Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Hypoglycaemic Molecules for the Management of Diabetes Mellitus from Marine Sources
Authors Chellappan DK , Chellian J , Rahmah NSN, Gan WJ, Banerjee P , Sanyal S, Banerjee P , Ghosh N, Guith T, Das A, Gupta G, Singh SK, Dua K, Kunnath AP, Norhashim NA , Ong KH, Palaniveloo K
Received 8 April 2023
Accepted for publication 12 July 2023
Published 25 July 2023 Volume 2023:16 Pages 2187—2223
DOI https://doi.org/10.2147/DMSO.S390741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Dinesh Kumar Chellappan,1 Jestin Chellian,1 Nur Suraiza Nabila Rahmah,2 Wee Jin Gan,2 Priyanka Banerjee,3 Saptarshi Sanyal,3 Pradipta Banerjee,4 Nandini Ghosh,4 Tanner Guith,4 Amitava Das,4 Gaurav Gupta,5– 7 Sachin Kumar Singh,8,9 Kamal Dua,9,10 Anil Philip Kunnath,11 Nur Azeyanti Norhashim,12,13 Kuan Hung Ong,13 Kishneth Palaniveloo13
1Department of Life Sciences, International Medical University, Kuala Lumpur, 57000, Malaysia; 2School of Pharmacy, International Medical University, Kuala Lumpur, 57000, Malaysia; 3Department of Pharmaceutical Technology, School of Medical Sciences, Adamas University, Kolkata, West Bengal, India; 4Indiana University School of Medicine, Indianapolis, IN, USA; 5School of Pharmacy, Suresh Gyan Vihar University, Jaipur, Rajasthan, 302017, India; 6Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, Uttarakhand, 248007, India; 7Center for Global Health Research, Saveetha Medical College, Saveetha Institute of Medical and Technical Science, Chennai, India; 8School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, 144411, India; 9Faculty of Health, Australian Research Centre in Complementary and Integrative Medicine, University of Technology Sydney, Ultimo, NSW, 2007, Australia; 10Discipline of Pharmacy, Graduate School of Health, University of Technology Sydney, Sydney, NSW, 2007, Australia; 11Division of Applied Biomedical Science and Biotechnology, School of Health Sciences, International Medical University, Kuala Lumpur, 57000, Malaysia; 12Division of Cardiovascular Sciences, Faculty of Biology, Medicine and Health, Core Technology Facility, The University of Manchester, Manchester, M13 9NT, UK; 13Institute of Ocean and Earth Sciences, University of Malaya, Kuala Lumpur, 50603, Malaysia
Correspondence: Dinesh Kumar Chellappan; Anil Philip Kunnath, Tel +6012 636 1308 ; +6012 618 7831, Email [email protected]; [email protected]
Abstract: Diabetes mellitus (DM) is a chronic metabolic disorder recognized as a major health problem globally. A defective insulin activity contributes to the prevalence and expansion of DM. Treatment of DM is often hampered by limited options of conventional therapies and adverse effects associated with existing procedures. This has led to a spike in the exploration for potential therapeutic agents from various natural resources for clinical applications. The marine environment is a huge store of unexplored diversity of chemicals produced by a multitude of organisms. To date, marine microorganisms, microalgae, macroalgae, corals, sponges, and fishes have been evaluated for their anti-diabetic properties. The structural diversity of bioactive metabolites discovered has shown promising hypoglycaemic potential through in vitro and in vivo screenings via various mechanisms of action, such as PTP1B, α-glucosidase, α-amylase, β-glucosidase, and aldose reductase inhibition as well as PPAR alpha/gamma dual agonists activities. On the other hand, hypoglycaemic effect is also shown to be exerted through the balance of antioxidants and free radicals. This review highlights marine-derived chemicals with hypoglycaemic effects and their respective mechanisms of action in the management of DM in humans.
Keywords: diabetes mellitus, marine organisms, bioactive metabolites, α-amylase inhibition, α-glucosidase inhibition, PTP1B inhibition, antioxidants
Introduction
Diabetes mellitus (DM) is one of the chronic metabolic disorders, which often has been recognized as a major health problem globally due to unrelenting hyperglycaemia which contributes to morbidity and mortality.1,2 The International Diabetes Federation reported that approximately 425 million adults suffered from diabetes in 2017, and by 2045 it is expected to hit 629 million.3 According to the World Health Organization (WHO), the number of diabetics rose from 108 million in the year 1980 to 422 million in the year 2014. Prevalence of diabetes has been on the rise more rapidly in low- and middle-income countries as compared to high-income countries. Diabetes has been the principal cause of blindness, kidney disease, heart diseases, stroke, and lower limb amputations. There has been a 3% agewise increase in the diabetes-related mortality rates between the years 2000 and 2019. The severity of this disease is associated with microvascular complications causing destruction of neurons, retina, and nephrons affecting nerves, eyes, and kidneys. This metabolic disorder is also associated with heart-related problems caused by multiple genetical factors and environmental factors,4–6 such as oversecretion of fats, proteins, and carbohydrate in an individual.7 DM is a disease of the endocrine system caused by the decreased secretion of insulin by the β-cells.8 Insulin is produced and secreted by β-pancreatic cells2 which function by stimulating glucose uptake and synthesis of lipid.9–11 Persistently elevated blood glucose levels in diabetes can result from either defective insulin role or defective insulin secretion, or both.6 DM is classified into type-1 (T1DM) and type-2 (T2DM) where T1DM occurs due to the damage of β-cells caused by an autoimmune response with no insulin production.12 The triggering factors for such a response remain unknown, and it could result from both genetic and environmental factors.13 Patients with T1DM are fully dependent on insulin therapy for life. In T2DM, the β-cells are either not capable of generating insulin sufficiently for proper functioning of the cells or the body has undergone resistance towards insulin usage. Insulin resistance has been a key factor in the pathogenesis of T2DM (Figure 1). In this case, adopting a healthy lifestyle by regular exercising and a low-calorie diet, together with daily insulin injections and prescribed drugs, may help patients to control the progression of the disease. This type of DM is associated with obesity occurring due to a sedentary lifestyle and high-calorie diet. T2DM has a higher prevalence compared to T1DM, accounting for greater than 90% of all diabetes cases.8 Pharmacological strategies investigated for controlling T1DM include inhibition of gluconeogenesis, increasing glucose transportation, stimulating the release of insulin, and bringing about reduction in the glucose absorption specifically in the intestines.14
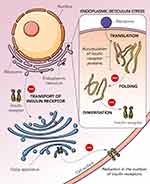 |
Figure 1 Mechanisms leading to insulin resistance in diabetes mellitus. |
Glycaemic regulation is crucial as it is important to keep the blood glucose levels at an optimum. Defects in the signalling mechanisms of GLUT4 leads to diminished levels of glucose intake by the muscle and fat cells (Figure 2) increasing the burden on the beta cells to produce more insulin. This progressive cascade of events over a period results in the pathogenesis of diabetes. Regulation of glucose levels in diabetics can be improved using insulin supplements and other anti-hyperglycaemic medications, either separately or combined.15 The pharmaceutical industry utilizes modern technology to develop new drugs through high-throughput synthesis and combinatorial chemistry-based drug development. Since the 1980s, pharmaceutical companies, despite a considerable effort towards research and development of newer drugs, are still facing challenges to increase drug productivity and efficiency.16 Some of the existing drugs have been found to be either less effective or known to cause lethal side effects.17
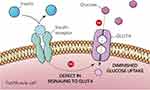 |
Figure 2 Pathophysiology of type 2 diabetes mellitus. |
Therefore, there has been a considerable amount of interest in the development of anti-diabetic drugs from natural products, with keen focus on exploring the marine environment for more substitutes. Marine bioresources are rich sources of bioactive constituents such as polyunsaturated fatty acids, polyphenols, proteins, sulphated polysaccharides, sterols, and pigments due to the extreme conditions and active interactions in the marine environment.18–22 Several studies have reported on the potential of marine drugs as a possible therapeutic option for the management of diabetes and related conditions. The importance of marine-derived metabolites has been the topic of discussion by several authors as they lay the basis for the discovery of potent, novel agents for the treatment of diabetes. For instance, in addition to exhibiting anti-viral and anti-microbial properties, macroalgae are known for synthesizing antioxidants which are used to control low blood pressure, hypolipidemia.23,24 These marine plants have been also reported to synthesize terpenoids, alkaloids, and peptides, associated to various in vivo pharmacological activities.25 Marine sponge is a source for cytarabine which is known to exhibit anti-cancer activity used in the treatment of lymphoma and acute myelocytic leukemia,26,27 ziconotide that is useful for treating pain sensations, whether acute or chronic,28 and vidarabine which is used for the treatment of herpes.29 This shows the potential of marine natural resources. Therefore, this review highlights the various compounds that possess hypoglycaemic effects to manage DM in human.
Methodology
Data collection for the published studies on the bioactive components of marine organisms was carried out in PubMed-indexed and Google Search databases using the topic search term (marine* OR bioactive* OR algae* OR sponges* OR fungal*) AND organisms AND (hypoglycemic* OR antidiabetic* OR antioxidant* OR antiinflammatory*) on 30th September 2020. Each paper’s title, year of publication, abstract, authors, and digital object identifier (DOI) were thoroughly screened to filter out duplication, and only publications related to this manuscript were eventually considered.
Anti-Diabetic Mechanisms of Marine Biomolecules in Modifying Enzyme Function in Carbohydrate Metabolism
α-Glucosidase, aldose reductase, α-amylase, dipeptidyl peptidase 4, glucose-6-phosphatase, glycogen synthase kinase-3β, glucose transporter 4 (Glut4), hexokinase, and N-acetyl-glycosaminidase are the enzymes which are notably involved in the metabolism of glucose. These enzymes play a vital role in inhibiting the diabetic effects in both animal model assays and patients.30–37 α-Amylase and α-glucosidase are majorly utilized for the breakdown and digestion of carbohydrates. The reduction or delay of post-prandial hyperglycaemia is triggered by reducing glucose absorption and inhibiting the carbohydrate breakdown or hydrolases particularly in the gastrointestinal tract.
Alpha-glucosidase inhibitors act along the brush border membrane in the small intestines through competitive inhibition. Therefore, these agents are typically called competitive inhibitors. Generally, they are saccharides that break down sugars. The inhibition is typically observed on enzymes that are essential to digest sugars. A common example of these enzymes would be the alpha-glucosidase enzymes. The mechanism by which the alpha-glucosidase enzyme works is by breaking down sugars to monosaccharides.30 Aldose reductase,38 the initial enzyme involved in the polyol pathway, converts glucose to fructose. When the concentration of glucose increases in the cells, aldose reductase converts glucose into sorbitol which is oxidized to fructose by sorbitol dehydrogenase. The mechanism by which aldose reductase functions is typical of a cytosolic NADPH-dependent oxidoreductase which assists in the reduction of sugars. The enzyme generally reduces monosaccharides to sorbitol.31 Enzymes such as dipeptidyl peptidase 4, glucose-6-phosphatase, hexokinase, maltase, and sucrase, which are involved in the glucose metabolism, can be used to investigate the anti-diabetic effects of biomolecules. DPP-4 inhibitors act by reducing the levels of the hormone glucagon and in turn blood glucose levels. Additionally, the principal action of DPP-4 inhibitors is to raise the level of the hormone incretins, namely GLP-1 and GIP. These actions will result in the inhibition of the hormone glucagon. These cascades eventually result in the increased release of insulin.32 In addition, Glut4 plays a role in the transport of glucose regulated by insulin, while glycogen synthase kinase-3β (GSK-3β) mediates the addition of phosphate molecules onto threonine and serine amino acid residues and has been associated with diseases such as T2DM, Alzheimer’s disease, cancer, and inflammation.39 In anti-diabetic assays tyrosine phosphatase-1B (PTP1B) is a common protein target. The protein is a non-transmembrane enzyme utilized as a therapeutic target for diabetes, obesity, and breast cancer, and the enzyme plays a significant role both as a negative insulin regulator and in leptin signalling. Aldose reductase and protein tyrosine phosphatase also function as long-term energy storage.40,41 The PTP1B enzyme reduces the activation state of the insulin receptor kinase by unlocking the insulin signalling leading to the post-receptor signalling inhibition in tissues responding to insulin, leading to the development of T2DM.2,42–44 Clinical values such as plasma glucose level, plasma insulin, total cholesterol, concentrations of triacylglycerol, blood pressure, body weight, and histopathology of intestine are usually assessed in diabetic animal models and/or patients.7,45–50 Screenings of these parameters mainly target possible compounds for treating T2DM, whereas identification of molecules with pancreatic β-cell-protective effect through reduction of oxidative processes and inflammation could help in screening for the more severe T2DM.2
Primary and Secondary Marine Metabolites Having Anti-Diabetic Activity
Several bioactive metabolites of marine origin have been investigated over the years. This section provides an overview of various categories of bioactive compounds that have been investigated for their anti-diabetic activity using various enzyme-guided anti-diabetic assays. The overall structure of this section is shown in the schematic diagram in Figure 3.
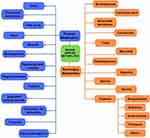 |
Figure 3 Schematic flowchart summarizing the various marine-derived metabolites. |
Primary Metabolites
Chitin
A study by Nguyen et al investigated six kinds of chitinous materials which were used as sole carbon/nitrogen (C/N) sources for producing α-glucosidase inhibitors (aGI) by Paenibacillus sp. TKU042.51 Demineralized crab shell powder (deCSP) and shrimp shell powder (deSSP) as the C/N source produced the highest aGI productivity in the culture supernatants. Compared to acarbose (1095 μg mL−1, 74%), fermented deCSP (38 μg mL−1, 98%) has a higher maximum inhibitory activity and a lower IC50 against the α-glucosidase in the intestine of rat. However, there was no inhibitory activity against porcine α-amylase and Bacillus subtilis α-amylase. Hence, fermented deCSP is a good candidate for anti-diabetic drugs. On the other hand, unfermented deCSP was tested for the presence of α-glucosidase inhibition, and there was no activity observed, which may have been due to aGI produced by the fermentation process.51 Homogentisic acid (HGA) isolated from squid pens fermented by four species of Paenibacillus is a type of non-sugar-based α-glucosidase inhibitor,52 and has a higher α-glucosidase activity compared to acarbose.53 Table 1 exhibits the properties of chitin from Paenibacillus sp. TKU042.
 |
Table 1 Microbial-Derived Chitin Possessing Anti-Diabetic Potential |
Chromenyl Derivatives
Two chromenyl derivatives, 11-(3,4,4α,5,8,8α-hexahydro-8-methoxy-4-methyl-1H-isochromen-4-yloxy)-11-hydroxyethylpentanoate (CD-1) and methyl 9-(4,4α,5,8-tetrahydro-3-oxo-3H-isochromen-5-yl)hexanoate (CD-2) were isolated from the spineless marine cuttlefish, Sepiella inermis.54 The anti-hyperglycaemic efficacies of chromenyl chemotypes were evaluated using the carbolytic enzymes inhibition such as α-glucosidase and α-amylase as well as dipeptidyl peptidase IV (DPP-IV). Inhibition of the carbolytic enzymes reduced the absorption of carbohydrates in the intestine followed by DPP-IV inhibition, which is crucial for the secretion of insulin. CD-1 showed greater DPP-IV, α-glucosidase, and α-amylase inhibitory potential compared to CD-2.54 The study concluded that CD-1 could be beneficial in managing hyperglycaemic-related disorders by maintaining the glucose homeostasis. Characteristics of CD-1 and CD-2 are shown in Table 2.
 |
Table 2 Invertebrate-Derived Chromenyl Derivatives Having Anti-Diabetic Potential |
Fatty Acids
Two fatty acids, 1,3-dipalmitolein and cis-9-octadecenoic acid, isolated from internal organ of sea cucumber, Stichopus japonicus, demonstrated potent α-glucosidase inhibition activity.55 1,3-Dipalmitolein has a more potent inhibitory activity against Saccharomyces cerevisiae α-glucosidase than cis-9-octadecenoic acid. The inhibitory activity is affected by the number and position of double bonds in fatty acids. An even number of unsaturated fatty acid has a weaker inhibitory activity compared to an odd number.54 Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) isolated from marine microalgae Isochrysis galbana and Nannochloropsis oculata controlled lipid and glucose metabolism in diabetic rats.2 Omega-3 fatty acid, eicosapentaenoic acid present in various microalgae (eg Chlorella zofingiensis, Chlorella protothecoides, and Nitzschia laevis) can be used as a possible preventive agent in patients with diabetic retinopathy as well as other ocular diseases.56 High amounts of DHA and EPA may contribute to the reduction in weight, blood glucose, triacylglycerol, and cholesterol levels in diabetic rat models.49 Unsaturated fatty acids such as 7(Z)-octadecenoic acid and 7(Z),10(Z)-octadecadienoic acid have potent α-glucosidase activity and mildly inhibited intestinal sucrase and maltase.57 Two unsaturated fatty acids isolated again from Sillago japonicus, 7(Z)-octadecenoic acid and 7(Z),10(Z)-octadecadienoic acid, were found to possess a mild rat-intestinal sucrase and maltase inhibitory activity.57 These compounds are highlighted with their mechanisms of actions in Table 3. The fatty acids also inhibited Saccharomyces cerevisiae α-glucosidase with IC50 values of 0.51 and 0.67 μg mL−1, respectively, and Bacillus stearothermophilus α-glucosidase with IC50 values of 0.49 and 0.60 μg mL−1, thus showing potential as therapeutic agent in treating DM.57 Furthermore, synthetic oxo-fatty acids such as (7E)-9-oxohexadec-7-enoic acid and (10E)-9-oxohexadec-10-enoic acid can be used in the management of diabetes by inducing anti-diabetic gene programmes in the adipocytes.58
 |
Table 3 Invertebrate-Derived Ether Derivatives Having Anti-Diabetic Potential |
Fibres
High consumption of dietary fibre can reduce the incidence of type 2 diabetes mellitus (T2DM).59,60 The recommended amounts of fibre for healthy individuals and diabetic patients are 30 g day−1 and 50 g day−1, respectively.61,62 In the management of T2DM patients, small amounts of seaweed consumption can achieve high fibre intake with a low glycaemic load.63 A serving of 8 g of seaweed can meet 12.5% of a person’s daily fibre intake.63 Diabetics who consumed seaweed supplements containing Undaria pinnatifida and Saccharina japonica were found to have reduced blood glucose levels, blood lipid levels and increased antioxidant enzyme activities.64 Similarly, consumption of a large amount of dietary fibre reduced insulin resistance biomarkers.65 Dietary fibre is crucial in managing metabolic disease like diabetes as its post-prandial glycaemic responses in human subjects that consume arroz-caldo with lambda-carrageenan were reduced compared to control groups.66 Nori (genus Porphyra) reduced sharp blood glucose peak and glycaemic response from 100% to 68% in healthy volunteers who consumed carbohydrate (white bread).67 Dietary porphyran isolated from the red alga Porphyra yezoensis enhanced glucose metabolism in diabetic patients by increasing the level of adiponectin.68 Algal polysaccharides (three seaweed fibres of different viscosities) isolated from Palmaria palmata, Eucheuma cottonii, or Laminaria digitata, were compared to purified cellulose. Among the polysaccharides, only high viscous alginates decreased the blood glucose level and insulin responses in pigs.69 Isolated alginates from L. digitata lead to decreased glucose absorption of up to 50% over 8 h by the reduction of blood glucose and insulin responses. Sodium alginate formulation has a potential to manage T2DM as it can reduce the average daily consumption of macronutrients and saturated fat in individuals such as healthy, over-weight, and obese people.70 Consumption of alginate-based supplements can reduce blood glucose levels.71,72 Table 4 shows samples of marine-derived fibres.
 |
Table 4 Macroalgae-Derived Fibres with Anti-Diabetic Potential |
Minerals
Many macroalgae species such as Kappaphycus alvarezii and Eucheuma denticulatum contain magnesium (Table 5). Every 100 g of dried macroalgae can provide around 30% to 90% of magnesium.73 The magnesium present in the red macroalgae possesses hypoglycaemic activity. Barbagallo et al proposed that the higher the concentration of free magnesium in the intracellular region, the lower the fasting blood glucose level.74 In addition, Barbagallo et al also proposed that magnesium is involved in the glucose metabolism as well as in insulin homeostasis.74 Ramadass et al also found that serum magnesium level is inversely proportional to HbA1c levels, a parameter to diagnose type 2 diabetes.75
 |
Table 5 Macroalgae-Derived Magnesium Possessing Anti-Diabetic Potential |
Metalloprotein
Vanadium-containing proteins (VCPs) isolated from the sea cucumber, Apostichopus japonicus, are known to manage insulin resistance and decrease fasting blood glucose and serum insulin levels.76 High-fat high-sugar diet (HFSD) mice treated with VCP were documented to have reduction in body weight. In addition, the diabetic rats fed with VCP diet had lower adipose tissue weight. VCP administration also reduced fasting blood glucose and increased insulin sensitivity in HFSD mice. It also increased serum adiponectin levels and reduced TNF-α, resistin, and leptin levels.76 The study evidenced that VCP exhibited anti-diabetic activity by the downregulation of hyperglycaemia and adipokines. Some examples of metalloproteins are shown in Table 6.
 |
Table 6 Marine-Derived Metalloproteins Having Anti-Diabetic Potential |
Pigment-Protein Complex
Phycocyanin isolated from Spirulina fusiformis, a microalga, is known to reduce the elevated blood glucose level.77 Gershwin and Belay report that phycocyanin is one of the biopigments having anti-hyperglycaemic effect.78 Table 7 shows the phycocyanin from Spirulina fusiformis.
 |
Table 7 Microalgae Spirulina fusiformis-Derived Pigments and Their Anti-Diabetic Potential |
Oligosaccharides
Oligosaccharides isolated from a brown seaweed, Sargassum confusum, administered for 30 days and 60 days in HFSD-fed hamsters, decreased fasting blood glucose.79 It exhibited anti-diabetic effect through the regulation of insulin receptor substrate 1/phosphatidylinositol 3-kinase and c-Jun N-terminal kinase pathways. Sargassum confusum was found to exhibit anti-diabetic activity in HepG2 cells in vitro and in vivo using hamsters.79 The administration of S. confusum reduced body weight compared to normal control group. The oral glucose tolerance test showed that S. confusum treatment also improved the impaired glucose tolerance of the HFSD-fed hamsters. Furthermore, S. confusum also has some effect on the mRNA expression in signalling pathways by reducing the expression of the gene (JNK1 and JNK2) in the treatment group compared to the model group in the hepatic cells. Hence, S. confusum can help to enhance the hepatic insulin resistance as well as the hypoglycaemic effect by controlling the pathways of IRS1/P13K and JNK. Sargassum confusum exhibited high anti-diabetic activity due to its low molecular weight.79 Kumar et al proposed that there is a relationship between the blood glucose-lowering action and the low total cholesterol level after the treatment of S. confusum for 60 days.80 Moreover, Oh et al reported that S. confusum reduced inflammation and, thereby, maintained normal insulin secretion in a long-term HFSD-induced animal model.81 Table 8 summarizes the marine-derived oligosaccharide anti-diabetic mechanism of action.
 |
Table 8 Macroalgae Sargassum confusum-Derived Pigment and Their Anti-Diabetic Potential |
Peptides
Peptides play a major role in diabetics. Oligopeptides isolated from salmon skin (Oncorhynchus kern) were investigated for their anti-diabetic effects in a rat model.2 The administration of oligopeptides reduced fasting blood glucose levels and apoptosis of pancreatic islet cells where the serum levels of TNF-α, interferon-gamma, and malondialdehyde were also observed to be reduced. The oligopeptides increased the serum levels of superoxide dismutase and glutathione.2 Similarly, through in vitro evaluation, bioactive peptides have been observed to decrease the generation of cytokines, IL-1β and TNF-α, when pancreatic β-cells are exposed to high glucose, suggesting that damage or apoptosis of pancreatic β-cells can be prevented from diabetic-induced oxidative stress.48 Marine collagen isolated from fish was shown not only to regulate metabolic nuclear receptors but also reduce free fatty acids in T2DM patients with or without hypertension.82 Undigested Goby fish (Zosterisessor ophiocephalus) muscle protein and their hydrolysates administered to HFHD-induced oxidative stress rats can manage hyperglycaemia, restore the status of antioxidant, and ameliorate renal damage.83 A milk-derived bioactive peptide, β-casomorphin-7, also acts as an antioxidant84 by reducing the blood glucose level in the streptozotocin-induced diabetic rat.
Common bean peptides too can upregulate insulin-like growth factor 2 (IGF-II), alleviate obesity-associated insulin resistance, and contribute in anti-inflammation.85 Rice bran and soybean flour are the example plant proteins that possess DPP-IV inhibitory activity.86,87 Nutripeptin® and Hydro MN Peptide® are marine protein hydrolysates that are effective in reducing the post-prandial blood glucose level and managing T2DM symptoms.88 Evidence of lowering both blood glucose and HbA1c levels was shown by the use of cholera toxin B sub-unit and the use of an active peptide derived from shark liver (CTB-APSL) fusion protein through.7 It also can promote the secretion of insulin and enhance insulin resistance. Recently, anti-diabetic enzymatic protein hydrolysates and peptides have been gaining interest. Suetsuna and Saito showed that hypoglycaemic activity became apparent from isolated Porphyra yezoensis when boiled laver mixture hydrolysate fractions were decomposed by pepsin.89 Similarly, Harnedy and FitzGerald reported precursors for generation of peptides which possessed DPP IV inhibitory activity from 3 hydrolysed fractions of Palmaria palmata protein.35
Sulphated Polysaccharide
Sulphated polysaccharide isolated from Cymodocea nodosa (CNSP) inhibited α-amylase activity and reduced the blood glucose level by protecting pancreatic β-cells.90 It can also increase the insulin secretion in the blood, leading to improved metabolism and body weight.90 In addition, CNSP also prevents the conversion of starch to simple sugars by inhibiting the enzyme responsible in the conversion process.90 Hypoglycaemic effect is present due to the stimulation of Langerhans islets, the enhancement of insulin sensitivity, and strong antioxidant effect. Polysaccharides contain the hydroxyl (OH) functional group, which may facilitate glucose binding leading to reduced blood glucose level. In a study conducted by Li et al, administration of marine polysaccharides reduced blood glucose level and exhibited a high antioxidant activity.91 Wijesekara et al reported that sulphated polysaccharides possessed anti-diabetic activity and antioxidant activity demonstrating its effectiveness in reducing blood glucose level.92 Huang et al reported that sulphated polysaccharides possess anti-diabetic activity that can lead to weight gain and glycaemic regulation.93 Carrageenan isolated from a red seaweed, Kappaphycus alvarezii, elicits its anti-diabetic activity through α-glucosidase inhibition.94 The soluble fibre present in carrageenan reduces the amount of carbohydrates reaching the bloodstream by slowing down the absorption in the small intestine.66 Presence of free radicals in the body can trigger the development of diabetes. Sulphated polysaccharides have protective effect against oxidative stress due to the presence of natural antioxidants and, hence, demonstrate their potential as a therapy for DM.95 Table 9 highlights some marine-derived polysaccharides with anti-diabetic potential.
 |
Table 9 Marine-Derived Polysaccharides and Their Anti-Diabetic Potential |
Chromium (III) Derivatives
Chromium (III) is a common metal which can be used as a nutritional supplement for T2DM patients.99 It stimulates glucose metabolism by promoting insulin activity in the peripheral tissue. Similarly, the action of chromium directly affects insulin receptors and the affinity towards insulin.100 It also involves phosphorylation that leads to the modulation of signal multiplying protein.100 Polysaccharide chromium (III) derivative has gained much importance in improving T2DM. Chromium supplementation plays a major role in insulin-resistant animals as it potentiates the effect of insulin, enhances the PI3K/PKB insulin signalling pathways, and improves AMPK activity.101 Sulphated polysaccharide isolated from Enteromorpha prolifera was used in the preparation of rhamnan-type sulphated polysaccharide derivatives (SPED) which was effective in enhancing glucose metabolism.102 Polysaccharides-chromium (III) complex isolated from Inonotus obliquus enhanced the oral glucose tolerance test in mice with T2DM and showed that it could enhance the glucose tolerance capacity.99 Moreover, SPED improves the insulin sensitivity by alleviating the pancreatic β-cell dysfunction103 and ameliorates hyperglycaemia by increasing the production of hepatic glycogen in insulin-resistant mice.102 In another study, Enteromorpha prolifera contains sulphated rhamnose polysaccharides, and a complex of sulphated rhamnose polysaccharides chromium (III) (SRPC) was synthesized. SRPC was found to possess hypoglycaemic effect, enhanced glucose tolerance, and improved insulin resistance in high-fat high-sucrose diet (HFSD)-induced T2DM mice.104
Fucoidan
Fucoidan resembles heparin due to its highly sulphated skeleton and can be found in brown marine algae. Fucoidan exhibits α-glucosidase and α-amylase activity and can prevent incidence of DM by preventing oxidative stress.96,104 It is more potent than other α-D-glucosidase inhibitors such as acarbose with an IC50 range of 0.013–0.047 mg mL−1.97 The inhibitory activities of fucoidan are affected by the structure and composition of the compound.97 Fucoidan with a low molecular weight and high sulphate content is suitable for inhibitory activity against α-amylase and α-glucosidase activity.97 Treatment with fucoidan in diabetic rats lowered blood glucose level and improved insulin sensitivity.105 Administration of fucoidan can reduce the SOD activity and increase malondialdehyde (MDA) level and decrease in HbA1c levels.105 On the other hand, fucoidan from sea cucumber Cucumaria frondosa normalized the PI3K/PKB pathway and GLUT4 translocation.106 Hence, fucoidan may be a potential therapy for DM.
Glycosaminoglycan
Glycosaminoglycan isolated from Urechis unicinctus reduced the blood glucose level, insulin resistance in homeostatic model assessment (HOMA-IR), and area under the curve in diabetic rats.98 It also enhanced the glucose tolerance and eased the symptoms in the diabetic mice, suggesting glycosaminoglycan exhibits good anti-diabetic effect.98 Glycosaminoglycan exhibits antioxidant effect by improving the glutathione peroxidase (GSH-Px) and SOD activity in the liver of diabetic mice and significantly reduces the concentration of MDA.98 Glycosaminoglycan possesses hypoglycaemic activity and enhances the capacity of antioxidant by repairing pancreas and liver tissue.98
Secondary Metabolites
Bromophenols
Bromophenols are compounds that exhibit numerous biological activities beneficial to human, and they can be found widely distributed in marine algae. Bromophenols isolated from marine algae demonstrate anti-diabetic potential through their antioxidant activity and inhibition of enzymes related to diabetes. Two bromophenols, 2,4-dibromophenol and 2,4,6-tribromophenol, isolated from a red alga, Grateloupia elliptica, exhibited potent α-glucosidase inhibitory activity. These compounds also exhibited a mild inhibition against rat intestinal maltase (IC50 4.8 and 5.0 mM, respectively) and rat-intestinal sucrase (IC50 3.6 and 4.2 mM, respectively).31 Another bromophenol, bis(2,3-dibromo-4,5-dihydroxybenzyl)ether (BDDE), isolated from Polyopes lancifolia, exhibited a strong α-glucosidase inhibitory activity.107 Liu et al investigated the BDDE-α-glucosidase inhibitory mechanism. It was observed that the α-glucosidase enzyme changes conformation when synthetic BDDE is attached to it. The study suggested that BDDE-α-glucosidase interaction was driven by both hydrogen bonds and hydrophobic forces.108 Kurihara et al found that inhibitory potencies of bromophenol could be enhanced by lowering the degree of methyl substitution and elevating the degree of bromo substitution per benzene ring.109 Hence, these findings could be a stepping stone for developing novel α-glucosidase inhibitors for effective treatment of diabetes.108 Apart from being a potent α-glucosidase inhibitor, BDDE can also elevate the glucose uptake in insulin-resistant HepG2 cells. In addition, in vivo study evidenced that BDDE remarkably lowered the blood glucose, triglyceride, and glycosylated haemoglobin (HbA1c) levels. It also activated the phosphorylation of IR-β in gastrocnemii and decreased the expression of PTP1B. At high doses, BDDE reduced the body weight without affecting both water and food intake.110
The bromophenols 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether, bis(2,3,6-tribromo-4,5-dihydroxyphenyl)-methane and 1,2-bis(2,3,6-tribromo-4,5-dihydroxyphenyl)-ethane isolated from Symphyocladia latiuscula, a species of marine red alga, demonstrated a high PTP1B inhibitory activity with IC50 values of 3.9, 4.3, and 3.5 μmol L−1, respectively.111 Another four bromophenol derivatives, 2,2′,3,3′-tetrabromo-4,4′,5,5′-tetra-hydroxydiphenyl methane, 3-bromo-4,5-bis(2,3-dibromo-4,5-dihydroxybenzyl) pyrocatechol, bis(2,3-dibromo-4,5-dihydroxybenzyl)-ether and 2,2′,3-tribromo-3′,4,4′,5-tetrahydroxy-6′-ethyloxy-methyl diphenylmethane isolated from ethanolic extract from red alga, Rhodomela confervoides, showed in vivo anti-hyperglycaemic potential believed to be partially attributed to their strong PTP1B inhibitory activities.112 In a study by Shi et al three bromophenols, 3,4-dibromo-5-(methoxymethyl)-1,2-benzenediol, 2-methyl-3-(2,3-dibromo4,5-dihydroxy)-propylaldehyde, and 3-(2,3-dibromo-4,5-dihydroxy-phenyl)-4-bromo-5,6-dihydroxy-1,3-dihydroiso-benzofuran isolated from R. confervoides exhibited potent inhibitory activity against PTP1B.113 In an in vivo study, 3,4-dibromo-5-(2-bromo-3,4-dihydroxy-6-(isopropoxymethyl)benzyl)-benzene-1,2-diol (HPN), a synthetic analogue of bromophenol also isolated from R. confervoides, showed significant reduction in serum blood glucose (P < 0.01) after eight weeks of treatment. In addition, HPN was also found to decrease serum triglycerides and total cholesterol concentration in a dose-dependent manner. Moreover, HPN also significantly reduced the HbA1c levels (P < 0.05) in medium- and high-dose group. There was a reduction in the PTP1B levels in pancreatic tissue, and the intraperitoneal glucose tolerance test in rats showed HPN exhibits a blood lowering activity like rosiglitazone.43 Another two bromophenols, 3′,5′,6′,6-tetrabromo-2,4-dimethyldiphenyl ether and 2′,5′,6′,5,6-pentabromo-3′,4′,3,4-tetramethoxybenzo-phenone isolated from Laurencia similis demonstrated potent PTP1B inhibitory activities with IC50 values of 2.97 and 2.66 μM, respectively.114 These are also shown in Table 3.
A methyl derivative of the sole bioactive component of Lamellodysidea herbacea strongly inhibits PTP1B (IC50 1.7 μM) with no apparent cytotoxicity.115 A synthetic highly brominated compound derived from bromophenol isolated from R. confervoides demonstrated a promising PTP1B inhibitory activity and a propitious in vivo anti-diabetic activity.116 Bromophenols isolated from Symphyocladia latiuscula, especially the highly brominated agents, may represent novel anti-diabetic drugs as they inhibit both PTP1B and α-glucosidase as well as having the ability to enhance the glucose uptake and insulin sensitivity.117 Table 10 compiles the marine-derived bromophenols and their anti-diabetic potentials.
 |
Table 10 Marine-Derived Bromophenols Possessing Anti-Diabetic Potential |
Carboxylic Acid
Phthalic acid, a potent bioactive compound, isolated from ascidian Phallusia nigra, was investigated for T2DM activity (Table 11). The results from the docking studies and data analysis had indicated that phthalic acid demonstrated a prominent anti-diabetic effect compared to fidarestat.118
 |
Table 11 Marine-Derived Carboxylic Acid Having Anti-Diabetic Potential |
Butenolide Derivatives
Three butenolide derivatives (2,3-disubstituted disubstituted γ-butenolide), namely flavipesolide A, flavipesolide B, and flavipesolide C, were isolated from Aspergillus flavipes HN4-13 along with several other inactive butenolide derivatives.119 Although all the three butanolide derivatives act as strong α-glucosidase inhibitors, flavipesolide A and B are more potent than flavipesolide C.119 The butenolide derivatives phenyl- and benzyldisubstituted γ-butenolide isolated from a coral-associated fungus Aspergillus sp. showed α-glucosidase inhibition activity.120 Table 12 compiles the marine-derived butenolide derivatives and their anti-diabetic potentials.
 |
Table 12 Microbial-Derived Butenolide Derivatives Possessing Anti-Diabetic Potential |
Carotenoids
Hosokawa et al proposed that carotenoids exhibit anti-diabetic activities.121 Carotenoids such as fucoxanthin and astaxanthin possess strong anti-diabetic activity.122 Fucoxanthin is generally found in macroalgae such as Eisenia bicyclis and Undaria pinnatifida that have brown complexion. In an animal study, glucose levels and insulin levels in the plasma were found to be reduced when fucoxanthin was introduced.122 Rats administered with a diet consisting of fucoxanthin (0.2%) indirectly reduced insulin resistance. Maeda et al proposed that this reduction was due to suppression of TNF-α mRNA and levels of plasma leptin.123 Fucoxanthin supplementation also successfully decreased glucose and HbA1c levels as well as insulin in the plasma.124 D’Orazio et al proposed that diabetes can be prevented using fucoxanthin. The diabetic mice undergo mRNA downregulation of various inflammatory mediators like tumour necrosis factor and interleukins, which is known to increase the glucose levels in the blood through upregulation of Glut4.12 Similarly, Jung et al proposed that fucoxanthin can also act as advanced glycation end-products (AGE) inhibitor and PTP1B inhibitor.125 As AGE accumulate in the body, diabetes and its complications tend to manifest.126 Thus, owing to its AGE and PTP1B inhibitory properties, fucoxanthin displays a promising potential as a therapeutic agent for diabetes and its associated complications.125 Astaxanthin can be a therapeutic agent in diabetics due to its high antioxidant and anti-glycative abilities.127 It also can be used as a preventive agent for diabetics suffering from diabetic retinopathy and other diseases like macular degeneration.128 Astaxanthin (50 mg kg−1 day−1) administered in rats for 18 weeks lowered the fasting blood glucose compared to control group.122 Arunkumar et al proposed that astaxanthin (6 mg kg−1 day−1) in mice can enhance the insulin signalling through the beta receptor activation which simultaneously leads to greater release of reactive oxygen species and pro-inflammatory mediators like cytokines.129 It has been observed that 20 mg−1 kg−1 b.w. of astaxanthin can be administered in alloxane-induced diabetic rats, continuously for 7 days, which can normally bring down the catalase, superoxide dismutase, and glutathione peroxidase activity in saliva.122
The changes in the antioxidant system of the saliva shows that astaxanthin has potential enough for treating diabetes.122 Extract of Chlorella zofingiensis, a microalga, contains different content of astaxanthin. The extract rich in astaxanthin demonstrated higher antioxidant abilities and greater anti-glycative capacities, including the inhibition of AGEs formation, glucose autoxidation, as well as glycation-induced protein oxidation.56 Intraperitoneal glucose tolerance test of astaxanthin treatment indicated it could preserve the islet cells' function to secrete insulin.130 Hence, astaxanthin can be an alternative therapeutic agent for diabetes. The marine-derived carotenoids are listed in Table 13.
 |
Table 13 Algal-Derived Carotenoids Having Anti-Diabetic Potential |
Furan
Hyrtiosal, isolated from Hyrtios erectus, a marine sponge, possessed a dose-dependent non-competitive inhibitory effect against PTP1B (IC50 42 μM). It also demonstrated extensive cellular effects on glucose transport, PI3K/AKT activation, and TGFbeta/Smad2 signalling. Additionally, hyrtiosal potentially showed that a potent amount can completely abolish the AKT translocation of the membrane. In fact, Glut4 in overexpressed PTB1B CHO cells was enhanced by increasing Glut4, and modulation of the insulin-mediated inhibition was activated via Smad2. Thus, the study suggested that further research could be carried out on hyrtiosal to establish its potential in treating diabetes.131 Table 14 shows the structure of hyrtiosal.
 |
Table 14 Anti-Diabetic Hyrtiosal, Isolated from the Marine Sponge Hyrtios erectus |
Macrolide
The macrolide des-O-methyllasiodiplodin, isolated from Cerbera manghas, has the potential ability to reduce the blood glucose levels and HbA1c in mice. The in vivo results also showed that it rectified the pro-inflammatory factor and genes related to ROS expressions.132 Marine-derived epoxypukalide possesses the ability to induce the replication of β-cells. It enhanced the β-cell proliferation and activation 2.5-fold through the ERK1/2 signalling pathway, and upregulations of cyclin D2 and cyclin E simultaneously happened. Furthermore, epoxypukalide has demonstrated a protective role via basal and cytokine-mediated β-cell apoptosis in the islets. Thus, epoxypukalide may serve as a new therapeutic for diabetes.133 The marine-derived macrolides are exhibited in Table 15.
 |
Table 15 Marine-Derived Glycolipids Having Anti-Diabetic Potential |
Plastoquinones
Plastoquinones such as sargahydroquinoic acid, sargachromenol, and sargaquinoic acid isolated from Sargassum serratifolium have potential as a therapy for T2DM.134 Although all these plastoquinones exhibit PTP1B inhibitory activity, it has been found that two compounds, namely sargachromenol and sargaquinoic acid, also exhibit inhibition of α-glucosidase enzyme.134 Sargahydroquinoic acid and sargaquinoic acid activate both PPARα and PPARγ and, thereby, enhance the metabolic disorders without the side effects such as weight gain and renal failure observed in the previous PPAR agonists.135 Among the three plastoquinones, sargachromenol showed the best α-glucosidase inhibition, followed by sargaquinoic acid, while sargahydroquinoic acid was inactive.136 Kim et al identified Sargassum yezoense-derived dual agonists sargaquinoic acid (SQA) and sargahydroquinoic acid (SHQA), where SQA demonstrated a stronger binding affinity with PPARγ compared to specific PPARγ agonist, troglitazone, which eventually activated PPARγ transcriptional activity. In addition, SQA- and SHQA-treated 3T3-L1 cells caused an increase in differentiation of adipocyte and elevated expression of PPARγ, aP2, adiponectin, resistin, Glut4, and C/EBPa. Hence, the study results suggested that both SQA and SHQA could be used to decrease the insulin resistance through adipogenesis regulation.137 Table 16 lists the marine-derived anti-diabetic plastoquinones.
 |
Table 16 Macroalgal-Derived Anti-Diabetic Plastoquinones and Their Mechanism of Action |
Saponins
The anti-diabetic activity of saponins is observed due to the atrophy of beta cells that leads to enhanced insulin secretion and glycogen in the liver138 (Table 17). When diabetic rats were compared to that of the healthy ones, decreased glucose levels, increased insulin levels comparative to healthy ones are obtained.139 Decrease in blood glucose levels generally occurs through varied pathways which lead to inhibition of glucose in the blood.140–142 The saponin-treated groups showed reduction in the α-amylase exhibiting as intestinal α-glucosidase and pancreatic α-amylase inhibitory activity.143 The presence of antioxidants in saponin can reduce oxidative stress related to the incidence of DM. In a study, diabetic rats treated with saponin had reduced serum IL-6 and TNF-α concentrations.139 Ginsenosides saponins can hinder the lipopolysaccharide-induced generation of TNF-α by inhibiting the transcription factor of nuclear factor kappa light-chain enhancer of activated B cells (NF-KB) through which the regulation of transcription of many genes related to inflammation occurs.144 Saponin possesses antioxidant activity as liver L-MDA was reduced upon its administration.145 The level of antioxidant enzymes such as SOD and CAT also improved.145 Saponin may have a functional ROS-protective mechanism that can help regenerate pancreatic β-cells and prevent the destruction of pancreatic islets from alloxan cytotoxic effects.145 In addition, saponin is a good metals chelator.145 The OH groups in the saponin increase antioxidant activity and prevent the formation of ROS.
 |
Table 17 Invertebrate-Derived Saponins and Their Anti-Diabetic Potential |
Sterols
Six different sterols, namely 24(S)-hydroxy-24-vinylcholesterol, fucosterol, 24(R)-hydroxy-24- vinylcholesterol, β-sitosterol, stigmasterol, and cholesterol, were isolated from Sargassum glaucescens and investigated for their anti-diabetic potential. In vitro α-amylase inhibitory test revealed a potent inhibition of the enzyme which was higher compared to acarbose (IC50 value 8.9 ± 2.4 mg mL−1).146 An in vivo study showed that oral administration of fucosterol (30 mg kg−1) isolated from Pelvetia siliquosa caused a prominent reduction in serum glucose concentrations and inhibited accumulation of sorbitol in lenses. The study concluded that fucosterol was a main contributor of anti-diabetic property in P. siliquosa.147 The promising application in treating diabetes is observed in the study which revealed that fucosterol exhibits aldose reductase inhibitory activity. Additionally, the study demonstrated that fucosterol inhibited PTP1B non-competitively.148 Thus, fucosterol can potentially be used as anti-diabetic agent. The sterols highlighted are shown in Table 18.
 |
Table 18 Macroalgae-Derived Sterols and Their Anti-Diabetic Potential |
Tannins
Marine algae-derived phlorotannin is extensively studied for its anti-diabetic potential due to various mechanisms like PTP1B, α-amylase, and α-glucosidase inhibitory activity, glucose utilizing effect in skeletal muscle, insulin sensitivity improvement in T2D db/db mice, and provides protection against diabetes complication.149 Phlorotannin-rich extract of Ascophyllum, an edible marine macroalga, was found to be effective in inhibiting α-glucosidase at low levels with an IC50 20 µg mL−1 GAE.32 An in vivo study confirmed the ability of phlorotannin extracts to lower the normal rise in post-prandial blood glucose by 90% and to decrease peak secretion of insulin by 40%.150 Moon et al isolated 6 different phlorotannins from two edible marine algae, Eisenia bicyclis and Ecklonia stolonifera. The compounds eckol, phlorofurofucoeckol-A, dieckol, and 7-phloroeckol were potent PTP1B inhibitors with IC50 values ranging from 0.56 to 2.64 μM, and they inhibited PTP1B non-competitively. With IC50 values ranging from 1.37 to 6.13 μM, phlorofurofucoeckol-A, dieckol, and 7-phloroeckol were considered to have the most potent α-glucosidase activity.151
Phlorotannins isolated from Eisenia bicyclis, fucofuroeckol A, and dioxinodehydroeckol showed significant α-glucosidase and α-amylase inhibitory activity.152 The in vivo study reveals that phlorotannin-rich methanolic extract of E. stolonifera demonstrated anti-hyperglycaemic effect and brought about decrease in lipid peroxidation in unfasted KK-Ay mice in a dose-dependent manner.153 A phlorotannin-rich Alaskan seaweed demonstrated α-glucosidase and α-amylase inhibitory activities with potential in limiting the sugar release from carbohydrate and, thus, alleviating the post-prandial hyperglycaemia.154 Three phlorotannins isolated from Ecklonia maxima were observed to have antioxidant effect on DPPH free radicals, and two of them, dibenzo-1,4-dioxin-2,4,7,9-tetraol and hexahydroxyphenoxydibenzo-1,4-dioxin, possessed more potent α-glucosidase inhibitory activity and antioxidant effect than positive controls.155 Phlorotannins exhibit prominent inhibitory activity against AGE, rat lens aldose reductase (RLAR), angiotensin converting enzyme (ACE), peroxynitrite, and reactive oxygen species (ROS).156
A seaweed-derived phlorotannin, diphlorethohydroxycarmalol (DPHC), possesses a protective effect against oxidative stress which is induced by hyperglycaemia as it significantly inhibited high glucose-induced toxicity and apoptosis. It decreased both thiobarbituric acid-reactive substrates (TBARS) and ROS generation and elevated nitric oxide level induced by glucose while increasing the activities of antioxidant enzymes.157 In addition, DPHC suppressed the over-expression of COX-2 proteins, inducible nitric oxide synthase (iNOS), and NF-κB activation induced by glucose.158 As stated by Heo et al, Ishige okamurae-derived DPHC showed significant α-glucosidase and α-amylase inhibitory activities with IC50 values of 0.16 and 0.53 mM, respectively, which reflected more potent effects than acarbose.159 According to Fernando et al, treatment with DPHC suppressed the dilation in retinal diameter and vessel formation induced by high glucose. It inhibited expression of high glucose-induced vascular endothelial growth factor receptor 2 and its signalling cascade. Hence, it was concluded to be potential therapy against diabetic-induced angiogenesis.160
Octaphlorethol A (OPA), a phlorotannin isolated from Ishige foliacea, is reported to have anti-diabetic potential. Lee et al found OPA to increase the uptake of glucose in differentiated L6 rat myoblast cells dose-dependently relative to control. It also increased Glut4 translocation to the plasma membrane which was mediated by PI3-K/Akt and AMPK activation.161 Lee et al discovered that OPA had a higher α-glucosidase inhibitory activity compared to acarbose (IC50 0.11 mM). Molecular modelling studies indicated that OPA interacted with Arg526, Asp542, Asp203, His600, Lys480, Met444, Phe450, Phe575, Ser448, and Tyr605. As compared to normal, OPA significantly suppressed the increase in post-prandial blood glucose levels in the diabetic mice.162 Apart from its anti-diabetic effects, Lee et al also reported the in vivo antioxidant activity of OPA showing protective effect against all biochemical parameters studied. It also suppressed apoptosis and was associated with reductions in pro-apoptotic Bax and cleaved caspase-3 expressions and elevated anti-apoptotic BclxL expression.163 Lee et al also concluded that the mechanism of OPA activity might involve the activation of AMPK which leads to glucose uptake in skeletal muscle through Glut4-mediated and inhibition of PEPCK and G6Pase activity in the liver leading to the suppression of gluconeogenesis. Thus, the findings provide a new opportunity through the clinical application of OPA as a potential anti-diabetic agent.18
Phloroglucinol was also one of the phlorotannins that could be potentially used as therapeutic for diabetes. Phloroglucinol, isolated from the brown alga Eisenia bicyclis, inhibits glycation and α-amylase.164 Five phloroglucinol derivatives isolated from Ecklonia cava demonstrated evident α-glucosidase and α-amylase inhibitory activity dose-dependently, with IC50 values at 10.8 μmol L−1 and 124.9 μmol L−1, respectively.165 In an in vivo study phloroglucinol exhibited protective effects against AGE formation which were generated in a non-enzymatic glycation process which is associated with ageing, diabetes, and other chronic illnesses.166 A new phloroglucinol derivative, 2-(4-(3,5-dihydroxyphenoxy)-3,5-dihydroxyphenoxy)-benzene-1,3,5-triol (DDBT) isolated from Sargassum patens, showed a potent inhibitory activity against carbohydrate-hydrolysing enzymes anticipated to help in diabetes prevention.167 Ishophloroglucin A isolated from Ishige okamurae was also found to exhibit the highest α-glucosidase inhibitory effect.168 Lee et al reported potent α-glucosidase and α-amylase inhibitory activities of dieckol, a phloroglucinol derivative isolated from brown algae, Ecklonia cava, with an IC50 values of 0.24 and 0.66 mM, respectively, a more potent effect than acarbose. In vivo study showed suppression of post-prandial blood glucose levels after dieckol administration to diabetes-induced or normal mice.169 Two years later, Lee et al conducted another in vivo study which showed that the animals fed with dieckol-rich extract (AG-dieckol) had significantly reduced glucose in the blood, plasma insulin levels were increased as well as HbA1c levels, while drastic improvement in glucose tolerance was also observed. In contrast to the control group, the AG-dieckol group had markedly reduced plasma and hepatic lipids concentration.170 Consequently, a randomized double blind, placebo-controlled trial was conducted to investigate the safety and efficacy of AG-dieckol extract in humans. AG-dieckol supplementation decreased post-prandial glucose level compared to placebo after a period of 12 weeks. Nevertheless, no adverse effects were observed during the intervention period.171
Kang et al reported that intraperitoneal administration of dieckol to diabetes-induced animals significantly reduced body weight, blood glucose, and serum insulin level compared to the saline-administered group.172 In addition, increased activities of antioxidant enzymes and reduction in TBARS were also observed. Western blotting analysis showed that phosphorylation levels of AMPK and Akt were elevated. The study concluded that dieckol is a potential therapeutic agent for diabetes.172 Another study evidenced that dieckol protected rat insulinoma cells from damage due to hyperglycaemic condition due to the suppression of apoptosis related with increased expression of anti-apoptotic Bcl-2 and decreased expression of pro-apoptotic cleaved caspase.173 Dieckol treatment also decreased the overexpression of proteins such as COX-2, inducible nitric oxide synthase (iNOS), and NF-kB which were often induced by high glucose condition.174 Overall, the anti-diabetic effect of dieckol helped in improving insulin sensitivity175 as well as plasma glucose and hepatic glucose metabolic regulation.176 Another phlorotannin, 6,6′-bieckol (BEK), isolated from Ecklonia cava, inhibited cytotoxicity induced by high glucose level. It decreased the high glucose-induced TBARS, nitric oxide level, and ROS generation. It also lowered the overexpression of COX-2, inducible nitric oxide synthase (iNOS), and NF-κB. The study concluded that BEK had potential in preventing endothelial dysfunction related to diabetes and other complications.177 Phlorofucofuroeckol A isolated from the same alga also demonstrated higher inhibitory activities against α-glucosidase and α-amylase compared to acarbose with IC50 values of 19.52 and 6.34 μM, respectively. Hence, it was a potent agent in alleviating post-prandial hyperglycaemia and had potential to be therapeutic as an anti-diabetic agent.178 Table 19 contains a list of marine-derived tannins and their anti-diabetic mechanism of action.
 |
Table 19 Edible Macroalgae-Derived Tannins Possessing Anti-Diabetic Potential |
Terpenes
Sesquiterpene
The sesquiterpene quinones purified from marine sponge Dysidea sp. have been evaluated for PTP1B inhibitory activity in vitro previously.180–182 Li et al successfully isolated 21-dehydroxybolinaquinone, a new sequiterpene quinone, along with two analogues, bolinaquinone and dysidine, from Hainan sponge Dysidea villosa. Among the analogues, dysidine appeared to have the most potent PTP1B inhibitory activity (IC50 6.70 μM). Bolinaquinone which had a PTP1B inhibitory activity (IC50 5.45 μM), also demonstrated cytotoxic activity against the HeLa cell line, whereas 21-dehydroxybolinaquinone moderately inhibited PTP1B (IC50 39.50 μM) and demonstrated moderate cytotoxicity against the HeLa cell line (IC50 19.45 μM).180 In a study by Zhang et al, dysidine exhibited insulin-sensitizing activity, which increases the glucose uptake in 3T3-L1 cells as well as effectively activating the insulin pathway wherein its specific inhibitory activity against PTP1B could have played a role. Hence, dysidine could be a potential lead compound in developing alternative adjuvant to insulin therapy.181 Another sesquiterpene, dehydroeuryspongin A, isolated from the sea sponge Euryspongia sp., exhibited PTP1B inhibitory activity (IC50 3.6 μM).44
Diterpene
Liang et al investigated the PTP1B inhibitory effects of diterpenoids isolated from soft coral Sarcophyton trocheliophorum.183,184 The diterpenoids, sarsolilide A and sarsolilide B, purified from the soft coral, exhibited in vitro PTP1B inhibitory activity.184 Due to the resemblance in terms of efficiency with the hypoglycaemic agent chlorpropamide, azorellanol may act on the pancreatic β-cells, whereas mulinolic acid could be acting upon glucose production or utilization in the liver.185
Sesterterpene
Five sesterpenoids were isolated from marine sponge, like Hippospongia lachne, which is collected from Yongxin Island in the South China Sea. Among these compounds, 9-oxa-2-azabicyclo-[3,3,1]-nona-3,7-diene derivative and 2-(aminomethylene)-hepta-3,5-dienedial moiety exhibited moderate inhibition of PTP1B with IC50 values of 5.2 μM and 8.7 μM, respectively.186
Triterpene
Hopane-6α,22-diol and brialmontin 1 were isolated from methanolic extract of Lecidella carpathica, a type of Antartic lichen, and along with atraric acid were found to have dose-dependent PTP1B inhibitory activity with IC50 values of 3.7, 14.0, and 51.5 μM, respectively.187 Kinetic analysis of the inhibitory activity showed that hopane-6α,22-diol and brialmontin 1 competitively inhibited PTP1B. Compared to other protein tyrosine phosphatases such as TCPTP (IC50 8.4 μM), LAR (IC50 >68 μM), SHP-2 (IC50 >68 μM), and protein tyrosine phosphatase receptor type C (PTPRC) (IC50 >68 μM), hopane-6α,22-diol selectively inhibited PTP1B (IC50 3.7 μM). Stellettin N, an isomalabaricane triterpene, was isolated from Hainan sponge Stelletta sp. along with other analogues, stellettin H, rhabdastrellic acid A, stellettin G, stellettin D, and 22,23-dihydrostellettin. Among the analogues, stellettin G was found to have a potent hPTP1B inhibitory activity (IC50 4.1 0.9 μM). It also demonstrated a weak cytotoxicity against HL-60 and A549 cells at a concentration of 10 μM.188 In addition, isomalabaricane triterpene cytotoxicity was found to be selective towards mouse lymphoma cell line L5178Y compared to other cell lines such as HeLa (human cervix carcinoma) and PC-12 (rat pheochromocytoma).189 Table 20 shows the chemical structure of the above-mentioned compounds.
 |
Table 20 Invertebrate-Derived Terpenes Possessing Anti-Diabetic Potential |
Others
Six different bioactive constituents, namely fructigenine A, cyclopenol, echinulin, flavoglaucin, viridicatol, and anhydrofulvic acid, isolated from marine fungal species, were found to have proven PTP1B inhibitory activity.190,191 Hence, bioactive constituents isolated from marine fungus could be a potential drug target in treating diabetes.190 Aquastatin A isolated from an ascomycete fungus, Cosmophora sp., also demonstrated a potent and selective PTP1B inhibitory activity (IC50 0.19 μM).191,192 Kinetic study also showed that it inhibited PTP1B in a competitive manner. It is known to have a low IC50 value against the PTP1B enzyme as well as selective PTP inhibitors such as LAR, CD45, TCPTP, and SHP-2.136 The study suggested that aquastatin A exhibited such an effect due to the presence of dihydroxypentadecyl benzoic acid moiety.191 Penstyrylpyrone isolated from a Penicillium sp. competitively inhibited PTP1B in a dose-dependent fashion. Apart from this, penstyrylpyrone was also found to inhibit NO and PGE2 via the inhibition of iNOS and cyclooxygenase-2 (COX-2) expression. Besides, through anti-inflammatory HO-1 expression, penstyrylpyrone successfully suppressed the pro-inflammatory mediator production via NF-κB pathway. Hence, by having both inhibitory and anti-inflammatory effects, penstyrylpyrone could be a potential lead agent to treat diabetes.149 The marine fungi-derived anti-diabetic compounds are shown in Table 21. In addition, long-chain polyunsaturated fatty acids, namely eicosapentaenoic acid and docosahexaenoic acid, are known to exert preventive effects on obesity and metabolic syndrome. Microalgae and marine fish oil are the principal sources of these compounds. A study conducted by Mayer et al showed that the microalga, Diacronema lutheri, showed significant protective effect from metabolic syndrome.193 Unnikrishnan et al conducted a comprehensive study involving extracts from the edible seaweed, Ulva reticulata; these were investigated for their anti-diabetic effect by examining their inhibitory effects on amylase, glucosidase, and DPP-IV and antioxidant (DPPH) potential in vitro and its purified fraction using animal models. The study reported highest activity in the methanolic extract and its fraction of the seaweed.194
 |
Table 21 Marine Organism-Derived Terpenes Having Anti-Diabetic Potential |
Future Perspectives and Limitations
Many of the compounds and metabolites isolated from marine sources have shown potent therapeutic activities that may be used in the treatment and prevention of various ailments. However, this would only correlate to a minimal percentage of what the marine ecosystem holds. Given the enormous body of unexplored marine organisms and sources, there remains a large conglomeration of ocean life yet to be studied. Furthermore, more in-depth studies are needed for the already identified potent molecules from the marine sources. This review is an attempt to segregate the potential marine biomolecules that were reported to possess promising hypoglycaemic potential. However, unpublished knowledge may have been overlooked. Moreover, reports published outside of the search focus duration may not have been reviewed here.
Conclusion
Due to the limited numbers of available anti-diabetic agents, marine-derived compounds (Table 22) are considered as unexploited resources of bioactive and structural compounds that have sparked significant interest among the scientific community and are anticipated to contribute to the discovery of novel hypoglycaemic agents. There is a need to pay more attention to identifying the structure–activity relationships and mechanisms of actions of these marine-derived compounds. However, limited yields of these compounds may hinder effective in vivo assessments. Hence, intensive investigations should be warranted in order to discover more effective and safer hypoglycaemic agents. Various bioactive compounds (Figure 4) isolated from marine sources compiled in this paper have exhibited hypoglycaemic effect through various mechanisms. Further studies such as toxicity tests need to be carried out in these compounds to identify the possible toxicities and identify safer hypoglycaemic agents to be used in the treatment of T2DM.
 |
Table 22 Anti-Diabetic Compounds Obtained from Various Marine Organisms at a Glance |
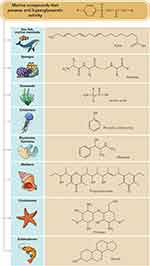 |
Figure 4 Anti-hyperglycaemic compounds obtained from various marine organisms at a glance. |
Acknowledgments
The authors would like to acknowledge the institutions that have supported this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. The authors also took part in drafting, revising, or critically reviewing the article and have given their final approval of the version to be published. All have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Funding
This project was funded by the International Medical University ID BP1-01/2019(35) research grant and the preliminary output for the work was supported by the Science and Technology Research Partnership for Sustainable Development (SATREPS) Program entitled “Development of Advanced Hybrid Ocean Thermal Energy Conversion (OTEC) Technology for Low Carbon Society and Sustainable Energy System: First Experimental OTEC Plant of Malaysia” funded by the Japan Science and Technology Agency (JST), the Japan International Cooperation Agency (JICA), and the Ministry of Higher Education Malaysia (MoHE) and was led by the Institute of Ocean Energy Saga University (IOES) of Japan, and UTM Ocean Thermal Energy Centre (UTM OTEC), Universiti Teknologi Malaysia (UTM). Registered Program Cost Centre: R.K130000.7809.4L887, Project [Cost Centre: Project No IF045-2019].
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Al-Lawati JA. Diabetes mellitus: a local and global public health emergency! Oman Med J. 2017;32(3):177–179. doi:10.5001/omj.2017.34
2. Barde SR, Sakhare RS, Kanthale SB, Chandak PG, Jamkhande PG. Marine bioactive agents: a short review on new marine anti-diabetic compounds. Asian Pac J Trop Dis. 2015;5:S209–S213. doi:10.1016/S2222-1808(15)60891-X
3. International diabetes federation- facts and figures. 2023. Available from: https://idf.org/about-diabetes/facts-figures/.
4. Goldenberg R, Punthakee Z. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome Canadian Diabetes Association clinical practice guidelines expert committee. Can J Diabetes. 2013;37:S8–S11. doi:10.1016/j.jcjd.2013.01.011
5. Groop L, Pociot F. Genetics of diabetes–are we missing the genes or the disease? Mol Cell Endocrinol. 2014;382(1):726–739. doi:10.1016/j.mce.2013.04.002
6. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–S24. doi:10.2337/dc17-S005
7. Liu Y, Gao Z, Guo Q, et al. Anti-diabetic effects of CTB-APSL fusion protein in type 2 diabetic mice. Mar Drugs. 2014;12(3):1512–1529. doi:10.3390/md12031512
8. Reimann M, Bonifacio E, Solimena M, et al. An update on preventive and regenerative therapies in diabetes mellitus. Pharmacol Ther. 2009;121(3):317–331.
9. Joshi SR, Parikh RM, Das AK. Insulin–history, biochemistry, physiology and pharmacology. J Assoc Physicians India. 2007;55:19–25.
10. Newsholme P, Cruzat V, Arfuso F, Keane K. Nutrient regulation of insulin secretion and action. J Endocrinol. 2014;221(3):R105–R120. doi:10.1530/JOE-13-0616
11. Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000;85(1):69–79. doi:10.1093/bja/85.1.69
12. D’Orazio N, Gammone MA, Gemello E, De Girolamo M, Cusenza S, Riccioni G. Marine bioactives: pharmacological properties and potential applications against inflammatory diseases. Mar Drugs. 2012;10:812–833. doi:10.3390/md10040812
13. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340–2348. doi:10.1016/S0140-6736(16)30507-4
14. Thilagam E, Parimaladevi B, Kumarappan C, Chandra Mandal S. α-Glucosidase and α- amylase inhibitory activity of senna surattensis. J Acupunct Meridian Stud. 2013;6(1):24–30. doi:10.1016/j.jams.2012.10.005
15. Jung M, Park M, Lee H, Kang Y-H, Kang E, Kim S. anti-diabetic agents from medicinal plants. Curr Med Chem. 2006;13(10):1203–1218. doi:10.2174/092986706776360860
16. Ngo LT, Okogun JI, Folk WR. 21st century natural product research and drug development and traditional medicines. Nat Prod Rep. 2013;30(4):584–592. doi:10.1039/c3np20120a
17. Ray SD. Side effects of drugs annual. In: Ray SD, editor. A Worldwide Yearly Survey of New Data in Adverse Drug Reactions. Vol. 39. Amsterdam, Netherlands: Elsevier; 2021:584.
18. Lee S-H, Ko S-C, Kang M-C, Lee DH, Jeon Y-J. Octaphlorethol A, a marine algae product, exhibits anti-diabetic effects in type 2 diabetic mice by activating AMP-activated protein kinase and upregulating the expression of glucose transporter 4. Food Chem Toxicol. 2016;91:58–64. doi:10.1016/j.fct.2016.02.022
19. Manikkam V, Vasiljevic T, Donkor ON, Mathai ML. A review of potential marine-derived hypotensive and anti-obesity peptides. Crit Rev Food Sci Nutr. 2016;56(1):92–112. doi:10.1080/10408398.2012.753866
20. Ruocco N, Costantini S, Guariniello S, Costantini M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules. 2016;21(5):551–567. doi:10.3390/molecules21050551
21. Saleh ASM, Zhang Q, Shen Q. Recent research in antihypertensive activity of food protein-derived hydrolyzates and peptides. Crit Rev Food Sci Nutr. 2016;56(5):760–787. doi:10.1080/10408398.2012.724478
22. Suleria HARHAR, Gobe G, Masci P, Osborne SA. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci Technol. 2016;50:44–55. doi:10.1016/j.tifs.2016.01.019
23. Choochote W, Suklampoo L, Ochaikul D. Evaluation of anti-oxidant capacities of green microalgae. J Appl Phycol. 2014;26(1):43–48. doi:10.1007/s10811-013-0084-6
24. Zhao C, Wu Y, Yang C, Liu B, Huang Y. Hypotensive, hypoglycaemic and hypolipidaemic effects of bioactive compounds from microalgae and marine micro-organisms. Int J Food Sci Technol. 2015;50:1705–1717. doi:10.1111/ijfs.12860
25. Pangestuti R, Kim SK. Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods. 2011;3:255–266. doi:10.1016/j.jff.2011.07.001
26. Jaspars M, de Pascale D, Andersen JH, Reyes F, Crawford AD, Ianora A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J Mar Biol Assoc UK. 2016;96(1):151–158. doi:10.1017/S0025315415002106
27. Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood. 2013;121:26–28. doi:10.1182/blood-2012-07-444851
28. Klotz U. Ziconotide- a novel neuron-specific calcium channel blocker for the intrathecal treatment of severe chronic pain- a short review. Int J Clin Pharmacol Ther. 2006;44:478–483. doi:10.5414/CPP44478
29. Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8(10):2619–2638. doi:10.3390/md8102619
30. Imada C. Enzyme inhibitors and other bioactive compounds from marine actinomycetes. Antonie Van Leeuwenhoek. 2005;87(1):59–63. doi:10.1007/s10482-004-6544-x
31. Kim KY, Nam KA, Kurihara H, Kim SM. Potent alpha-glucosidase inhibitors purified from the red alga grateloupia elliptica. Phytochemistry. 2008;69(16):2820–2825. doi:10.1016/j.phytochem.2008.09.007
32. Nwosu F, Morris J, Lund VA, Stewart D, Ross HA, McDougall GJ. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011;126(3):1006–1012. doi:10.1016/j.foodchem.2010.11.111
33. Sun Z, Chen F. Evaluation of the Green Alga Chlorella pyrenoidosa for management of diabetes. J Food Drug Anal. 2012;20:246–249.
34. Bidon-Chanal A, Fuertes A, Alonso D, et al. Evidence for a new binding mode to GSK-3: allosteric regulation by the marine compound palinurin. Eur J Med Chem. 2013;60:479–489. doi:10.1016/j.ejmech.2012.12.014
35. Harnedy PA, FitzGerald RJ. In vitro assessment of the cardioprotective, anti-diabetic and 805 anti-oxidant potential of Palmaria palmata protein hydrolysates. J Appl Phycol. 2013;25(6):1793–1803. doi:10.1007/s10811-013-0017-4
36. Pandey S, Sree A, Dash SS, Sethi DP, Chowdhury L. Diversity of marine bacteria producing beta-glucosidase inhibitors. Microb Cell Fact. 2013;12:35. doi:10.1186/1475-2859-12-35
37. Krish S, Das A. In vitro bioactivity of marine seaweed, Cladophora rupestris. Int J Pharm Biol Sci. 2014;5:898–908.
38. Suzen S, Buyukbingol E. Recent studies of aldose reductase enzyme inhibition for diabetic complications. Curr Med Chem. 2005;10(15):1329–1352. doi:10.2174/0929867033457377
39. Henriksen EJ, Dokken BB. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr Drug Targets. 2006;7(11):1435–1441. doi:10.2174/1389450110607011435
40. Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297(6):E1247–E1259. doi:10.1152/ajpendo.00274.2009
41. Patel AM, Anand IS, Suva MA. Role of protein tyrosine phosphatase-1B inhibitors in type 2 diabetes mellitus. J Pharm Sci Tech. 2014;4:2–6.
42. Sharifuddin Y, Chin Y-X, Lim P-E, Phang S-M. Potential bioactive compounds from seaweed for diabetes management. Mar Drugs. 2015;13(8):5447–5491. doi:10.3390/md13085447
43. Shi D, Guo S, Jiang B, et al. HPN, a synthetic analogue of bromophenol from red alga Rhodomela confervoides: synthesis and anti-diabetic effects in C57BL/KsJ-db/db mice. Mar Drugs. 2013;11(2):350–362. doi:10.3390/md11020350
44. Yamazaki H, Nakazawa T, Sumilat DA, et al. Three new unique sesquiterpenes from a marine sponge Euryspongia sp. Bioorg Med Chem Lett. 2013;23(7):2151–2154. doi:10.1016/j.bmcl.2013.01.102
45. Tamrakar AK, Tiwari P, Ahmad R, et al. Antihyperglycaemic activity of Sinularia firma and Sinularia erecta in streptozotocin-induced diabetic rats. Med Chem Res. 2008;17(2):62–73.
46. Tiwari P, Rahuja N, Kumar R, et al. Search for antihyperglycemic activity in few marine flora and fauna. Indian J Sci Technol. 2008;1(5):1–5. doi:10.17485/ijst/2008/v1i5.4
47. Kang C, Jin YB, Lee H, et al. Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food Chem Toxicol. 2010;48(2):509–516. doi:10.1016/j.fct.2009.11.004
48. Zhu CF, Peng HB, Liu GQ, Zhang F, Li Y. Beneficial effects of oligopeptides from marine salmon skin in a rat model of type 2 diabetes. Nutrition. 2010;26(10):1014–1020. doi:10.1016/j.nut.2010.01.011
49. Nuno K, Villarruel-Lopez A, Puebla-Perez AM, Romero-Velarde E, Puebla-Mora AG, Ascencio F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J Funct Foods. 2013;5(1):106–115. doi:10.1016/j.jff.2012.08.011
50. Popov AM, Krivoshapko ON. Protective effects of polar lipids and redox-active compounds from marine organisms at modeling of hyperlipidemia and diabetes. J Biomed Sci Eng. 2013;06(05):543–550. doi:10.4236/jbise.2013.65069
51. Nguyen VB, Wang SL. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar Drugs. 2017;15(11):350. doi:10.3390/md15110350
52. Nguyen V, Nguyen A, Wang S-L. Utilization of fishery processing by-product squid pens for α-glucosidase inhibitors production by Paenibacillus sp. Mar Drugs. 2017;15(9):274. doi:10.3390/md15090274
53. Nguyen VB, Nguyen TH, Doan CT, et al. Production and bioactivity-guided isolation of anti-oxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous materials. Molecules. 2018;23(5):1124. doi:10.3390/molecules23051124
54. Krishnan S, Chakraborty K, Joy M. First report of chromenyl derivatives from spineless marine cuttlefish Sepiella inermis: prospective antihyperglycemic agents attenuate serine protease dipeptidyl peptidase-IV. J Food Biochem. 2019;43(5):1–12. doi:10.1111/jfbc.12824
55. Nguyen TH, Kim SM. α-glucosidase inhibitory activities of fatty acids purified from the internal organ of sea cucumber Stichopus japonicus. J Food Sci. 2015;80(4):H841–H847. doi:10.1111/1750-3841.12810
56. Sun Z, Liu J, Zeng X, et al. Astaxanthin is responsible for antiglycoxidative properties of microalga Chlorella zofingiensis. Food Chem. 2011;126(4):1629–1635. doi:10.1016/j.foodchem.2010.12.043
57. Nguyen TH, Um BH, Kim SM. Two unsaturated fatty acids with potent α-glucosidase inhibitory activity purified from the body wall of sea cucumber (Stichopus japonicus). J Food Sci. 2011;76(9):H208–H214. doi:10.1111/j.1750-3841.2011.02391.x
58. Saether T, Paulsen SM, Tungen JE, et al. Synthesis and biological evaluations of marine oxohexadecenoic acids: pPARα/γ dual agonism and anti-diabetic target gene effects. Eur J Med Chem. 2018;155:736–753. doi:10.1016/j.ejmech.2018.06.034
59. de Munter JSL, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4(8):e261. doi:10.1371/journal.pmed.0040261
60. Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes. Arch Intern Med. 2007;167(9):956.
61. Mann JI, De Leeuw I, Hermansen KDSG, et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis. 2004;14(6):373–394.
62. Association AD. Standards of medical care in diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37(1):11–34.
63. MacArtain P, Gill CIR, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev. 2007;65(12):535–543. doi:10.1111/j.1753-4887.2007.tb00278.x
64. Kim MS, Kim JY, Choi WH, Lee SS. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and anti-oxidant enzyme activities in patients with type 2 diabetes 880 mellitus. Nutr Res Pract. 2008;2(2):62. doi:10.4162/nrp.2008.2.2.62
65. Pi-Sunyer X. Do glycemic index, glycemic load, and fiber play a role in insulin sensitivity, disposition index, and type 2 diabetes? Diabetes Care. 2005;28(12):2978–2979. doi:10.2337/diacare.28.12.2978
66. Dumelod BD, Ramirez RP, Tiangson CL, Barrios EB, Panlasigui LN. Carbohydrate availability of arroz caldo with lambda-carrageenan. Int J Food Sci Nutr. 1999;50(4):283–289.
67. Goni I, Valdivieso L, Garcia-Alonso A. Nori seaweed consumption modifies glycemic re- sponse in healthy volunteers. Nutr Res. 2000;20(10):1367–1375. doi:10.1016/S0271-5317(00)80018-4
68. Kitano Y, Murazumi K, Duan J, et al. Effect of dietary porphyran from the red alga, Porphyra yezoensis, on glucose metabolism in diabetic KK-Ay mice. J Nutr Sci Vitaminol (Tokyo). 2012;58(1):14–19. doi:10.3177/jnsv.58.14
69. Vaugelade P, Hoebler C, Bernard F, et al. Non-starch polysaccharides extracted from seaweed can modulate intestinal absorption of glucose and insulin response in the pig. Reprod Nutr Dev. 2000;40(1):33–47. doi:10.1051/rnd:2000118
70. Paxman JR, Richardson JC, Dettmar PW, Corfe BM. Daily ingestion of alginate reduces energy intake in free-living subjects. Appetite. 2008;51(3):713–719. doi:10.1016/j.appet.2008.06.013
71. Wolf BW, Lai CS, Kipnes MS, et al. Glycemic and insulinemic responses of nondiabetic healthy adult subjects to an experimental acid-induced viscosity complex incorporated into a glucose beverage. Nutrition. 2002;18(7–8):621–626. doi:10.1016/S0899-9007(02)00750-5
72. Williams JA, Lai CS, Corwin H, et al. Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J Nutr. 2004;134(4):886–889. doi:10.1093/jn/134.4.886
73. Balasubramaniam V, Mustar S, Mustafa Khalid N, et al. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J Appl Phycol. 2013;25(5):1405–1412.
74. Barbagallo M, Dominguez LJ, Galioto A, et al. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med. 2003;24(1–3):39–52. doi:10.1016/S0098-2997(02)00090-0
75. Ramadass S, Basu S, Srinivasan AR. SERUM magnesium levels as an indicator of status of diabetes mellitus type 2. Diabetes Metab Syndr. 2015;9(1):42–45. doi:10.1016/j.dsx.2014.04.024
76. Liu Y, Zhou Q, Zhao Y, et al. Enrichment, distribution of vanadium-containing protein in vanadium-enriched sea cucumber Apostichopus japonicus and the ameliorative effect on insulin resistance. Biol Trace Elem Res. 2016;171(1):167–175. doi:10.1007/s12011-015-0517-y
77. Setyaningsih I, Bintang M, Madina N. Potentially antihyperglycemic from biomass and phycocyanin of spirulina fusiformis voronikhin by in vivo test. Procedia Chem. 2015;14:211–215. doi:10.1016/j.proche.2015.03.030
78. Gershwin ME, Belay A. Spirulina in Human Nutrition and Health. CRC press; 2007:328.
79. Yang CF, Lai SS, Chen YH, et al. Anti-diabetic effect of oligosaccharides from seaweed Sargassum confusum via JNK-IRS1/PI3K signaling pathways and regulation of gut microbiota. Food Chem Toxicol. 2019;131:110562. doi:10.1016/j.fct.2019.110562
80. Kumar SG, Rahman MA, Lee SH, Hwang HS, Kim HA, Yun JW. Plasma proteome analysis for anti-obesity and anti-diabetic potentials of chitosan oligosaccharides in ob/ob mice. Proteomics. 2009;9(8):2149–2162. doi:10.1002/pmic.200800571
81. Oh JH, Kim J, Lee Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr Res Pract. 2016;10(1):42–48. doi:10.4162/nrp.2016.10.1.42
82. Zhu CF, Li GZ, Peng H-B, Zhang F, Chen Y, Li Y. Effect of marine collagen peptides on markers of metabolic nuclear receptors in type 2 diabetic patients with/without hypertension. Biomed Environ Sci. 2010;23(2):113–120. doi:10.1016/S0895-3988(10)60040-2
83. Nasri R, Abdelhedi O, Jemil I, et al. Ameliorating effects of goby fish protein hydrolysates on high-fat-high-fructose diet-induced hyperglycemia, oxidative stress and deterioration of kidney function in rats. Chem Biol Interact. 2015;242:71–80. doi:10.1016/j.cbi.2015.08.003
84. Xia EQ, Zhu SS, He MJ, Luo F, Fu CZ, Zou TB. Marine peptides as potential agents for the management of type 2 diabetes mellitus-a prospect. Mar Drugs. 2017;15(4):88. doi:10.3390/md15040088
85. Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi:10.1016/j.metabol.2015.11.006
86. Hatanaka T, Inoue Y, Arima J, et al. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012;134(2):797–802. doi:10.1016/j.foodchem.2012.02.183
87. Velarde-Salcedo AJ, Barrera-Pacheco A, Lara-González S, et al. In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem. 2013;136(2):758–764. doi:10.1016/j.foodchem.2012.08.032
88. Cheung RCF, Ng TB, Wong JH. Marine peptides: bioactivities and applications. Mar Drugs. 2015;13(7):4006–4043. doi:10.3390/md13074006
89. Suetsuna K, Saito M; Shirako Co Ltd. Enzyme-decomposed materials of laver and uses thereof. U.S. Patent 6217879; 2001.
90. Ben Abdallah Kolsi R, Ben Gara A, Jardak N, et al. Inhibitory effects of Cymodocea nodosa sulphated polysaccharide on α-amylase activity, liver-kidney toxicities and lipid profile disorders in diabetic rats. Arch Physiol Biochem. 2015;121(5):218–227. doi:10.3109/13813455.2015.1107588
91. Li F, Zhang Y, Zhong Z. Antihyperglycemic effect of ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci. 2011;12(9):6135–6145. doi:10.3390/ijms12096135
92. Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym. 2011;84:14–21.
93. Huang L, Wen K, Gao X, Liu Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm Biol. 2010;48(4):422–426. doi:10.3109/13880200903150435
94. Suganya AM, Sanjivkumar M, Chandran MN, Palavesam A, Immanuel G. Pharmacological importance of sulphated polysaccharide carrageenan from red seaweed Kappaphycus alvarezii in comparison with commercial carrageenan. Biomed Pharmacother. 2016;84:1300–1312. doi:10.1016/j.biopha.2016.10.067
95. Baluchnejadmojarad T, Roghani M, Homayounfar H, Hosseini M. Beneficial effect of aqueous garlic extract on the vascular reactivity of streptozotocin-diabetic rats. Ethnopharmacol. 2003;85(1):139–144. doi:10.1016/S0378-8741(02)00372-0
96. Kim KT, Rioux LE, Turgeon SL. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014;98:27–33. doi:10.1016/j.phytochem.2013.12.003
97. Kumar TV, Lakshmanasenthil S, Geetharamani D, Marudhupandi T, Suja G, Suganya P. Fucoidan–a α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int J Biol Macromol. 2015;72:1044–1047. doi:10.1016/j.ijbiomac.2014.10.013
98. Yuan C, Liu P, Han X, Cui Q. Hypoglycemic effects of glycosaminoglycan from Urechis unicinctus in diabetic mice. J Med Food. 2015;18(2):190–194. doi:10.1089/jmf.2013.3139
99. Wang C, Chen Z, Pan Y, Gao X, Chen H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem Toxicol. 2017;108:498–509. doi:10.1016/j.fct.2017.01.007
100. Mackowiak P, Krejpcio Z, Sassek M, et al. Evaluation of insulin binding and signaling activity of newly synthesized chromium (III) complexes in vitro. Mol Med Rep. 2010;3(2):347–353. doi:10.3892/mmr_00000264
101. Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012;23:313–319. doi:10.1016/j.jnutbio.2011.11.001
102. Cui JF, Ye H, Zhu YJ, Li YP, Wang JF, Wang P. Characterization and hypoglycemic activity of a rhamnan-type sulfated polysaccharide derivative. Mar Drugs. 2019;17(1):1–14. doi:10.3390/md17010021
103. Dabhi B, Mistry KN. Oxidative stress and its association with TNF-α-308 G/C and IL-1α-889 C/T gene polymorphisms in patients with diabetes and diabetic nephropathy. Gene. 2015;562(2):197–202. doi:10.1016/j.gene.2015.02.069
104. Ye H, Shen Z, Cui J, et al. Hypoglycemic activity and mechanism of the sulfated rhamnose polysaccharides chromium(III) complex in type 2 diabetic mice. Bioorg Chem. 2019;88:102942. doi:10.1016/j.bioorg.2019.102942
105. Yang XD, Liu CG, Tian YJ, Gao DH, Li WS, Ma HL. Inhibitory effect of fucoidan on hypoglycemia in diabetes mellitus anim. Int J Clin Exp Med. 2017;10(5):8529–8534.
106. Wang Y, Wang J, Zhao Y, Hu S, Shi D, Xue C. Fucoidan from sea cucumber Cucumaria frondosa exhibits anti-hyperglycemic effects in insulin resistant mice via activating the PI3K/PKB pathway and GLUT4. J Biosci Bioeng. 2016;121(1):36–42. doi:10.1016/j.jbiosc.2015.05.012
107. Kim KY, Nguyen TH, Kurihara H, Kim SM. α-glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J Food Sci. 2010;75(5):H145–H150. doi:10.1111/j.1750-3841.2010.01629.x
108. Liu M, Zhang W, Wei J, Lin X. Synthesis and α-glucosidase inhibitory mechanisms of bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a potential marine bromophenol α-glucosidase inhibitor. Mar Drugs. 2011;9(9):1554–1565. doi:10.3390/md9091554
109. Kurihara H, Mitani T, Kawabata J, Takahashi K. Inhibitory potencies of bromophenols from rhodomelaceae algae against alpha-glucosidase activity. Fish Sci. 1999;65(2):300–303. doi:10.2331/fishsci.65.300
110. Xu F, Wang F, Wang Z, Lv W, Wang W, Wang Y. Glucose uptake activities of Bis (2,3- Dibromo-4,5-Dihydroxybenzyl) ether, a novel marine natural product from red alga Odonthalia corymbifera with protein tyrosine phosphatase-1B inhibition, in vitro and in vivo. PLoS One. 2016;11(1):e0147748. doi:10.1371/journal.pone.0147748
111. Liu X, Li X, Gao L, et al. Extraction and PTP1B inhibitory activity of bromophenols from the marine red alga Symphyocladia latiuscula. Chin J Oceanol Limnol. 2011;29(3):686–690. doi:10.1007/s00343-011-0136-1
112. Shi D, Xu F, He J, Li J, Fan X, Han L. Inhibition of bromophenols against PTP1B and anti-hyperglycemic effect of Rhodomela confervoides extract in diabetic rats. Sci Bull. 2008;53(16):2476–2479. doi:10.1007/s11434-008-0353-y
113. Shi D-Y, Xu F, Li J, Guo S-J, Su H, Han L-J. PTP1B inhibitory activities of bromophenol derivatives from algae. Zhongguo Zhong Yao Za Zhi. 2008;33(19):2238–2240.
114. Qin J, Su H, Zhang Y, et al. Highly brominated metabolites from marine red alga Laurencia similis inhibit protein tyrosine phosphatase 1B. Bioorg Med Chem Lett. 2010;20(23):7152–7154. doi:10.1016/j.bmcl.2010.08.144
115. Yamazaki H, Sumilat DA, Kanno SI, et al. A polybromodiphenyl ether from an Indonesian marine sponge Lamellodysidea herbacea and its chemical derivatives inhibit protein tyrosine phosphatase 1B, an important target for diabetes treatment. J Nat Med. 2013;67(4):730–735. doi:10.1007/s11418-012-0735-y
116. Shi D, Li J, Jiang B, Guo S, Su H, Wang T. Bromophenols as inhibitors of protein tyrosine phosphatase 1B with anti-diabetic properties. Bioorg Med Chem Lett. 2012;22(8):2827–2832. doi:10.1016/j.bmcl.2012.02.074
117. Paudel P, Seong SH, Park HJ, Jung HA, Choi JS. Anti-diabetic activity of 2,3,6-tribromo- 4,5-dihydroxybenzyl derivatives from Symphyocladia latiuscula through PTP1B downregulation and α-glucosidase inhibition. Mar Drugs. 2019;17(3):166–185. doi:10.3390/md17030166
118. Ananthan G, Prabhu AS. New lead molecules from ascidian phallusia nigra (savigny, 1816) for type-2 diabetes mellitus targeting aldose reductase: an in silico approach. Univ J Appl Sci. 2014;2(4):73–76. doi:10.13189/ujas.2014.020401
119. Wang C, Guo L, Hao J, Wang L, Zhu W. α-glucosidase inhibitors from the marine-derived fungus Aspergillus flavipes HN4-13. J Nat Prod. 2016;79(11):2977–2981. doi:10.1021/acs.jnatprod.6b00766
120. Zhang LH, Feng BM, Zhao YQ, et al. Polyketide butenolide, diphenyl ether, and benzophenone derivatives from the fungus Aspergillus flavipes PJ03-11. Bioorg Med Chem Lett. 2016;26(2):346–350. doi:10.1016/j.bmcl.2015.12.009
121. Hosokawa M, Okada T, Mikami N, Konishi I, Miyashita K. Bio-functions of marine carotenoids. Food Sci Biotechnol. 2009;18:1–11.
122. Chuyen HV, Eun JB. Marine carotenoids: bioactivities and potential benefits to human health. Crit Rev Food Sci Nutr. 2017;57(12):2600–2610. doi:10.1080/10408398.2015.1063477
123. Maeda H, Hosokawa M, Sashima T, Miyashita K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J Agric Food Chem. 2007;55(19):7701–7706. doi:10.1021/jf071569n
124. Woo MN, Jeon SM, Kim HJ, et al. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem Biol Interact. 2010;186(3):316–322. doi:10.1016/j.cbi.2010.05.006
125. Jung HA, Islam M, Lee CM, et al. Promising anti-diabetic potential of fucoxanthin isolated from the edible brown algae Eisenia bicyclis and Undaria pinnatifida. Fish Sci. 2012;78(6):1321–1329. doi:10.1007/s12562-012-0552-y
126. Rigalleau V, Cougnard-Gregoire A, Nov S, et al. Association of advanced glycation end products and chronic kidney disease with macroangiopathy in type 2 diabetes. J Diabetes Complications. 2015;29(2):270–274.
127. Lauritano C, Ianora A. Marine organisms with anti-diabetes properties. Mar Drugs. 2016;14(12):220. doi:10.3390/md14120220
128. Sun Z, Liu J, Zeng X, et al. Protective actions of microalgae against endogenous and exogenous advanced glycation end products (AGEs) in human retinal pigment epithelial cells. Food Funct. 2011;2(5):251–258.
129. Arunkumar E, Bhuvaneswari S, Anuradha CV. An intervention study in obese mice with astaxanthin, a marine carotenoid–effects on insulin signaling and pro-inflammatory cytokines. Food Funct. 2012;3(2):120–126. doi:10.1039/C1FO10161G
130. Uchiyama K, Naito Y, Hasegawa G, Nakamura N, Takahashi J, Yoshikawa T. Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002;7(5):290–2933. doi:10.1179/135100002125000811
131. Sun T, Wang Q, Yu Z, et al. Hyrtiosal, a PTP1B inhibitor from the marine sponge Hyrtios erectus, shows extensive cellular effects on PI3K/AKT activation, glucose transport, and TGFβ/Smad2 signaling. ChemBioChem. 2007;8(2):187–193. doi:10.1002/cbic.200600349
132. Zhou R, Lin ZH, Jiang CS, et al. Marine natural product des-O-methyllasiodiplodin effectively lowers the blood glucose level in db/db mice via ameliorating inflammation. Acta Pharmacol Sin. 2013;34(10):1325–1336. doi:10.1038/aps.2013.47
133. López-Acosta JF, Moreno-Amador JL, Jiménez-Palomares M, et al. Epoxypukalide induces proliferation and protects against cytokine-mediated apoptosis in primary cultures of pancreatic β-cells. PLoS One. 2013;8(1):e52862. doi:10.1371/journal.pone.0052862
134. Ali Y, Kim DH, Seong SH, Kim HR, Jung HA, Choi JS. α-Glucosidase and protein tyrosine phosphatase 1b inhibitory activity of plastoquinones from marine brown alga Sargassum serratifolium. Mar Drugs. 2017;15(12):368. doi:10.3390/md15120368
135. Adeghate E. Medicinal chemistry and actions of dual and pan PPAR modulators. Open Med Chem J. 2011;5(1):93–98. doi:10.2174/1874104501105010093
136. Ezzat SM, Bishbishy MHE, Habtemariam S, et al. Looking at marine-derived bioactive molecules as upcoming anti-diabetic agents: a special emphasis on PTP1B inhibitors. Molecules. 2018;23(12):3334. doi:10.3390/molecules23123334
137. Kim S-N, Choi HY, Lee W, Park GM, Shin WS, Kim YK. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through PPARalpha/gamma activation in 3T3-L1 cells. FEBS Lett. 2008;582(23–24):3465–3472. doi:10.1016/j.febslet.2008.09.011
138. Raju K, Balaraman R. anti-diabetic mechanisms of saponins of Momordica cymbalaria. Pharmacogn Mag. 2008;4(15):197–206.
139. El Barky AR, Hussein SA, Alm-Eldeen AA, Hafez YA, Mohamed TM. Anti-diabetic activity of Holothuria thomasi saponin. Biomed Pharmacother. 2016;84:1472–1487. doi:10.1016/j.biopha.2016.10.002
140. Metwally NS, Mohamed AM, El Sharabasy FS. Chemical constituents of the Egyptian plant Anabasis articulata (Forssk) moq and its anti-diabetic effects on rats with streptozotocin induced diabetic hepatopathy. J Appl Pharm Sci. 2012;2(4):54–65.
141. Elekofehinti OO, Kamdem JP, Kade IJ, Rocha JBT, Adanlawo IG. Hypoglycemic, antiperoxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. S Afr J Bot. 2013;88:56–61. doi:10.1016/j.sajb.2013.04.010
142. Priya SP, Gladys JR. Validation of anti-diabetic potential of Avirai kudineer a Siddha herbal formulation-a review. IOSR J Dent Med Sci. 2015;14(7):7–15.
143. Nagmoti DM, Juvekar AR. In vitro inhibitory effects of Pithecellobium dulce (Roxb.) Benth. seeds on intestinal α-glucosidase and pancreatic α-amylase. J Biochem Technol. 2013;4(3):616–621.
144. Wu CF, Bi XL, Yang JY, et al. 1095 differential effects of ginsenosides on NO and TNF-α production by LPS-activated N9 microglia. Int Immunopharmacol. 2007;7(3):313–320. doi:10.1016/j.intimp.2006.04.021
145. Elekofehinti OO, Adanlawo IG, Saliu JA, Sodehinde SA. Saponins from Solanum anguivi fruits exhibit hypolipidemic potential in Rattus norvegicus. Der Pharm Lett. 2012;4(3):811–814.
146. Payghami N, Jamili S, Rustaiyan A, Saeidnia S, Nikan M, Gohari A. Alpha-amylase inhibitory activity and sterol composition of the marine algae, Sargassum glaucescens. Pharmacognosy Res. 2015;7(4):314. doi:10.4103/0974-8490.167893
147. Lee YS, Shin KH, Kim B-K, Lee S. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch Pharm Res. 2004;27(11):1120. doi:10.1007/BF02975115
148. Jung HA, Islam MN, Lee CM, et al. Kinetics and molecular docking studies of an anti-diabetic complication inhibitor fucosterol from edible brown algae Eisenia bicyclis and Ecklonia stolonifera. Chem Biol Interact. 2013;206(1):55–62.
149. Lee S-H, Jeon Y-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia. 2013;86:129–136. doi:10.1016/j.fitote.2013.02.013
150. Roy M-C, Anguenot R, Fillion C, Beaulieu M, Bérubé J, Richard D. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res Int. 2011;44(9):3026–3029. doi:10.1016/j.foodres.2011.07.023
151. Moon HE, Islam MN, Ahn BR, et al. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci Biotechnol Biochem. 2011;75(8):1472–1480. doi:10.1271/bbb.110137
152. Eom SH, Lee SH, Yoon NY, et al. α-Glucosidase-and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. J Sci Food Agric. 2012;92(10):2084–2090.
153. Iwai K. anti-diabetic and anti-oxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Foods Hum Nutr. 2008;63(4):163–169. doi:10.1007/s11130-008-0098-4
154. Kellogg J, Grace M, Lila M. Phlorotannins from Alaskan seaweed inhibit carbolytic enzyme activity. Mar Drugs. 2014;12(10):5277–5794. doi:10.3390/md12105277
155. Rengasamy KRR, Aderogba MA, Amoo SO, Stirk WA, Van Staden J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) papenfuss. Food Chem. 2013;141(2):1412–1415. doi:10.1016/j.foodchem.2013.04.019
156. Jung HA, Yoon NY, Woo MH, Choi JS. Inhibitory activities of extracts from several kinds of seaweeds and phlorotannins from the brown alga Ecklonia stolonifera on glucose-mediated protein damage and rat lens aldose reductase. Fish Sci. 2008;74(6):1363–1365.
157. Lee SH, Choi JI, Heo SJ, et al. Diphlorethohydroxycarmalol isolated from Pae (Ishige okamurae) protects high glucose-induced damage in RINm5F pancreatic β cells via its anti-oxidant effects. Food Sci Biotechnol. 2012;21(1):239–246. doi:10.1007/s10068-012-0031-3
158. Heo SJ, Hwang JY, Choi JI, et al. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against high glucose-induced-oxidative stress in human umbilical vein endothelial cells. Food Chem Toxicol. 2010;48(6):1448–1454. doi:10.1016/j.fct.2010.02.025
159. Heo S-J, Hwang J-Y, Choi J-I, Han J-S, Kim H-J, Jeon Y-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown alga, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2009;615(1–3):252–256. doi:10.1016/j.ejphar.2009.05.017
160. Fernando K, Yang H-W, Jiang Y, Jeon Y-J, Ryu B. Diphlorethohydroxycarmalol isolated from Ishige okamurae represses high glucose-induced angiogenesis in vitro and in vivo. Mar Drugs. 2018;16(10):375. doi:10.3390/md16100375
161. Lee S-H, Kang S-M, Ko S-C, Lee D-H, Jeon Y-J. Octaphlorethol A, a novel phenolic compound isolated from a brown alga, Ishige foliacea, increases glucose transporter 4-mediated glucose uptake in skeletal muscle cells. Biochem Biophys Res Commun. 2012;420(3):576–581. doi:10.1016/j.bbrc.2012.03.036
162. Lee S-HS-H, Kang S-MS-M, Ko S-CS-C, et al. Octaphlorethol a: a potent α-glucosidase inhibitor isolated from Ishige foliacea shows an anti-hyperglycemic effect in mice with streptozotocin-induced diabetes. Food Funct. 2014;5(10):2602–2608. doi:10.1039/C4FO00420E
163. Lee SH, Kang N, Kim EA, et al. Antidiabetogenic and antioxidative effects of octaphlorethol A isolated from the brown algae Ishige foliacea in streptozotocin-induced diabetic mice. Food Sci Biotechnol. 2014;23(4):1261–1266. doi:10.1007/s10068-014-0173-6
164. Okada Y, Ishimaru A, Suzuki R, Okuyama T. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: potential for the effective treatment of diabetic complications. J Nat Prod. 2004;67(1):103–105. doi:10.1021/np030323j
165. Lee SH, KimKaradeniz MMF, Kim MM, Kim SK, Kim S-K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J Sci Food Agric. 2009;89(9):1552–1558. doi:10.1002/jsfa.3623
166. Shakambari G, Ashokkumar B, Varalakshmi P. Phlorotannins from brown algae: inhibition of advanced glycation end products formation in high glucose induced Caenorhabditis elegans. Indian J Exp Biol. 2015;53(6):371–379.
167. Kawamura-Konishi Y, Watanabe N, Saito M, et al. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga Sargassum patens. J Agric Food Chem. 2012;60(22):5565–5570. doi:10.1021/jf300165j
168. Ryu B, Jiang Y, Kim HS, et al. Ishophloroglucin A, a novel phlorotannin for standardizing the anti-α-glucosidase activity of Ishige okamurae. Mar Drugs. 2018;16(11):436. doi:10.3390/md16110436
169. Lee SH, Park MH, Heo SJ, et al. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem Toxicol. 2010;48(10):2633–2637. doi:10.1016/j.fct.2010.06.032
170. Lee SH, Min KH, Han JS, et al. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ- db/db mice, a model of type 2 diabetes mellitus. Food Chem Toxicol. 2012;50(3–4):575–582. doi:10.1016/j.fct.2011.12.032
171. Lee S-H, Jeon Y-J. Efficacy and safety of a dieckol-rich extract (AG-dieckol) of brown algae, Ecklonia cava, in pre-diabetic individuals: a double-blind, randomized, placebo-controlled clinical trial. Food Funct. 2015;6(3):853–858. doi:10.1039/C4FO00940A
172. Kang MC, Wijesinghe WAJP, Lee SH, et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type diabetes in db/db mouse model. Food Chem Toxicol. 2013;53:294–298. doi:10.1016/j.fct.2012.12.012
173. Lee SH, Park MH, Kang SM, et al. Dieckol isolated from Ecklonia cava protects against high-glucose induced damage to rat insulinoma cells by reducing oxidative stress and apoptosis. Biosci Biotechnol Biochem. 2012;76(8):1445–1451. doi:10.1271/bbb.120096
174. Lee S-H, Han J-S, Heo S-J, Hwang J-Y, Jeon Y-J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol Vitr. 2010;24(2):375–381. doi:10.1016/j.tiv.2009.11.002
175. Jeon HJ, Yoon KY, Koh EJ, et al. Seapolynol and dieckol improve insulin sensitivity through the regulation of the PI3K pathway in C57BL/KsJ-db/db mice. J Food Nutr Res. 2015;3(10):648–652. doi:10.12691/jfnr-3-10-5
176. Kim EA, Lee SH, Lee JH, et al. A marine algal polyphenol, dieckol, attenuates blood glucose levels by Akt pathway in alloxan induced hyperglycemia zebrafish model. RSC Adv. 2016;6(82):78570–78575. doi:10.1039/C6RA12724J
177. Park MH, Heo SJ, Park PJ, et al. 6, 6′-bieckol isolated from Ecklonia cava protects oxidative stress through inhibiting expression of ROS and proinflammatory enzymes in high-glucose-induced human umbilical vein endothelial cells. Appl Biochem Biotechnol. 2014;174(2):632–643. doi:10.1007/s12010-014-1099-4
178. Lee H-A, Lee J-H, Han J-S. A phlorotannin constituent of Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Pharm Biol. 2017;55(1):1149–1154. doi:10.1080/13880209.2017.1291693
179. You H-N, Lee H-A, Park M-H, Lee J-H, Han J-S. Phlorofucofuroeckol A isolated from Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2015;752:92–96. doi:10.1016/j.ejphar.2015.02.003
180. Li Y, Zhang Y, Shen X, Guo Y-W. A novel sesquiterpene quinone from Hainan sponge Dysidea villosa. Bioorg Med Chem Lett. 2009;19(2):390–392. doi:10.1016/j.bmcl.2008.11.068
181. Zhang Y, Li Y, Guo Y, Jiang H, Shen X. A sesquiterpene quinone, dysidine, from the sponge Dysidea villosa, activates the insulin pathway through inhibition of PTPases. Acta Pharmacol Sin. 2009;30(3):333–345. doi:10.1038/aps.2009.5
182. Jiao WH, Huang XJ, Yang JS, et al. Dysidavarones A–D, new sesquiterpene quinones from the marine sponge Dysidea avara. Org Lett. 2012;14(1):202–205. doi:10.1021/ol202994c
183. Liang L-F, Gao L-X, Li J, Taglialatela-Scafati O, Guo Y-W. Cembrane diterpenoids from the soft coral Sarcophyton trocheliophorum Marenzeller as a new class of PTP1B inhibitors. Bioorg Med Chem. 2013;21(17):5076–5080. doi:10.1016/j.bmc.2013.06.043
184. Liang LF, Kurtán T, Mándi A, et al. Sarsolenane and capnosane diterpenes from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller as PTP1B inhibitors. European J Org Chem. 2013;2014(9):1841–1847. doi:10.1002/ejoc.201301683
185. Fuentes NL, Sagua H, Morales G, et al. Experimental antihyperglycemic effect of diterpenoids of llareta azorella compacta (Umbelliferae) Phil in rats. Phytother Res. 2005;19(8):713–716. doi:10.1002/ptr.1740
186. Piao SJ, Jiao WH, Yang F, et al. New hippolide derivatives with protein 1B inhibitory activity from the marine sponge Hippospongia lachne. Mar Drugs. 2014;12(7):4096–4109. doi:10.3390/md12074096
187. Seo C, Han Yim J, Kum Lee H, Oh H. PTP1B inhibitory secondary metabolites from the Antarctic lichen Lecidella carpathica. Mycology. 2011;2(1):18–23. doi:10.1080/21501203.2011.554906
188. Xue D-QL, Mao L, Yu X-Q, Guo Y-W. Isomalabaricane triterpenes with potent protein- tyrosine phosphatase 1B (PTP1B) inhibition from the Hainan sponge Stelletta sp. Biochem Syst Ecol. 2013;49:101–106. doi:10.1016/j.bse.2013.03.001
189. Fouad M, Edrada RA, Ebel R, et al. Cytotoxic isomalabaricane triterpenes from the Marine Sponge Rhabdastrella globostellata. J Nat Prod. 2006;69(2):211–218. doi:10.1021/np050346t
190. Sohn JH, Lee YR, Lee DS, Kim YC, Oh H. PTP1B inhibitory secondary metabolites from marine-derived fungal strains Penicillium spp. and Eurotium sp. J Microbiol Biotechnol. 2013;23(9):1206–1211. doi:10.4014/jmb.1303.03078
191. Seo C, Sohn JH, Oh H, Kim BY, Ahn JS. Isolation of the protein tyrosine phosphatase 1B inhibitory metabolite from the marine-derived fungus Cosmospora sp. SF-5060. Bioorg Med Chem Lett. 2009;19(21):6095–6097. doi:10.1016/j.bmcl.2009.09.025
192. Debbab A, Aly AH, Lin WH, Proksch P. Bioactive compounds from marine bacteria and fungi: minireview. Microb Biotechnol. 2010;3(5):544–563. doi:10.1111/j.1751-7915.2010.00179.x
193. Mayer C, Côme M, Ulmann L, et al. The potential of the Marine Microalga diacronema lutheri in the prevention of obesity and metabolic syndrome in high-fat-fed wistar rats. Molecules. 2022;27:4246. doi:10.3390/molecules27134246
194. Unnikrishnan PS, Animish A, Madhumitha G, Suthindhiran K, Jayasri MA. Bioactivity guided study for the isolation and identification of antidiabetic compounds from edible seaweed—ulva reticulata. Molecules. 2022;27(24):8827. doi:10.3390/molecules27248827
195. Lee DS, Jang JH, Ko W, et al. PTP1B inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus Penicillium sp. JF-55. Mar Drugs. 2013;11(4):1409–1426. doi:10.3390/md11041409
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
