Back to Journals » Journal of Inflammation Research » Volume 16
Identification of Ferroptosis-Related Genes in Heart Failure Induced by Transverse Aortic Constriction
Authors Gu JJ, Du TJ, Zhang LN, Zhou J, Gu X , Zhu Y
Received 27 August 2023
Accepted for publication 20 October 2023
Published 31 October 2023 Volume 2023:16 Pages 4899—4912
DOI https://doi.org/10.2147/JIR.S433387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jian Jun Gu,1,2,* Tian Jian Du,1,2,* Li Na Zhang,3,* Jing Zhou,4 Xiang Gu,2 Ye Zhu2
1Department of Cardiology, Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou, Jiangsu, People’s Republic of China; 2Department of Cardiology, Northern Jiangsu People’s Hospital, Yangzhou, Jiangsu, People’s Republic of China; 3Department of Cardiology, The Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, Jiangsu, People’s Republic of China; 4Key Laboratory of Animal Breeding Reproduction and Molecular Design for Jiangsu Province, College of Animal Science and Technology, Yangzhou University, Yangzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ye Zhu; Xiang Gu, Department of Cardiology, Northern Jiangsu People’s Hospital, 98 Nantong West Road, Yangzhou, Jiangsu, People’s Republic of China, Email [email protected]; [email protected]
Background: Heart failure (HF) is a common clinical syndrome due to ventricular dysfunction and is a major cause of mortality worldwide. Ferroptosis, marked by excessive iron-dependent lipid peroxidation, is closely related to HF. Therefore, the purpose of this study is to explore and validate ferroptosis-related markers in HF by bioinformatics analysis and animal experiments validation.
Materials and Methods: The gene expression profiles (GSE36074) of murine transverse aortic constriction (TAC) were obtained from the Gene Expression Omnibus (GEO); From the FerrDb database, ferroptosis-related genes (FRGs) were identified. Using GEO2R, differential expressed genes (DEGs) were screened. An overlapping analysis was conducted among DEGs and FRGs to identify ferroptosis-related DEGs (FRDEGs). We then performed clustering, functional enrichment analysis, and protein-protein interaction (PPI) analyses. In addition, the key FRDEGs were extracted by cytoHubba plugin and the networks of transcription factors (TFs)-key FRDEGs and microRNA-key FRDEGs were constructed. Lastly, the key FRDEGs were carried by quantitative reverse transcription PCR (RT-qPCR) and immunohistochemistry (IHC).
Results: Fifty-nine FRGs showing significantly different expression were identified from a total of 1918 DEGs in mice heart by transverse aortic constriction. GO and KEGG functional enrichment analysis revealed that these 59 ferroptosis-related DEGs mostly associated with positive regulation of apoptotic process, FoxO signaling pathway, VEGF signaling pathway, Apoptosis, Ferroptosis. Five key FRDEGs (Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2) were identified using PPI networks; Based on TFs-key FRDEGs networks, we found that Mapk14, Hif1a, Tlr4 and Ptgs2 were regulated by 3, 4, 5, and 29 TFs, respectively; however, Ddit3 was not regulated by any TF; By analyzing the miRNA–key FRDEGs networks, we found that 39, 74, 11, 28, and 18 miRNAs targets regulate the expression of Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2, respectively. Lastly, five key FRDEGs were validated at the mRNA and protein levels by RT-qPCR and IHC, which were in line with our bioinformatics analysis.
Conclusion: Our findings reveal that Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2 may be involved in the development of HF through regulating ferroptosis and as potential targets for HF.
Keywords: heart failure, transverse aortic constriction, ferroptosis, bioinformatics, key genes
Introduction
Heart failure (HF) represents the final stage of many cardiovascular diseases (CVDs), in which heart is incapable of pumping and supplying blood to the body effectively.1 Globally, approximately thirty million people suffered from HF, according to a previous report.2 However, this does not include undiagnosed or misdiagnosed cases.3 World Health Organization data indicated that HF was a major cause of mortality and morbidity throughout the world.4 In addition, there is evidence that numerous genes and signaling pathways are involved in HF.5 However, the exact mechanisms of HF remain unclear. Thus, understanding the pathogenesis of HF is crucial to its diagnosis and treatment.
Unlike apoptosis, autophagy, and necrosis, ferroptosis is a unique type of programmed cell death, which is induced by iron accumulation and lipid peroxidation.6 Previous studies have proven that ferroptosis is related to various diseases, such as cancer7, ischemic stroke8 and ulcerative colitis.9 Moreover, increasing evidence has indicated that many CVDs are associated with ferroptosis, including myocardial infarction,10 cardiomyopathy11 and HF.12 Iron is an essential trace element in the body, participating in energy metabolism and nucleotide synthesis.13 Excessive iron and reactive oxygen species (ROS) in cardiomyocytes can induce ferroptosis.11 Recent research showed that ferroptosis inhibitors can alleviate cardiac remodeling in transverse aortic constriction (TAC) mice.14 In addition, Wang et al found that MAP3K11 could induce ferroptosis in a TAC mouse model.15 Thus, exploring markers associated with ferroptosis in HF is of great importance.
Transverse aortic constriction (TAC) is a commonly used experimental model for mechanical stress-induced cardiac remodeling. Initially, TAC leads to compensated hypertrophy of the heart; In the end stage, chronic hemodynamic overload leads to cardiac dilatation and HF. Murine TAC models have been widely used to mimic human cardiovascular diseases and investigate the signaling processes involved in cardiac hypertrophic response and HF. It provides a more gradual time course for the development of HF.16
In this study, we aimed to screen and validate the key FRDEGs in heart failure by bioinformatic analysis and animal experiments, which may provide new insight into its treatment.
Materials and Methods
Data Acquisition
The RNA expression profile (GSE36074) was obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The dates were from the platform GPL1261 [Mouse430_2] Affymetrix Mouse Genome 430 2.0 Array. GSE36074 included 5 Sham-surgery and 7 TAC samples.
Differential Ferroptosis-Related Genes Screening
DEGs were screened according to adj.p-value of < 0.05 and|Log2FC(fold change)|≥0.05 by GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/). FRGs were extracted from the FerrDb website (http://www.zhounan.org/ferrdb/). FRDEGs were obtained from the DEGs and FRGs using the Draw Venn Diagram tool.
Functional Enrichment Analysis for FRDEGs
Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were processed to analyze the functions and significant pathways of FRDEGs using DAVID 6.8. Statistical significance was determined by a p value cut-off of 0.05.
Construction of Protein-Protein Interaction (PPI) Network and Identification of Key Genes
PPI network was built by STRING online tool (http://string-db.org), and the cytoHubba plugin of Cytoscape was then used to analyze PPI network modules. Key genes were considered with degrees of ≥11.
Regulatory Networks
Transcription factor (TF) and miRNA play vital roles in regulating the expression of mRNA. Therefore, based on key FRDEGs, the transcription factor (TF) was predicted by the TRRUST2.0 online tool, and the miRNAs were predicted by using miRNANet online tool (https://www.mirnet.ca). Then, the TF-FRDEGs and miRNA-FRDEGs networks were also visualized via Cytoscape.
Animals
Ten male C57BL/6 mice (8–10 weeks, 20–23g, specific pathogen-free) were obtained from Yangzhou University Comparative Medical Center, and they were raised in an environment with a temperature of 26 ◦C, humidity of 55% and 12 h light/dark cycle. Mice were fed a standard pellet diet and water ad libitum, and the rearing environment was cleaned regularly by professionals. All mice were anesthetized with ketamine (80 mg/kg) and xylazine (5 mg/kg) by intraperitoneal injection and euthanized with cervical dislocation on the 28th day after transverse aortic constriction. All the animal experiments were approved by the Yangzhou University Ethics Committee (No. 20210398). All the applied procedures were followed the Chinese guidelines for the welfare of the laboratory animals (GB/T 35823–2018).
Transverse Aortic Constriction (TAC) Mouse Model
After seven days of acclimatization, the mice were randomly divided into TAC or sham surgery group. Before surgery, the mice were tested to obtain baseline level of the echocardiogram, which showed no difference between the two groups. Mice were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.), and when mice lost the response to foot squeeze, the surgical procedures were performed according to previous study.17 Briefly, the left chest of mouse was opened to identify the thoracic aorta by blunt dissection in the 2nd intercostal space, Then the ascending aorta was ligated with a 7–0 silk suture, over a 26 G blunted needle in order to get the same degree of stenosis in all mice. Lastly, the thoracotomy was closed with a 5–0 silk suture. Sham-operated mice were subjected to a similar surgical procedure without ligation of the aorta. No animals died after surgery.
Echocardiographic Measurement
Four weeks after operation, echocardiography was performed with a Vevo 3100 machine (Visual Sonics Toronto, Canada). First, B-mode long-axis image was obtained by positioning the probe parallel to the long axis of the left ventricular (LV). Next, the probe was rotated at 90° to obtain a M-mode short-axis image of the LV. Echocardiographic images were recorded at least two cardiac cycles. Echocardiographic parameters of left ventricular fractional shortening (LVFS), Left ventricular ejection fraction (LVEF), left ventricular internal diameter at end-diastole (LVIDd) and left ventricular internal diameter at end-systole (LVIDs) were analyzed using the Vevo3100 cardiac analysis package, and the mean values over three heartbeats were calculated.
Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
With RT-qPCR, the levels of FRDEGs mRNA expression were determined. Total RNA extraction was performed on ventricular tissues using Trizol reagent (Takara, China), subsequently, reverse-transcribed RNA into cDNA with a reverse transcription kit (Takara, China). RT-qPCR primers (forward/reverse) can be found in Table 1. GAPDH was used to normalize all mRNA levels.
 |
Table 1 Pairs of Forward-Reverse Primers |
Immunohistochemistry (IHC)
IHC was conducted in accordance with the previous description.18 Briefly, the ventricular tissues were cut into 5-µm thick sections after being fixed in formalin and embedded in paraffin; then the sections were stained with anti-Mapk14 (Santa Cruz), anti-Hif1a (Santa Cruz), anti-Ddit3 (Santa Cruz), anti-Tlr4 (Santa Cruz) and anti-Ptgs2 (Santa Cruz) antibodies following the manufacturer’s instructions. Three randomly selected areas in each slice (×200) were used to evaluate the images. Relative expression was compared using average optical density (IOD/area), as measured using IPP6.0 software.
Statistical Analysis
The data were described as mean ± standard deviation. Statistical analysis was performed by GraphPad Prism (version 7.0). A two-sample unpaired Student’s t-test was used for two-group comparisons. P value < 0.05 was considered statistically significant.
Results
GEO and FerrDb databases were used to obtain FRGs in HF. As shown in Figure 1, this study provides an overview.
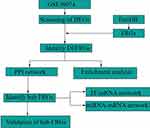 |
Figure 1 Study protocol. A flow chart of our study protocol. |
Identification of FRDEGs in Heart Failure
In order to explore ferroptosis-related markers in HF, the gene expression profiles (GSE36074) were obtained from the GEO, ferroptosis-related genes (FRGs) were screened from the FerrDb database; A total of 1918 DEGs were identified in the GSE36074 dataset; and 388 FRGs were found in FerrDb (Figure 2A). Moreover, 59 FRDEGs were obtained after intersection between DEGs and FRGs (Figure 2B). Clustering analysis of FRDEGs revealed that the samples in the same group clustered closely (Figures 2C).
FRDEGs Functional Enrichment Analysis
To elucidate the functions of FRDEGs, GO enrichment and KEGG pathway analyses were conducted. For GO enrichment analysis, FRDEGs were predominantly related to positive regulation of apoptotic process, lipopolysaccharide-mediated signaling pathway and cytosol (Figure 3A). Additionally, for KEGG enrichment analysis, FRDEGs were mainly associated with FoxO signaling pathway, VEGF signaling pathway, Apoptosis, MAPK signaling pathway, mTOR signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, PI3K-Akt signaling pathway, Toll-like receptor signaling pathway and Ferroptosis. (Figure 3B).
 |
Figure 3 Functional enrichment analysis of FRDEGs. (A) GO enrichment of FRDEGs. (B) KEGG pathway analysis of FRDEGs. BP, biological processes; CC, cellular component. MF, molecular function. |
Construction of the PPI Networks and Prediction of TFs for Key FRDEGs
To evaluate the interactions among the FRDEGs, we constructed a PPI network using the STRING online database, as shown in Figure S1. Then, the top 5 key FRDEGs (Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2) were obtained by CytoHubba plugin (Figure 4A). Moreover, TFs are key regulatory proteins that are essential for regulation of gene expression. Therefore, the TFs of the 5 key FRDEGs were predicted using the TRRUST2.0 database. We found that Mapk14, Hif1a, Tlr4 and Ptgs2 were regulated by 3, 4, 5, and 29 TFs, respectively; however, Ddit3 was not regulated by any TF (Figure 4B).
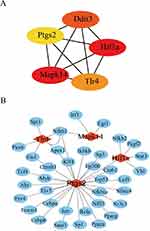 |
Figure 4 The screened key FRDEGs and predicted TFs. (A) Top five key FRDEGs screened by Degree method. (B) The TFs predicted for key FRDEGs. |
Generation of miRNA-Key FRDEGs Networks
MiRNANet was used to identify potential miRNAs target of key FRDEGs. According to the miRNA–key FRDEGs networks, we found that Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2 were regulated by 39, 74, 11, 28, and 18 miRNAs, respectively (Figure 5). In addition, based on miRNA- key FRDEGs networks, we found that miR-22-3p simultaneously regulated Mapk14, Hif1a, Ddit3 and Tlr4 expression; miR-92a-3p regulated Mapk14, Hif1a, Tlr4 and Ptgs2 expression; and miR-155-5p regulated the expression of Hif1a, Ddit3 and Ptgs2.
 |
Figure 5 Construction of miRNA- FRDEGs regulatory network. miRNA- FRDEGs network construction. |
Establishment of a Heart Failure Model in Mice
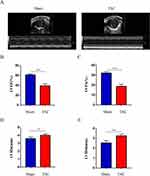
To investigate cardiac function after 4 weeks of TAC, echocardiographic examination was performed. Representative echocardiograms from each group are shown in Figure 6A. According to echocardiographic evaluation, the TAC group started to show significant reductions in LVEF and LVFS compared to the sham group (p<0.001) (Figure 6B and C). While, LVIDs and LVIDd were markedly increased in the TAC group compared with the sham group (p<0.01) (Figure 6D and E); Based on these results, we have successfully established a TAC-induced HF model in mice.
Validation of Key Genes
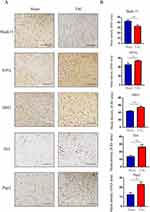
The key FRDEGs from bioinformatics analysis were further validated via establishing a mouse model of HF induced by TAC. According to the RT-qPCR analysis, A significant increase in Hif1a, Ddit3, Tlr4, and Ptgs2 mRNA levels was observed in the TAC group, whereas Mapk14 mRNA levels were substantially reduced (Figure 7). Furthermore, we further verified the expression of these FRDEGs by immunohistochemistry. Figure 8A and B showed the immunohistochemical results of Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2 proteins and its average optical densities. It was observed that the TAC group showed lower expression of Mapk14 protein (P < 0.01), while Hif1a, Ddit3, Tlr4 and Ptgs2 proteins were significantly higher (P<0.05). Those results supported bioinformatic analysis.
Discussion
With high morbidity and mortality, heart failure has become a major healthcare burden worldwide. Loss of terminally differentiated cardiomyocytes is a critical pathogenic factor in lethal heart failure, however, the mechanisms of cardiomyocyte death are unknown. Ferroptosis is a unique type of cell death driven by an iron-dependent increase in ROS.19 In recent studies, A crucial role of ferroptosis has been demonstrated in HF. Research from Fang et al reports that mice lacking ferritin H (Fth) produce more ROS and develop HF after 6 months.12 In addition, it has been shown that inhibition of ferroptosis reduces cardiac cell death in HF.20 Nevertheless, further research is needed to elucidate the molecular mechanisms underlying ferroptosis in cardiomyocytes. Hence, we conducted a bioinformatic analysis to explore the ferroptosis-related markers in HF, which may provide novel biomarkers for HF diagnosis and treatment.
In this study, we found that 59 FRDEGs in TAC group compared with that in sham group. Then, based on the functional enrichment analysis, we found that these FRDEGs were mostly related to FoxO signaling pathway, VEGF signaling pathway, Apoptosis, MAPK signaling pathway, mTOR signaling pathway, and Ferroptosis. Battiprolu et al found that the FoxO transcription factors are increased in mice models of HF,21 and in end-stage failing human heart.22 VEGF signaling pathway and MAPK signaling pathway also play key roles in HF.23,24 Moreover, a study showed that mTOR inhibited lipid-derived ROS production, thus preventing ferroptosis in cardiomyocytes.10 Therefore, these 59 FRDEGs may also play important roles in HF through these pathways. Then, by constructing a PPI network, we obtained five key FRDEGs, including Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2.
MAPK14 (also known as p38α) is expressed widely and plays an important role in producing cytokines and responding to a variety of stresses. Previous study showed that cardiomyocyte-specific knockout of p38α lead to accelerating heart failure after pressure overload.25 Ferroptosis is regulated by the RAS/MAPK pathway.26 As an oxygen-regulated transcription factor, Hif1a is activated when oxygen availability in the cellular environment decreases, which can regulate cell metabolism, survival and angiogenesis, and activate the gene expression of glycolytic enzymes in the failing heart.27 Moreover, Zou et al found that Hif1a could regulate ferroptosis and is related to GPX4.28 As we all know, cardiovascular hypertrophy and heart failure are associated with endoplasmic reticulum stress (ERS). Additionally, research has shown that ERS could induce unfolded proteins to cause ferroptosis.29,30 Ddit3 (also known as CHOP) is a transcription factor induced by ERS,31 and in patients with low ejection fraction heart failure, Sabirli et al confirmed that CHOP may be useful as a predictor of hospital stay.32 The current study found that CHOP was very rare in the study of ferroptosis. TLRs (Toll-like receptors) are single transmembrane receptors on cell surfaces that play roles in the innate immune response. It is generally believed that TLRs exist as homodimers on immune cells, B lymphocytes, macrophages and mast cells. Chen et al has revealed that knock-down of TLR4 slowed ferroptosis, reduced myocytes death and alleviated left ventricular remodeling in rats with HF significantly, suggesting TLR4 may be a potential therapeutic target for HF.20 Previous research has shown that Ptgs2 can regulate prostaglandins expression levels, which plays a key role in inflammation.33 Ptgs2 genetic reductions has been linked to a lower CVDs risk.34 Liu et al identified that ferroptosis-related marker (PTGS2) was upregulated in acute myocardial infarction.35 Furthermore, recent studies have found that Ptgs2 can be used as a marker for ferroptosis.12,36 Nevertheless, the exact regulatory mechanisms of Ptgs2 still require further exploration in HF.
It is believed that key genes play a crucial role in a wide range of biological processes. Some researches demonstrate that TFs and miRNAs could regulate a variety of target genes in HF. Hence, we constructed the TF-mRNA networks and miRNA-mRNA networks of 5 key FRDEGs. We found that except Ddits, the other four FRDEGs could be regulated by more than one TFs. Additionally, our findings also suggest that NF-κB1 is a key TF, because it can regulate three key FRDEGs simultaneously. Prior studies showed the NF-κB1 participated in regulating numerous biological processes. Frantz et al demonstrated that the absence of NF-κB1 improved early survival and reduced left ventricular dilatation after myocardial infarction, which might be an attractive target for treating heart failure.37 In addition, the miRNA network analysis showed that the miR-22-3p and miR-92a-3p could simultaneously regulate four key FRDEGs. Previous studies have confirmed that miR-22-3p is a biomarker for adverse outcome in patients with chronic heart failure.38 Marques et al reported that coronary sinus plasma level of the miR-92a-3p was substantially decreased, while miR-155-5p was substantially increased in HF patients.39 Furthermore, the five key FRDEGs were verified by RT-qPCR and IHC, which were consistent with our bioinformatic analysis result.
However, our study still has some limitations. Firstly, the ferroptosis-related genes are incomplete in our study, due to the updated continuously FerrDb database. In addition, more laboratory evidences are needed to support the role of FRGs in HF.
Conclusion
In conclusion, our results suggest that these five key FRGs (Mapk14, Hif1a, Ddit3, Tlr4 and Ptgs2) are closely associated with ferroptosis in HF, and serve as potential targets for HF.
Abbreviations
HF, Heart failure; TAC, Transverse aortic constriction; FRDEGs, Ferroptosis-related differential expressed genes; PPI, Protein-protein interaction; RT-qPCR, Quantitative reverse transcription PCR; IHC, Immunohistochemistry; CVDs, Cardiovascular diseases.
Data Sharing Statement
The data is available in NCBI GEO, accession number: GSE36074 and ementary material.
Ethics Statement
In this study, there does not involve human research, infringe on the privacy of human, and include any human biological samples. Therefore, the ethics were approval waived by Medical College, Yangzhou University.
Acknowledgments
We are indebted to Prof. Skrbic B who shared their data in GEO.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.81800250), China Postdoctoral Science Foundation (No.2022M711417), Yangzhou science and technology plan social development project (No. SSF2023000133).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Raffaa H, Alasmari BA, Abadi SA, et al. Adherence of heart failure patients to heart failure medications and its determinants in the Aseer region, Southern Saudi Arabia. J Family Med Prim Care. 2020;9(9):5041–5045. doi:10.4103/jfmpc.jfmpc_904_20
2. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi:10.15420/cfr.2016:25:2
3. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–378. doi:10.1038/nrcardio.2016.25
4. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi:10.1161/CIR.0000000000000757
5. Kolur V, Vastrad B, Vastrad C, Kotturshetti S, Tengli A. Identification of candidate biomarkers and therapeutic agents for heart failure by bioinformatics analysis. BMC Cardiovasc Disord. 2021;21(1):329. doi:10.1186/s12872-021-02146-8
6. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
7. Lin W, Chen Y, Wu B, Chen Y, Li Z. Identification of the pyroptosis-related prognostic gene signature and the associated regulation axis in lung adenocarcinoma. Cell Death Discov. 2021;7(1):161. doi:10.1038/s41420-021-00557-2
8. Weiland A, Wang Y, Wu W, et al. Ferroptosis and its role in diverse brain diseases. Mol Neurobiol. 2019;56(7):4880–4893. doi:10.1007/s12035-018-1403-3
9. Xu M, Tao J, Yang Y, et al. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020;11(2):86. doi:10.1038/s41419-020-2299-1
10. Baba Y, Higa JK, Shimada BK, et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314(3):H659–H668. doi:10.1152/ajpheart.00452.2017
11. Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672–2680. doi:10.1073/pnas.1821022116
12. Fang X, Cai Z, Wang H, et al. Loss of cardiac Ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res. 2020;127(4):486–501. doi:10.1161/CIRCRESAHA.120.316509
13. Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93(4):1721–1741. doi:10.1152/physrev.00008.2013
14. Ito J, Omiya S, Rusu MC, et al. Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. Elife. 2021;10:
15. Wang J, Deng B, Liu Q, et al. Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis. 2020;11(7):574. doi:10.1038/s41419-020-02777-3
16. DeAlmeida AC, van Oort RJ. Wehrens XH: transverse aortic constriction in mice. J Vis Exp. 2010;38:1729.
17. Ma ZG, Dai J, Zhang WB, et al. Protection against cardiac hypertrophy by geniposide involves the GLP-1 receptor / AMPKalpha signalling pathway. Br J Pharmacol. 2016;173(9):1502–1516. doi:10.1111/bph.13449
18. Kolkova Z, Noskova V, Ehinger A, Hansson S, Casslen B. G protein-coupled estrogen receptor 1 (GPER, GPR 30) in normal human endometrium and early pregnancy decidua. Mol Hum Reprod. 2010;16(10):743–751. doi:10.1093/molehr/gaq043
19. Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi:10.3389/fphys.2019.00139
20. Chen X, Xu S, Zhao C, Liu B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun. 2019;516(1):37–43. doi:10.1016/j.bbrc.2019.06.015
21. Battiprolu PK, Hojayev B, Jiang N, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122(3):1109–1118. doi:10.1172/JCI60329
22. Galasso G, De Rosa R, Piscione F, et al. Myocardial expression of FOXO3a-Atrogin-1 pathway in human heart failure. Eur J Heart Fail. 2010;12(12):1290–1296. doi:10.1093/eurjhf/hfq102
23. Zhang Y, Li C, Meng H, et al. BYD ameliorates oxidative stress-induced myocardial apoptosis in heart failure post-acute myocardial infarction via the P38 MAPK-CRYAB signaling pathway. Front Physiol. 2018;9:505. doi:10.3389/fphys.2018.00505
24. Tual-Chalot S, Garcia-Collado M, Redgrave RE, et al. Loss of endothelial endoglin promotes high-output heart failure through peripheral arteriovenous shunting driven by VEGF signaling. Circ Res. 2020;126(2):243–257. doi:10.1161/CIRCRESAHA.119.315974
25. Nishida K, Yamaguchi O, Hirotani S, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24(24):10611–10620. doi:10.1128/MCB.24.24.10611-10620.2004
26. Nguyen T, Mahalakshmi B, Velmurugan BK. Functional role of ferroptosis on cancers, activation and deactivation by various therapeutic candidates-an update. Chem Biol Interact. 2020;317:108930. doi:10.1016/j.cbi.2019.108930
27. Kakinuma Y, Miyauchi T, Yuki K, Murakoshi N, Goto K, Yamaguchi I. Novel molecular mechanism of increased myocardial endothelin-1 expression in the failing heart involving the transcriptional factor hypoxia-inducible factor-1alpha induced for impaired myocardial energy metabolism. Circulation. 2001;103(19):2387–2394. doi:10.1161/01.CIR.103.19.2387
28. Zou Y, Palte MJ, Deik AA, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10(1):1617. doi:10.1038/s41467-019-09277-9
29. Lee YS, Lee DH, Choudry HA, Bartlett DL, Lee YJ. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Cancer Res. 2018;16(7):1073–1076. doi:10.1158/1541-7786.MCR-18-0055
30. Zheng DW, Lei Q, Zhu JY, et al. Switching apoptosis to ferroptosis: metal-organic network for high-efficiency anticancer therapy. Nano Lett. 2017;17(1):284–291. doi:10.1021/acs.nanolett.6b04060
31. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. doi:10.1038/sj.cdd.4401373
32. Sabirli R, Koseler A, Mansur N, et al. Predictive value of endoplasmic reticulum stress markers in low ejection fractional heart failure. In Vivo. 2019;33(5):1581–1592. doi:10.21873/invivo.11640
33. Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38(10):1654–1661. doi:10.1016/j.biocel.2006.03.021
34. Ross S, Eikelboom J, Anand SS, et al. Association of cyclooxygenase-2 genetic variant with cardiovascular disease. Eur Heart J. 2014;35(33):2242–2248. doi:10.1093/eurheartj/ehu168
35. Liu K, Chen S, Lu R. Identification of important genes related to ferroptosis and hypoxia in acute myocardial infarction based on WGCNA. Bioengineered. 2021;12(1):7950–7963. doi:10.1080/21655979.2021.1984004
36. Li Y, Feng D, Wang Z, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26(11):2284–2299. doi:10.1038/s41418-019-0299-4
37. Frantz S, Hu K, Bayer B, et al. Absence of NF-kappaB subunit p50 improves heart failure after myocardial infarction. FASEB J. 2006;20(11):1918–1920. doi:10.1096/fj.05-5133fje
38. van Boven N, Akkerhuis KM, Anroedh SS, et al. Serially measured circulating miR-22-3p is a biomarker for adverse clinical outcome in patients with chronic heart failure: the Bio-SHiFT study. Int J Cardiol. 2017;235:124–132. doi:10.1016/j.ijcard.2017.02.078
39. Marques FZ, Vizi D, Khammy O, Mariani JA, Kaye DM. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur J Heart Fail. 2016;18(8):1000–1008. doi:10.1002/ejhf.517
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.