Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Identification of Genes Related to Endoplasmic Reticulum Stress (ERS) in Chronic Obstructive Pulmonary Disease (COPD) and Clinical Validation
Authors Tao S, Jing J, Wang Y , Li F , Ma H
Received 15 October 2023
Accepted for publication 15 December 2023
Published 27 December 2023 Volume 2023:18 Pages 3085—3097
DOI https://doi.org/10.2147/COPD.S440692
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Siming Tao,1,* Jing Jing,1,2,* Yide Wang,1,3,* Fengsen Li,1,2 Hongxia Ma1,2
1Department of Respiratory and Critical Care Medicine, Fourth Affiliated Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China; 2Xinjiang Laboratory of Respiratory Disease Research, Traditional Chinese Medicine Hospital Affiliated to Xinjiang Medical University, Urumqi, People’s Republic of China; 3Department of Respiratory Medicine, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fengsen Li; Hongxia Ma, Department of Respiratory and Critical Care Medicine, Fourth Affiliated Hospital of Xinjiang Medical University, Urumqi, 830000, People’s Republic of China, Tel +86 13999980996 ; +86 13999995669, Email [email protected]; [email protected]
Objective: Endoplasmic reticulum stress (ERS) is key in chronic obstructive pulmonary disease (COPD) incidence and progression. This study aims to identify potential ERS-related genes in COPD through bioinformatics analysis and clinical experiments.
Methods: We first obtained a COPD-related mRNA expression dataset (GSE38974) from the Gene Expression Omnibus (GEO) database. The R software was then used to identify potential differentially expressed genes (DEGs) of COPD-related ERS (COPDERS). Subsequently, the identified DEGs were subjected to protein-protein interaction (PPI), correlation, Gene Ontology (GO) enrichment, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. Following that, qRT-PCR was used to examine the RNA expression of six ERS-related DEGs in blood samples obtained from the COPD and control groups. The genes were also subjected to microRNA analysis. Finally, a correlation analysis was performed between the DEGs and key clinical indicators.
Results: Six ERS-related DEGs (five upregulated and one downregulated) were identified based on samples drawn from 23 COPD patients and nine healthy individuals enrolled in the study. Enrichment analysis revealed multiple ERS-related pathways. The qRT-PCR and mRNA microarray bioinformatics analysis results showed consistent STC2, APAF1, BAX, and PTPN1 expressions in the COPD and control groups. Additionally, hsa-miR-485-5p was identified through microRNA prediction and DEG analysis. A correlation analysis between key genes and clinical indicators in COPD patients demonstrated that STC2 was positively and negatively correlated with eosinophil count (EOS) and lymphocyte count (LYM), respectively. On the other hand, PTPN1 showed a strong correlation with pulmonary function indicators.
Conclusion: Four COPDERS-related key genes (STC2, APAF1, BAX, and PTPN1) were identified through bioinformatics analysis and clinical validation, and the expressions of some genes exhibited a significant correlation with the selected clinical indicators. Furthermore, hsa-miR-485-5p was identified as a potential key target in COPDERS, but its precise mechanism remains unclear.
Keywords: endoplasmic reticulum stress, chronic obstructive pulmonary disease, bioinformatics analysis, clinical prediction
Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory illness characterized by a not completely reversible and progressive airflow restriction, which seriously affects patients’ quality of life. A 2018 report revealed that the COPD incidence in China was 13.7%, with approximately 100 million people having the disease.1 Furthermore, COPD has become a major public health concern, given its huge economic burden on patients’ families and society. Hitherto, the pathogenesis of COPD remains unclear. Increasing evidence suggests that COPD incidence and progression may lead to a functional endoplasmic reticulum stress (ERS) imbalance, which is involved in the COPD pathophysiological process. According to research, ERS is one of the early pathological manifestations of COPD and the major cause of apoptosis in alveolar epithelial cells.2–4 In a recent study, therapeutic benefits were observed in a COPD animal model through drugs alleviating ERS or inhibiting key molecules in the associated signaling pathway, highlighting potential avenues for COPD treatment despite its intricate pathogenesis.5 The biological effects of ERS might be associated with emphysema, and considering the varying severity levels among COPD patients, it is reasonable to speculate that the severity of COPD could be linked to this specific biological phenotype, ERS.
To ensure the correct protein folding and maintenance of Endoplasmic Reticulum (ER) function, ERS triggers a series of cellular processes, including initiating the Unfolded Protein Reaction (UPR), reprogramming gene transcription, mRNA translation, and protein modification to eliminate unfolded or misfolded proteins; hence, restoring protein homeostasis.6,7 Recent research has demonstrated that Ursolic Acid (UA) can not only suppress the UPR signaling pathway to alleviate ERS-related apoptosis and Oxidative Stress (OS) but can also eliminate the Cigarette Smoke (CS)-induced airway remodeling via regulating three UPR pathways, thereby exerting therapeutic effects on COPD. The exogenous 14, 15-EET can prevent CS-induced cell apoptosis by inhibiting the UPR signaling pathway. Furthermore, adiponectin alleviated apoptosis in the alveolar epithelial cells of COPD-infected rats by inhibiting ERS. It has also been reported that CS can activate the ER-related signaling pathway PERK/eIF2 α/ ATF4/CHOP to upregulate caspase-3, caspase-8, caspase-12, and BCL-2-related Bax, and to downregulate BCL-2.8,9 The stabilization of ER function may play a critical role in COPD progression. However, there are still many unknowns about COPD and ERS-related genes. Therefore, more research is required to further explore and reveal the potential ERS-related genes in COPD, thus providing potential targets for COPD prevention and treatment.
Ezzie et al10 created a COPD-related dataset (GSE38974) based on lung tissue samples and an analysis of DEGs of COPD patients and healthy individuals using microchip technology. They discovered that 70 miRNAs and 2667 mRNAs were differentially expressed in the two groups. Herein, we conducted a secondary analysis of GSE38974 from an ERS perspective. Specifically, the COPD- and ERS-related (COPDERS) DEGs were generalized and summarized by analyzing the GSE38974 dataset. Subsequently, the identified ERS-related DEGs were subjected to bioinformatics analysis. Following that, the underlying mechanism of ERS-related genes associated with COPD incidence and progression was preliminarily explored by validating clinical specifications.
Materials and Methods
A total of 293 genes were obtained from the Human Gene Database (https://www.genecards.org/) using “ERS” as the keyword. The GSE38974 dataset was downloaded from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). The dataset is found in the GPL4133 platform (Agilent-014850 Whole Human Genome Microarray 4x44K G4112F) and contains 23 COPD and nine normal lung tissue samples. The screening criteria was set as FDR<0.05.
Analysis of ERS-Related DEGs
The normalized expression matrix for microarray data was first downloaded from GSE38974. The probe was then annotated using the annotation file from the dataset. The repeatability of data in GSE38974 was verified through Principal Component Analysis (PCA). The “limma” package in R software was used to identify ERS-related differentially expressed genes (DEGs). Genes with an adjusted P-value < 0.05 and an absolute folding change value > 1.5 were considered differentially expressed. The “heatmap” and “ggplot2” packages in R software were used to draw heat maps, volcanic maps, and box plots and to perform the protein-protein interaction (PPI) and correlation analyses.
ERS-Related DEGs
The STRING database (https://STRING-db.org/) and Cytoscape (Version 3.8.1) were used for the PPI analysis of ERS-related genes. For correlation analysis, the ERS-related DEGs were subjected to the Spearman correlation analysis using the “corrplot” package in R software.
ERS-Related Genes GO and KEGG Pathway Enrichment
The “GO plot” package in R software was used to perform the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. Cellular Component (CC), Biological Process (BP) and Molecular Function (MF) were included in GO analysis as the three main cellular categories.
Validation of Clinical Specimens
Herein, 40 participants were enrolled and divided into two groups, each with 20 individuals: COPD and control (healthy individuals). The participants had consistent ages and were recruited from the Fourth Affiliated Hospital of Xinjiang Medical University, China, between January 2023 and June 2023. The COPD diagnosis followed the Global Initiative for Chronic Obstructive Lung Disease (GOLD) standards: patients with a FEV1/FVC value < 70% and a FEV1 predicted value < 80% after treatment with a bronchodilator agent. Patients with bronchial asthma, bronchiectasis, pulmonary fibrosis, lung tumors, and tuberculosis were excluded from the study. The 20 healthy individuals were recruited from the hospital’s health examination center. This study complies with the Declaration of Helsinki and was ethically approved by the hospital’s Medical Ethics Committee. Written informed consent was obtained from all participants before the study commenced. Venous blood was collected from all study participants.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
We employed Ficoll solution for the isolation of Peripheral Blood Mononuclear Cells (PBMCs), with the primary steps involving the use of two 15mL centrifuge tubes. Initially, 5mL of Ficoll solution was added to each tube. Subsequently, diluted blood was gently layered onto the Ficoll upper layer in both tubes, with 10mL of diluted blood in each tube. Centrifugation was then conducted at 2000rpm for 20 minutes, resulting in the final acquisition of PBMCs. Each participant’s blood sample was processed to isolate PBMCs using the Ficoll solution (Solarbio, China). An RNA extraction kit (Omega, China) was used to extract total RNA from the PBMCs. Reverse transcription was performed using the Prime Script RT Master Mix Kit (Takara, China). Following the manufacturer’s instructions, the TB Green Premix Ex Taq Kit (Takara, China) was used to evaluate the mRNA levels. The primers used in RNA extraction are listed in Supplementary Table 1. The 2−ΔΔCt method was used to calculate mRNA expression after normalization to ACTB. The detailed process of this study is depicted in Figure 1.
 |
Figure 1 The detailed analysis process of this study. |
Results
Differential Expressions of COPD ERS-Related Genes
The clinical characteristics of COPD patients in the public dataset (GSE38974) are detailed in Supplementary Table 2. We performed PCA to evaluate the repeatability of data within GSE38974. The results showed that GSE38974 had good data repeatability (Figure 2A). Among the 23 COPD patients and nine healthy individuals, six ERS-related genes [one upregulated gene and five downregulated genes (Table 1)] were identified based on the standards described earlier (an adjusted P-value < 0.05 and an absolute fold change value >1.5) (Figure 2B). The six ERS-related DEGs of the COPD and normal groups were displayed in volcano and heat maps after analyzing the GSE38974 dataset using R software (Figure 2C and D). The chromosomal localization of key genes is shown in Figure 2E. ERS-related genes and the DEG list for the GSE38974 dataset are shown in Supplementary Tables 3 and 4.
 |
Table 1 The Specific Expression Levels of ERS-Related Genes |
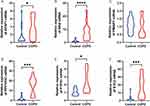
The box plots show the expression patterns of six ERS-related DEGs in the COPD and normal samples (Figure 3). They specifically show one downregulated gene (PPP1R3C) and five upregulated genes (APAF1, BAX, PMAIP1, PTPN1, and STC2).
 |
Figure 3 Expressions of ERS-related genes in the GSE38974 dataset. **Indicates p<0.01, ***Indicates p < 0.001. |
PPI Network and Correlation Analyses of ERS-Related DEGs
The interactions among ERS-related DEGs were determined through PPI analysis. The results showed that the six ERS-related genes interacted with each other (Figure 4A), and the number of interactions for each gene is shown in Figure 4B. A correlation analysis was performed to explore the expression correlation of the identified ERS genes. The results showing the relationship among the six ERS-related DEGs in the GSE38974 dataset are depicted Figure 4C.
GO and KEGG Enrichment Analysis of ERS-Related DEGs
The GO and KEGG enrichment analyses were performed using R software to analyze the potential biological functions of ERS-related genes expressed in different sources. The results showed that the DEGs were significantly enriched in three main categories: BP (DNA metabolism regulation, calcium ion homeostasis, and aromatic compound catabolism); CC (outer membrane, organelle outer membrane, and mitochondrial outer membrane); and MF (phosphatase binding, phosphatase activity, and phosphate ester hydrolase activity) (Figure 5A and B and Supplementary Table 5).
 |
Figure 5 Enrichment analysis of ERS genes. Notes: (A and B) Conducting differential gene enrichment analysis using R software for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). |
The GSEA analysis revealed that ERS-related DEGs mainly participated in apoptosis pathways related to JAK-STAT, TP53, IL-10, and IL-11, as well as NF-κB and other inflammation-related signaling pathways (Figure 6A–I and Supplementary Table 6).
Validation of ERS-Related DEGs in COPD Patients
The expression levels of the six ERS-related DEGs were further identified in clinical samples through qRT-PCR to verify the reliability of GSE38974. Consistent with the results of mRNA microarray in lung tissue samples, the APAF1, BAX, PPP1R3C, PTPN1, and STC2 expression levels were significantly higher in COPD blood samples than in normal blood samples (Figure 7A, B and D–F). The low expression gene PMAIP1 defied the trend predicted by bioinformatics analysis, and there was no significant difference in PMAIP1 expression levels between the two groups (Figure 7C). Table 2 summarizes the clinical pathological changes in the COPD and control groups. Supplementary Table 7 presents the basic information for the included patients.
 |
Table 2 Correlation Analysis Between the ERS-Related Gene Expression Levels and Clinical Indicators |
Key Genes-Related MicroRNA Analysis
MicroRNA analysis was performed on the above-screened DEGs, and the results revealed that the predicted microRNAs corresponding to PPP1R3C and PMAIP1 were not intersected. On the other hand, several intersecting microRNAs were identified (Figure 8), and hsa-miR-485-5p was particularly related to STC2, APAF1, BAX, and PTPN1.
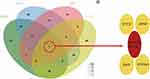 |
Figure 8 Key genes-related MicroRNA analysis. |
Correlation Analysis Between the ER-Related Gene Expression Levels and Clinical Indicators
Here, the correlation between the ERS-related gene expression levels and clinical pulmonary function indicators, such as eosinophil count (EOS), and lymphocyte count (LYM) was analyzed (Table 2). According to the results, PTPN1 was negatively correlated with FEV1% and FEV1/FVC (P<0.05) (Figure 9A and B), while STC2 was positively correlated with EOS and negatively correlated with LYM (P<0.05) (Figure 9C and D).
Discussion
Emphysema, chronic inflammation of the airway and lung parenchyma, and systemic inflammatory reactions are among the main pathological changes of COPD. On the other hand, the main causes of COPD incidence and progression are exposure factors such as biofuels, CS, and air pollution. Studies have shown that ERS-induced cell apoptosis is critically involved in COPD pathogenesis. Furthermore, it has been confirmed that COPD incidence and progression and hormone resistance in emphysema patients are related to ERS,11 although the precise mechanism needs to be further investigated.
In COPD, exposure to toxic substances such as CS induces inflammatory cell infiltration in lung tissues and increases the release of several inflammatory factors, including TNF-ɑ, IL-1, IL-6, and INF-γ. These cytokines can activate ERS and UPR. Furthermore, ERS-induced cell apoptosis is associated with Reactive Oxygen Species (ROS). The inhibition of glutathione peroxidase 1 enhances the CS-induced ERS and cell apoptosis. As a result, ERS may be a pathological event associated with oxidative and antioxidant imbalance in COPD.12 It has been reported that ERS can trigger HRD1 to protect alveolar type II epithelial cells from Cigarette Smoke Extract (CSE)-induced apoptosis.13 Protein Disulfide Isomerase (PDI) levels are often increased in smokers. Acute CS exposure alters PDI expression levels, affecting the folding of oxidative proteins in the ER, leading to ERS incidence and progression.14 Additionally, UPR has been shown to play a key role in COPD incidence.15,16 Specifically, UPR activity is severely impaired in COPD patients, with the accumulation of ubiquitin proteins as its specific manifestation, indicating elevated levels of unfolded and/or misfolded proteins leading to ERS induction and UPR activation.11 Furthermore, mRNA and protein levels of UPR markers and their downstream effectors, including ATF6, IRE1a, p-PERK, p-eIF2a, ATF4, GRP94, EDEM, and CHOP, are upregulated in human primary epithelial cells isolated from the lungs of COPD patients.12
Herein, DEGs were investigated using GO and KEGG enrichment analyses to clarify the ERS-related biological functions. The results revealed that ERS-related DEGs are primarily involved in biological processes such as DNA metabolism regulation, calcium ion homeostasis, and aromatic compound catabolism; cell components such as outer membrane, organelle outer membrane, and mitochondrial outer membrane; and molecular functions such as phosphatase binding, phosphatase activity, and phosphate ester hydrolase activity. Moreover, ERS has been reported to be involved in COPD occurrence and development, whether in COPD animal models or in vitro experimental studies such as those involving lung tissues of COPD-infected rats, where significantly upregulated ERS key proteins (CHOP and GRP78) and mRNA were detected.17,18 Furthermore, the GRP78, ATF4, ATF6, and CHOP protein expression levels, as well as the PERK and IRE1 phosphorylation levels, were significantly increased in lung tissues of CS-induced COPD mice.4 A previous study reported that the expression of UPR-related proteins in rat lung tissue rose significantly with the increasing duration of CS exposure.19 Several in vitro studies have confirmed that CSE exposure triggers ERS in airway epithelial cells and other related cells, such as lung cancer cells and alveolar macrophages.20,21 Under CS-induced ERS conditions, intracellular protein homeostasis is disrupted due to the reduced proteasome degradation of misfolded proteins, resulting in the build-up of damaged proteins and reduced new protein synthesis.22 The biological functions of ERS-related DEGs may be further explored in future research.
Based on the bioinformatics analysis results, the qRT-PCR was employed to further identify the expression levels of six ERS-related genes in clinical samples. According to the results, the expression levels of the four upregulated genes (APAF1, BAX, PTPN1, and STC2) were consistent with the bioinformatics mRNA microarray analysis results. Furthermore, no statistical difference was found between healthy individuals and COPD patients in PMAIP1 gene validation. Additionally, the low-expression gene PPP1R3C defied the predictions of bioinformatics analysis. Therefore, the remaining four high-expression genes were the focus of subsequent analyses. Some of these genes have been confirmed to be associated with COPD occurrence and development, with the most reported genes being APAF1 and BAX,23–25 which have specifically been linked directly to the apoptotic phenotype of COPD cells. According to research, PTPN1 and STC2 are yet to be associated with COPD, but STC2 has been frequently reported in tumor research.26 In addition to enhancing the cell processing of stress conditions and preventing cell apoptosis, STC2 also promotes the development of acquired resistance to chemotherapy and radiotherapy. However, the specific mechanisms of these genes in COPDERS are yet to be comprehensively explored, necessitating further investigation.
Multiple studies have proposed ERS as a potential target for COPD treatment. For instance, Wang et al27 discovered that 4-PBA prevented CS-induced emphysema, alveolar apoptosis, and inflammation by inhibiting ERS and NF-κB signaling. Additionally, H2S, as an endogenous modulator, alleviated CS-induced rat lung cell apoptosis and Epithelial-Mesenchymal Transition (EMT) by suppressing endoplasmic enzyme stress, thereby impeding the progression of lung function decline and emphysema formation.28–30 It has also been reported that melatonin inhibits ERS and mitochondrial dysfunction, counteracting CS-induced inflammasome activation and apoptosis.31,32
The specific relationships between key genes and clinical indicators were also analyzed in the study. Notably, PTPN1 was negatively correlated with pulmonary function indicators. Salazar et al33 measured several phenotypes associated with obesity markers and obesity-related biochemical and clinical parameters in 1057 European adolescents via five polymorphic genotyping analyses of PTPN1. The results showed that PTPN1 variants were associated with obesity, and physical activity appeared to regulate genetic tendencies. Furthermore, an epidemiologic study34 discovered that fat was nonlinearly related to pulmonary function indicators and that an excessively high fat level was negatively correlated with pulmonary function indicators. Therefore, it is reasonable to hypothesize that PTPN1 significantly affects pulmonary function indicators and that the effect of PTPN1 on COPD patients’ pulmonary function may be related to the regulation of fat metabolism-related gene expression. Recent clinical trials have demonstrated that EOS and LYM are strongly associated with response, risk of exacerbation, mortality rate, and length of hospitalization in COPD.35,36 Herein, STC2 was positively correlated with EOS and negatively correlated with LYM. Therefore, modulating STC2, which in turn affects EOS and LYM, may affect or improve the status of COPD patients.
Nevertheless, there are several limitations to this study. First, specimens for bioinformatics analysis were obtained from the lungs of nine healthy smokers and 26 COPD patients, while the clinical validation specimens were drawn from the peripheral blood, and this could have caused some errors in the experimental results. However, it is noteworthy that the microarray sequencing of the lung tissue specimen increased the sensitivity and reliability of the bioinformatics analysis results. Second, the sample size was relatively small when clinical specimens were validated, necessitating further multi-center and large-sample clinical trials for further verification. Third, this COPDERS study was only a preliminary bioinformatics analysis and a DEG expression level detection. Therefore, the precise ERS mechanism needs to be further explored in future animal and/or cell model experiments.
Conclusions
Herein, four key COPDERS-related genes were confirmed by bioinformatics analysis and clinical validation. In COPD samples, STC2 was positively and negatively correlated with EOS and LYM, respectively. On the other hand, PTPN1 showed a significant correlation with pulmonary function indicators. Moreover, hsa-miR-485-5p was found to be a potential key target in the ERS mechanism. Nonetheless, future studies may involve other animal and/or cell models to further validate the precise ERS mechanism of action, providing a new basis and direction for COPD prevention and treatment.
Data Sharing Statement
The corresponding authors will provide Supplemental Material and all other data used this study upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Xinjiang Uygur Autonomous Region Chinese Medicine Hospital (IRB ID: 2016XE-YS093).
Consent for Publication
All participants were informed of the study’s potential risks and provided a written informed consent.
Acknowledgments
We would like to thank all the participants and data collectors who were involved in this study.
Author Contributions
All authors contributed significantly to various key aspects of this study, including conception, study design, execution, acquisition of data, analysis, and interpretation, as well other areas, such as writing the manuscript (drafting, revising, or critically reviewing the article), giving final approval of the version to be published, agreeing on the journal to which the article should be submitted, and agreeing to be accountable for all aspects of this report.
Funding
This study was supported by the 2023 Tianshan Innovation Team Plan Project “Research Innovation Team of Integrated Chinese and Western Medicine Prevention and Treatment of Chronic Obstructive Pulmonary Disease” (Project number: 2023D14004); Tianshan Talent - Youth Science and Technology Top Talent Project (Project number: 2022TSYCCX0028); and Xinjiang Uygur Autonomous Region Health Young Medical Science and Technology Talents Special Research Project (Project number: WJWY-201957).
Disclosure
The authors declare no conflict of interest.
References
1. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
2. Barreiro E, Salazar-Degracia A, Sancho-Munoz A, Aguilo R, Rodriguez-Fuster A, Gea J. Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dysfunction of patients with stable chronic obstructive pulmonary disease. J Appl Physiol. 2019;126:1572–1586. doi:10.1152/japplphysiol.00670.2018
3. Wu G, Yuan T, Zhu H, et al. Chrysophanol protects human bronchial epithelial cells from cigarette smoke extract (CSE)-induced apoptosis. Int J Mol Epidemiol Genet. 2020;11:39–45.
4. Wang HL, Chen FQ, Wu LJ. Ephedrine ameliorates chronic obstructive pulmonary disease (COPD) through restraining endoplasmic reticulum (ER) stress in vitro and in vivo. Int Immunopharmacol. 2022;103:107842. doi:10.1016/j.intimp.2021.107842
5. Naiel S, Tat V, Padwal M, et al. Protein misfolding and endoplasmic reticulum stress in chronic lung disease: will Cell-Specific targeting be the key to the cure? Chest. 2020;157:1207–1220. doi:10.1016/j.chest.2019.11.009
6. Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421–438. doi:10.1038/s41580-020-0250-z
7. Oakes SA. Endoplasmic reticulum proteostasis: a key checkpoint in cancer. Am J Physiol Cell Physiol. 2017;312:C93–C102. doi:10.1152/ajpcell.00266.2016
8. Lin L, Hou G, Han D, Kang J, Wang Q. Ursolic acid protected lung of rats from damage induced by cigarette smoke extract. Front Pharmacol. 2019;10:700. doi:10.3389/fphar.2019.00700
9. Lin L, Yin Y, Hou G, Han D, Kang J, Wang Q. Ursolic acid attenuates cigarette smoke-induced emphysema in rats by regulating PERK and Nrf2 pathways. Pulm Pharmacol Ther. 2017;44:111–121. doi:10.1016/j.pupt.2017.03.014
10. Ezzie ME, Crawford M, Cho JH, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67(2):122–131. doi:10.1136/thoraxjnl-2011-200089
11. Min T, Bodas M, Mazur S, et al. Critical role of proteostasis-imbalance in athogenesis of COPD and severe emphysema. J Mol Med. 2011;89(6):577–593. doi:10.1007/s00109-011-0732-8
12. Geraghty P, Baumlin N, Salathe MA, et al. Glutathione peroxidase-1 suppresses the unfolded protein response upon cigarette smoke exposure. Mediators Inflammation. 2016;2016:9461289. doi:10.1155/2016/9461289
13. Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev. 1999;79(3):683–701. doi:10.1152/physrev.1999.79.3.683
14. Kenche H, Baty CJ, Vedagiri K, et al. Cigarette smoking affects oxidative protein folding in endoplasmic reticulum by modifying protein disulfide isomerase. FASEB J. 2013;27(3):965–977. doi:10.1096/fj.12-216234
15. Korfei M, MacKenzie BA, Meiners S. The ageing lung under stress. Eur Respir Rev. 2020;29:200126. doi:10.1183/16000617.0126-2020
16. Kelsen SG. The unfolded protein response in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:S138–S145. doi:10.1513/AnnalsATS.201506-320KV
17. Tang F, Ling C. Curcumin ameliorates chronic obstructive pulmonary disease by modulating autophagy and endoplasmic reticulum stress through regulation of SIRT1 in a rat model. J Int Med Res. 2019;47:4764–4774. doi:10.1177/0300060519869459
18. Wang Y, Su NX, Pan SG, Ge XP, Dai XP. Fengbaisan suppresses endoplasmic reticulum stress by up-regulating SIRT1 expression to protect rats with chronic obstructive pulmonary diseases. Pharm Biol. 2020;58:878–885. doi:10.1080/13880209.2020.1806335
19. Gan G, Hu R, Dai A, et al. The role of endoplasmic reticulum stress in emphysema results from cigarette smoke exposure. Cell Physiol Biochem. 2011;28:725–732. doi:10.1159/000335766
20. Ito H, Yamashita Y, Tanaka T, et al. Cigarette smoke induces endoplasmic reticulum stress and suppresses efferocytosis through the activation of RhoA. Sci Rep. 2020;10:12620. doi:10.1038/s41598-020-69610-x
21. Yoo YM, Jung EM, Jeon BH, Tran DN, Jeung EB. Cigarette smoke extraxt influences intracellular calcium concentration in A549 cells. J Physiol Pharmacol. 2020;71:679–687.
22. Somborac-Bacura A, van der Toorn M, Franciosi L, et al. Cigarette smoke induces endoplasmic reticulum stress response and proteasomal dysfunction in human alveolar epithelial cells. Exp Physiol. 2013;98:316–325. doi:10.1113/expphysiol.2012.067249
23. Wu H, Miao Y, Shang LQ, Chen RL, Yang SM. MiR-31 aggravates inflammation and apoptosis in COPD rats via activating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(18):9626–9632. doi:10.26355/eurrev_202009_23051
24. Kuwano K, Yoshimi M, Maeyama T, Hamada N, Yamada M, Nakanishi Y. Apoptosis signaling pathways in lung diseases. Med Chem. 2005;1(1):49–56. doi:10.2174/1573406053402497
25. Foronjy RF, Salathe MA, Dabo AJ, et al. TLR9 expression is required for the progress of cigarette smoke-induced emphysema in mice. Am J Physiol Lung Cell Mol Physiol. 2016;311(1):L154–66. doi:10.1152/ajplung.00073.2016
26. Gao J, Lei T, Wang H, et al. Dimethylarginine dimethylaminohydrolase 1 protects PM2.5 exposure-induced lung injury in mice by repressing inflammation and oxidative stress. Part Fibre Toxicol. 2022;19(1):64. doi:10.1186/s12989-022-00505-7
27. Qie S, Sang N. Stanniocalcin 2 (STC2): a universal tumour biomarker and a potential therapeutical target. J Exp Clin Cancer Res. 2022;41(1):161. doi:10.1186/s13046-022-02370-w
28. Wang Y, Wu ZZ, Wang W. Inhibition of endoplasmic reticulum stress alleviates cigarette smoke-induced airway inflammation and emphysema. Oncotarget. 2017;8:77685–77695. doi:10.18632/oncotarget.20768
29. Lin F, Liao C, Zhang J, et al. Hydrogen sulfide inhibits bronchial epithelial cell epithelial mesenchymal transition through regulating endoplasm reticulum stress. Front Mol Biosci. 2022;9:828766. doi:10.3389/fmolb.2022.828766
30. Lin F, Liao C, Sun Y, et al. Hydrogen sulfide inhibits cigarette Smoke-Induced endoplasmic reticulum stress and apoptosis in bronchial epithelial cells. Front Pharmacol. 2017;8:675. doi:10.3389/fphar.2017.00675
31. Ding HB, Liu KX, Huang JF, Wu DW, Chen JY, Chen QS. Protective effect of exogenous hydrogen sulfide on pulmonary artery endothelial cells by suppressing endoplasmic reticulum stress in a rat model of chronic obstructive pulmonary disease. Biomed Pharmacother. 2018;105:734–741. doi:10.1016/j.biopha.2018.05.131
32. Mahalanobish S, Dutta S, Saha S, Sil PC. Melatonin induced suppression of ER stress and mitochondrial dysfunction inhibited NLRP3 inflammasome activation in COPD mice. Food Chem Toxicol. 2020;144:111588. doi:10.1016/j.fct.2020.111588
33. He B, Zhang W, Qiao J, Peng Z, Chai X. Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can J Physiol Pharmacol. 2019;97:386–391. doi:10.1139/cjpp-2018-0529
34. Salazar-Tortosa DF, Labayen I, González-Gross M, et al. Association between PTPN1 polymorphisms and obesity-related phenotypes in European adolescents: influence of physical activity. Pediatr Res. 2023;93(7):2036–2044. doi:10.1038/s41390-022-02377-1
35. Wang Y, Li Z, Li F. Nonlinear relationship between visceral adiposity index and lung function: a population-based study. Respir Res. 2021;22(1):161. doi:10.1186/s12931-021-01751-7
36. Russell REK, Bafadhel M. Investigating blood eosinophil count thresholds in patients with COPD. Lancet Respir Med. 2018;6(11):823–824. doi:10.1016/S2213-2600(18)30415-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.