidum infection (1.23%) (Table 7).
 |
Table 5 Comparison of Clinically Associated Risk Factors Among Patients with or Without the IFIs |
 |
Table 6 Demographic Characteristics Between Fungal Species Distribution and Significant Risk Factors for Clinically Diagnosed IFI Patients |
 |
Table 7 Potentially Featured Risk Factors for Clinically Diagnosed IFI Patients Infected with the Major Fungal Species |
Discussion
To date, an increasing number of clinical practices engage directly or indirectly with host immune systems, which inevitably poses a potential or substantial impact on the immune defense functions within patients, especially for those cancer patients receiving chemo/radio-therapies, as well as patients receiving transplantations and continuous immunosuppressant drugs. Though constant progress has been achieved from invasive medical interventions over the past decades, the incidence of clinically diagnosed invasive fungal infections has risen concomitantly.6,25–28 The increase in such infections gives rise to relatively high morbidity and mortality among the inpatients with immunosuppressive state. However, current clinical studies of the IFIs in immunosuppressed subjects are rarely involved in central part of China, indicating relatively limited knowledge is available for local areas in the country on the epidemiology, antifungal susceptibility patterns, and associated risk factors for clinically diagnosed IFI patients under immunosuppressive status. Therefore, this study provided potentially valuable information to bedside on the management of these patients with the infections in local regions.
With the ongoing outbreak of coronavirus disease 2019, the sample size of this study was relatively affected, however it may otherwise truly reflect the distinctive features of clinically diagnosed IFIs in immunosuppressed cases in the region during the special time period. We believe it would be highly informative to investigate whether the coronavirus would potentially be an important risk factor for the clinically diagnosed IFIs locally, though the present study did not access to any coronavirus-positive cases (with PCR assays).29,30 Additionally, with recent implementation of the policy of hierarchical medical system in China,31 severe patients have a priority to visit our tertiary teaching hospital with a higher medical hierarchy, relatively leading to a high local incidence of clinically diagnosed IFIs. Previous studies reported that the occurrence of invasive fungal infections was largely attributable to Candida spp., then Cryptococcus spp. and Aspergillus spp.32–35 The present study observed a similar phenomenon that Candida spp. remained a causative agent for clinically diagnosed IFIs in immunosuppressed patients; and Trichosporon and Aspergillus species were the two primary non-Candida spp. causing the clinically diagnosed invasive mycoses. The specimen sources in this research were of diverse origins. The top three sources of culture-positive specimens were urine, sputum, and peripheral blood. In general, the incidence and severity of clinically diagnosed IFIs were influenced by a variety of factors, for instance, patient age, hospitalization duration, underlying diseases, clinical invasive interventions, immune status and others.25,36 It turned out that patients’ age played a significant role in raising the clinically diagnosed IFI incidence. Those developing the infections in this study were almost elderly patients who also underwent significantly prolonged hospitalization duration, ie, over 70% of them were more than 50 years of age and experienced hospitalization for over two weeks. The highly relevant underlying diseases were mainly comprised of cancers (particularly with aberrant tumor biomarker expressions), organ transplantations and diabetes. These conditions, together with invasive procedures (eg, surgery, catheters and arteriovenous cannulation), immunosuppressive treatment and other infectious or featured factors (eg, virus- or mycoplasma-mediated infections, abnormal cytokines release, etc.), would likely offer massive opportunities for the IFIs or transmission of the causative fungal species among those inpatients. Interestingly, we observed that one subject was infected with influenza B virus who underwent kidney transplantation and developed C. orthopsilosis-mediated candidemia, holding a clue that influenza might be a potential risk factor for clinically diagnosed IFIs here.37 Other researchers stated that the risk factors for Trichosporon infections dealt with neutropenia (an abnormally low amount of neutrophils), transplantations, end-stage renal diseases, HIV infections, and use of immuno-regulatory agents and invasive medical devices.38 Our analyses echoed that statement, and it is worth noting that the clinically diagnosed IFIs caused by Trichosporon in immunocompromised patients are likely to be one of life-threatening complications.
The emergence of drug-resistant fungal strains is becoming a major health concern globally. We next made an in-depth analysis of the antifungal susceptibility features of clinically diagnosed IFI patients. Antifungal agents are generally categorized into four major classes including polyenes (to target cell membrane), fluoropyrimidine analogs (to block nucleic acid synthesis), echinocandins (to inhibit cell wall synthesis), and azoles (to inhibit ergosterol biosynthesis).5 Especially, the decreased sensitivity of azoles among Candida and Aspergillus species mounts a rigorous challenge in current clinical settings, and echinocandin- or multidrug-resistant (MDR) isolates of certain Candida spp. also become a compelling concern.33 Recent emergence of new drugs or disinfectant agents against MDR Candida spp. during invasive and ocular Candida infections holds a promising perspective particularly for immunosuppressed patients.15 For instance, ophthalmic solutions containing hexamidine diisethionate 0.05% (Keratosept) or povidone-iodine 0.6% (IODIM®) have been evaluated to control ocular fungal infections with potent antimicrobial activities against Candida spp. as recently reported by Pinna et al.13–15 Moreover, many plant essential oils have increasingly been assessed to present promising broad-spectrum antifungal activities to overcome MDR Candida spp. and even filamentous fungi, including the essential oils from Ruta graveolens, Myrtus communis and Austroeupatorium inulaefolium.16–18 In China, the antifungal susceptibility rate differs significantly from region to region.39 It is likely due to the extensive and/or irrational utilization of antifungals within clinical and veterinary settings.40 In the present study, we found that AMB was fully sensitive to all of the invasive Candida and Trichosporon species. And ITR sensitivity rate was the lowest among all Candida and Trichosporon species, followed by FLU, VOR and 5-FC. Since ITR currently has only oral formulations available and is regularly used for mucosal mycoses, it is not highly recommended for IFI patients that might prefer an intravenous formulation if applicable in future.41 Hence, the antifungal susceptibility assay for ITR here merely reflected an in vitro activity for Candida and Trichosporon species, but it still may provide instrumental information for possible cross resistance between azoles and combined fungal infections. To be specific, a substantial number of C. orthopsilosis and Trichosporon spp. were identified with remarkably lowered sensitivities to FLU, ITR and VOR, even prone to cross resistance. Owing to relatively limited sample size, the underlying mechanisms for azole resistance found here were yet fully discussed. However, incidentally through sequencing azole-resistance associated genes of ERG3 and ERG11 in the C. tropicalis resistant against azoles in this study, it might get a clue to a possible resistance mechanism that the existence of homozygous point mutations of A395T and C461T (causing Y132C and S154C amino acid residue changes) in ERG11 might contribute to such resistance at any rate in C. tropicalis here, which is expected to jeopardize the ability of ERG11 proteins to binding azoles.20 Other researchers also did gene sequencing studies on azole-resistant C. glabrata and seldom found significant antifungal missense mutations in local regions.42 Our ongoing project is proceeding to investigate genetic polymorphisms pertaining to the mechanisms of azole resistance in invasive fungal infections. And consistent with the reported synergistic effects of azole plus caspofungin on multidrug-resistant Candida infections,43–45 the limited data here also appear to imply that a combinatorial antifungal strategy of azole plus echinocandins might be of benefit to the immunosuppressed patients with clinically diagnosed IFIs, albeit more conclusive evidence needed.
Conclusion
Taken together, the current research concretely emphasized the fungal species distribution and antifungal sensitivity profiles, as well as clinical characteristics and the complexity of risk factors associated with immunocompromised patients that were infected with clinically diagnosed invasive mycoses during hospitalization in a comprehensive teaching hospital of a provincial capital city in Central China. It concluded that Candida species was the primary pathogen resulting in clinically diagnosed IFIs in patients under immunosuppressive state, followed by Trichosporon and Aspergillus species. The findings further imply that patient age, duration of hospitalization, multiple underlying diseases and diverse invasive interventions, as well as certain featured conditions would likely be regarded as the highly associated risk factors for clinically diagnosed IFIs. The critical gene mutations of ERG11 might contribute to attenuated sensitivity to azoles in C. tropicalis here. To maintain effective antifungal susceptibilities for better clinical outcomes of clinically diagnosed IFI patients, more efforts ought to be put to standardize the rational application of common antifungals at bedside.
Abbreviations
IFI, invasive fungal infection; ERG3, sterol Δ5,6-desaturase; ERG11, 14-ɑ-sterol demethylase; BALF, bronchoalveolar lavage fluid; MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry; MIC, minimum inhibitory concentration; MIC50 or MIC90, MIC at which 50% or 90% of the isolates tested were inhibited; AMB, amphotericin B; 5-FC, 5-fluorocytosine; FLU, fluconazole; ITR, itraconazole; VOR, voriconazole; CAS, caspofungin; CLSI, Clinical and Laboratory Standards Institute; PCR, polymerase chain reaction; PB, peripheral blood; AF, ascitic fluid; DF, drainage fluid; HBV, hepatitis B virus; IL, interleukin.
Ethical Considerations
The study protocol was reviewed, approved by and carried out following the recommendations of the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. Written informed consents were obtained from patients as per the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (21604079) (JX), and the Natural Science Foundation of Anhui Province (1608085QH183) (JX).
Disclosure
All the authors declared no any competing interests.
References
1. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. doi:10.1038/nrdp.2018.26
2. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi:10.1093/cid/ciz1008
3. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13. doi:10.1126/scitranslmed.3004404
4. Kaushik N, Pujalte GG, Reese ST. Superficial fungal infections. Prim Care. 2015;42(4):501–516. doi:10.1016/j.pop.2015.08.004
5. Pathakumari B, Liang G, Liu W. Immune defence to invasive fungal infections: a comprehensive review. Biomed Pharmacother. 2020;130:110550. doi:10.1016/j.biopha.2020.110550
6. Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–917. doi:10.1086/339202
7. Blumberg HM, Jarvis WR, Soucie JM, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis. 2001;33(2):177–186. doi:10.1086/321811
8. Gorschluter M, Mey U, Strehl J, et al. Invasive fungal infections in neutropenic enterocolitis: a systematic analysis of pathogens, incidence, treatment and mortality in adult patients. BMC Infect Dis. 2006;6:35. doi:10.1186/1471-2334-6-35
9. Zhang H, Zhu A. Emerging invasive fungal infections: clinical features and controversies in diagnosis and treatment processes. Infect Drug Resist. 2020;13:607–615. doi:10.2147/IDR.S237815
10. Kauffman CA. Fungal infections in older adults. Clin Infect Dis. 2001;33(4):550–555. doi:10.1086/322685
11. Jenks JD, Cornely OA, Chen SC, Thompson GR
12. Martino R, Subira M. Invasive fungal infections in hematology: new trends. Ann Hematol. 2002;81(5):233–243. doi:10.1007/s00277-002-0466-3
13. Pinna A, Donadu MG, Usai D, Dore S, Boscia F, Zanetti S. In-vitro antimicrobial activity of a new ophthalmic solution containing hexamidine diisethionate 0.05% (keratosept). Cornea. 2020;39(11):1415–1418. doi:10.1097/ICO.0000000000002375
14. Pinna A, Donadu MG, Usai D, et al. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM®). Acta Ophthalmol. 2020;98(2):e178–e180. doi:10.1111/aos.14243
15. Raj N, Vanathi M, Ahmed NH, Gupta N, Lomi N, Tandon R. Recent perspectives in the management of fungal keratitis. J Fungi. 2021;7(11):907. doi:10.3390/jof7110907
16. Donadu MG, Peralta-Ruiz Y, Usai D, et al. Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant Candida strains. J Fungi. 2021;7(5):383. doi:10.3390/jof7050383
17. Barac A, Donadu M, Usai D, et al. Antifungal activity of Myrtus communis against Malassezia sp. isolated from the skin of patients with pityriasis versicolor. Infection. 2018;46(2):253–257. doi:10.1007/s15010-017-1102-4
18. Bua A, Usai D, Donadu MG, et al. Antimicrobial activity of Austroeupatorium inulaefolium (H.B.K.) against intracellular and extracellular organisms. Nat Prod Res. 2018;32(23):2869–2871. doi:10.1080/14786419.2017.1385014
19. Berkow EL, Lockhart SR. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist. 2017;10:237–245. doi:10.2147/IDR.S118892
20. Arendrup MC, Patterson TF. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(suppl_3):S445–S451. doi:10.1093/infdis/jix131
21. Society of Critical Care Medicine CMA. Guidelines for the diagnosis and treatment of invasive fungal infections in critically ill patients. Chin J Intern Med. 2007;46(11):960–966.
22. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts.
23. Wang X, Liu P, Cao X. Genetic mutation of ERG11 and ERG3 and the relation between Candida and fluconazole resistance. Chin J Dermatovenereol. 2018;32(4):387–390.
24. Jiang C, Dong D, Yu B, et al. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J Antimicrob Chemother. 2013;68(4):778–785. doi:10.1093/jac/dks481
25. Von Lilienfeld-toal M, Wagener J, Einsele H, Cornely OA, Kurzai O. Invasive fungal infection. Dtsch Arztebl Int. 2019;116(16):271–278. doi:10.3238/arztebl.2019.0271
26. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi:10.1086/421946
27. Baddley JW, Stroud TP, Salzman D, Pappas PG. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001;32(9):1319–1324. doi:10.1086/319985
28. Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42(10):4419–4431. doi:10.1128/JCM.42.10.4419-4431.2004
29. Armstrong-James D, Youngs J, Bicanic T, et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur Respir J. 2020;56(4):2002554. doi:10.1183/13993003.02554-2020
30. Vijay S, Bansal N, Rao BK, et al. Secondary infections in hospitalized COVID-19 patients: Indian experience. Infect Drug Resist. 2021;14:1893–1903. doi:10.2147/IDR.S299774
31. Wu Q, Xie X, Liu W, Wu Y. Implementation efficiency of the hierarchical diagnosis and treatment system in China: a case study of primary medical and health institutions in Fujian province. Int J Health Plann Manage. 2021;1–14. doi:10.1002/hpm.3333
32. Hajjeh RA, Sofair AN, Harrison LH, et al. Incidence of bloodstream infections due to Candida species and in-vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42(4):1519–1527. doi:10.1128/JCM.42.4.1519-1527.2004
33. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383–e392. doi:10.1016/S1473-3099(17)30316-X
34. Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine. 2000;79(4):250–260. doi:10.1097/00005792-200007000-00006
35. Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, Beney J. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5(1):26–34. doi:10.1046/j.1524-4733.2002.51108.x
36. Muskett H, Shahin J, Eyres G, Harvey S, Rowan K, Harrison D. Risk factors for invasive fungal disease in critically ill adult patients: a systematic review. Crit Care. 2011;15(6):R287. doi:10.1186/cc10574
37. Schauwvlieghe A, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–792. doi:10.1016/S2213-2600(18)30274-1
38. Li H, Guo M, Wang C, et al. Epidemiological study of Trichosporon asahii infections over the past 23 years. Epidemiol Infect. 2020;148:e169. doi:10.1017/S0950268820001624
39. Xiao M, Sun ZY, Kang M, et al. Five-year national surveillance of invasive candidiasis: species distribution and azole susceptibility from the China hospital invasive fungal surveillance net (CHIF-NET) study. J Clin Microbiol. 2018;56(7). doi:10.1128/JCM.00577-18
40. Xiao Y, Zhang J, Zheng B, Zhao L, Li S, Li L. Changes in Chinese policies to promote the rational use of antibiotics. PLoS Med. 2013;10(11):e1001556. doi:10.1371/journal.pmed.1001556
41. Edwards JE
42. Yinzhong S, Hongzhou L, Yongxin Z. Analysis of ERG11 gene mutations in fluconazole-resistant Candida glabrata strains. Chin J Infect Dis. 2010;28(6):331–335.
43. Robbins N, Wright GD, Cowen LE. Antifungal drugs: the current armamentarium and development of new agents. Microbiol Spectr. 2016;4(5). doi:10.1128/microbiolspec.FUNK-0002-2016
44. Svetaz LA, Postigo A, Butassi E, Zacchino SA, Sortino MA. Antifungal drugs combinations: a patent review 2000–2015. Expert Opin Ther Pat. 2016;26(4):439–453. doi:10.1517/13543776.2016.1146693
45. Janeth Rimachi Hidalgo K, Carmello JC, Carolina Jordao C, et al. Antimicrobial photodynamic therapy in combination with nystatin in the treatment of experimental oral candidiasis induced by Candida albicans resistant to fluconazole. Pharmaceuticals. 2019;12(3):140. doi:10.3390/ph12030140
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.