Back to Journals » Nature and Science of Sleep » Volume 15
How is Obstructive Sleep Apnea Associated with High Blood Pressure and Diabetes Mellitus Type 2? Clues from a Two-Step Mendelian Randomized Study
Authors Shen Y, Wang H, Zhang W , Ou X, Liu S
Received 20 June 2023
Accepted for publication 14 September 2023
Published 29 September 2023 Volume 2023:15 Pages 749—765
DOI https://doi.org/10.2147/NSS.S423331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Yubin Shen,* Hongwei Wang,* Weiyu Zhang, Xiwen Ou, Song Liu
Department of Respiratory Medicine and Sleep Lab, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200092, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Song Liu, Department of Respiratory Medicine and Sleep Lab, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200092, People’s Republic of China, Tel +8613651713307, Email [email protected]
Background: Obstructive sleep apnea (OSA), high blood pressure (HBP), and type 2 diabetes mellitus (T2DM) have a close clinical relationship, but whether and how OSA affects HBP and T2DM is unclear.
Study Design and Methods: Two-step, two-sample Mendelian randomization techniques were applied using single-nucleotide polymorphisms as genetic instruments for exposure and mediators, thus minimizing bias due to confounding factors and reverse causality. The total effect of OSA on HBP and T2DM was categorized into direct and mediating effects based on the mediating factors.
Results: Two-sample MR analysis showed that OSA increased the risk of HBP (odds ratio [OR] = 1.010, 95% confidence interval [CI], 1.002– 1.018; P = 0.0121) and T2DM (OR = 1.140, 95% CI, 1.059– 1.228; P = 0.0005). In the process of OSA caused by HBP, sex hormone-binding globulin (SHBG) (female, 4.47% mediation; male, 2.76% mediation), total testosterone (TT) (male, 3.72% mediation), bioavailable testosterone (BioT) (female, 7.74% mediation), high-density lipoprotein cholesterol (HDL-C) (3.25% mediation), and apolipoprotein A1 (ApoA1) (1.31% mediation) were individual contributors. SHBG (female, 4.10% mediation; male, 1.58% mediation), TT (male, 3.69% mediation), BioT (female, 2.58% mediation), HDL-C (3.32% mediation), ApoA1 (2.14% mediation), and omega-6 fatty acids (2.33% mediation) may have mediating roles to varying degrees in the process of OSA caused by T2DM.
Interpretation: This MR study showed that OSA is a risk factor for HBP and T2DM, and the evaluation of mediators may help further reveal the specific mechanism by which OSA causes HBP and T2DM.
Keywords: high blood pressure, mediating factors, Mendelian randomization, obstructive sleep apnea, type 2 diabetes mellitus
Introduction
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder and is characterized by the narrowing of the upper airway, which leads to a series of pathophysiological changes owing to intermittent hypoxia, fragmented sleep, intrathoracic pressure swings, and increased sympathetic nervous activation.1 The prevalence of OSA is estimated to be 14% in males and 5% in females. It is higher in patients who have had transient ischemic attacks, coronary artery disease, congestive heart failure, arrhythmias, refractory hypertension, type 2 diabetes mellitus (T2DM), or polycystic ovary syndrome.1 Patients with OSA are prone to multiorgan and multisystem damage. Additionally, OSA is a potentially life-threatening disease that has become an important public healthcare problem that endangers human health.
High blood pressure (HBP) and T2DM are both prevalent diseases that have considerable impact on global public health due to their association with cardiovascular complications and mortality. While the association between OSA, HBP, and T2DM has been studied extensively, our understanding of the complex interplay of these conditions remains incomplete. In particular, there is limited insight into the influence of various confounding factors and mediators on the relationship between OSA and these two conditions. Therefore, the choice of HBP and T2DM as the focus of our study is compelled by their relevance to public health, as well as the remaining gaps in knowledge about their relationship with OSA. Our study aims to bridge this gap in knowledge using an innovative methodological approach.
Recent studies have hinted at the role of changes in testosterone and lipid levels in the relationship between OSA and HBP/T2DM.2,3 However, these observations have not been fully explored, let alone the specific pathways and mechanisms involved. Our study aims to delve deeper into these underlying factors using Mendelian randomization (MR), a well-accepted method for supporting causal inferences in observational data. This method uses single-nucleotide polymorphisms (SNPs) identified in genome-wide association studies (GWAS) that are closely associated with exposure as instrumental variables (IVs). MR provides estimates of the association between exposure and outcome under many assumptions, and this association is unlikely to be biased by unobserved confounding factors. Recent advances in MR methodology, such as two-sample and two-step MR, can be used to study mediating variables.
What sets our study apart from previous MR studies is our use of the most recent GWAS data and the application of a two-sample and two-step MR method, which allows us to investigate potential mediators in the association between OSA, HBP, and T2DM, an aspect rarely covered by previous investigations.4 By employing this innovative approach, we aim not only to confirm the causal relationship of OSA with HBP and T2DM but also to shed light on the potential mechanisms underpinning these relationships.
Materials and Methods
Study Design
A two-step, two-sample MR model was used to estimate the causal effects of OSA on HBP and T2DM and to explore whether and how mediators can mediate these effects. For causal estimates from MR studies to be valid, three main assumptions must be met: (I) the variant is associated with exposure; (II) the variant is not associated with the outcome via a confounding pathway; and (III) the variant does not affect the outcome directly, only possibly indirectly via exposure. The assumptions and design of the MR study are shown in Figure 1.
Two-step, two-sample MR was used to assess whether an intermediate trait had a mediation effect between exposure and outcome.5 First, SNPs were used to estimate the causal effect of OSA on potential mediators. Next, SNPs of potential mediating risk factors were used to make genetic predictions of these mediators and estimate their causal effects on outcomes. Subsequently, the total effect of OSA was decomposed into a direct effect (effect on HBP or T2DM independent of the mediator) and an indirect effect (effect on HBP or T2DM through the mediator). A checklist for the Strengthening the Reporting of Observational Studies in Epidemiology Using MR guideline is provided in Supplementary Table 1.6 Proxy SNPs were not used.
Data Sources
The data used in this study are available in public databases. Summary data related to OSA from FinnGen was used.7 Using the Finnish National Health register, this GWAS included 321,302 individuals and 27,976 patients with OSA. The diagnosis of OSA was based on subjective symptoms, clinical examination, and sleep registration applying apnea hypopnea index (AHI) ≥ 5/h or respiratory event index (REI) ≥ 5/h, as per the International Classification of Diseases, Tenth Revision (ICD-10) and Ninth Revision (ICD-9) codes (ICD-10: G47.3, ICD-9:3472). The ICD-10 data were collected from the Finnish National Hospital Discharge Registry and the Causes of Death Registry. The remaining data used for the MR study were obtained from the Integrative Epidemiology Unit Open GWAS database.8 Detailed information on data sources is presented in Table 1. Each group of summary data used for two-sample MR was obtained from different databases to reduce the effect of sample overlap and increase the reliability of results.
 |
Table 1 Detailed Information of Studies and Datasets Used for Analyses |
The Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa approved the FinnGen study protocol (Nr HUS/990/2017). The FinnGen study was approved by the Finnish Institute for Health and Welfare. The remaining analyses of publicly available data did not require ethical approval.
Validation of Instrumental SNPs
First, we identified SNPs related to OSA at a genome-wide significance threshold (P < 5×10−6) in a large GWAS meta-analysis by FinnGen7 (Table 2). The independence of SNPs was assessed using strict criteria (clumping r2 cutoff ≤ 0.001; clumping distance cutoff, 10,000 kb). An SNP was excluded from our analysis if its allele frequency was close to 50% with palindromic variants, and it was impossible to verify that the alleles were correctly oriented since it would produce inaccurate results in the MR analysis.
 |
Table 2 SNPs Associated with Obstructive Sleep Apnea |
The F-statistic was used in the MR to estimate weak IV bias. An often-cited rule of thumb is that an F-statistic less than 10 indicates the presence of weak instruments, which may lead to the reduced power of the test, violation of other core hypotheses, and cause bias in the causal association. When the F-statistic is >10, there is no weak IV, and the formula is as follows:
where R2 is the extent to which IV explains the exposure (coefficient of determination of the regression equation) and can be approximated as 2β2MAF(1-MAF), β is the genetic association with the exposure measured in standard deviation units of the exposure, MAF is the minor allele frequency, N is the sample size, and K is the number of instruments. For a single variant, the F-statistic is equal to the square of the genetic association, with the exposure divided by the square of its standard deviation:
Statistical Analysis
In addition to the inverse variance weighted (IVW) method for data analysis, we applied the constrained maximum likelihood, model average, and Bayesian information component (cML-MA-BIC) method for comparison. This method is better able to control Type I error rates and can use data perturbation (DP). This results in a greater measure of estimation error, which complements the instability of variable selection.9
The IVW method provides the highest statistical power; however, the results may be biased if there is horizontal pleiotropy in the IVs.10 Therefore, we performed sensitivity analyses, including heterogeneity tests, pleiotropy tests, leave-one-out sensitivity tests, and E-value calculations, to test the robustness and potential pleiotropy of our results. We assessed heterogeneity between each SNP using Cochran’s Q test; P < 0.05 indicated statistical significance, which reflected potential intergenic heterogeneity. We used the IVW random effects (IVW-RE) method to eliminate the effect of heterogeneity.11 MR-Egger regression methods were used to obtain corrected estimates of pleiotropy;12 P < 0.05 indicated the occurrence of pleiotropy.10 We corrected for horizontal pleiotropy by removing outliers using the MR-Pleiotropy Residual Sum and Outlier (PRESSO) method and tested for significant differences in causal estimates before and after outlier correction. The leave-one-out sensitivity test was used to assess the stability of the results by removing each SNP. Additionally, we used the E-value to assess the effect of unmeasured confounders on causal conclusions. The E-value represented the minimum amount of unmeasured confounding effect needed to erase the effect of the association between exposure and outcome obtained in the study.
IVW-RE was used as the main statistical method, while the cML-MA-BIC (DP) method was used as an adjunct to determine whether the results were reliable. The cML-MA-BIC-DP method was selected when GOF_P rejected the null hypothesis; otherwise, cML-MA-BIC method was selected. With different database sources for a type of mediator, a meta-analysis fixed-effects model was used to combine the results of each MR statistic from different data sources and test the existence of mediating effects for each mediating factor using the Sobel test; P < 0.05 was considered statistically significant.
All statistical analyses were conducted using R (version 4.2.1), which is available as Free Software under the terms of the Free Software Foundation’s GNU General Public License in source code form. The IVW method was performed using the “TwoSampleMR” package (version 0.5.6) and the cML-MA-BIC method was performed using the “MRcML” package (version 0.0.0.9000). The MR-PRESSO test was performed using the “MRPRESSO” package (version 1.0).
Results
Direct Effect on HBP
The MR analysis of OSA to HBP retained 58 independent genetic variants that reached genome-wide significance levels after significance screening (P < 5×10−6), removal of linkage disequilibrium (r2 < 0.001, kb = 10,000), and removal of outliers by MR-PRESSO (Table 3). These IVs explained approximately 6.1% of the total variance, with heterogeneity and no pleiotropy. All F-statistics of the included SNPs were higher than 20, which indicated that these SNPs were sufficient for predicting OSA in this study. Genetically predicted OSA increased the risk of HBP, with an odds ratio (OR) of 1.010 (95% confidence interval [CI], 1.002–1.018, P = 0.0121) in IVW-RE analysis and 1.007 (95% CI, 1.000–1.013, P = 0.0347) in cML-MA-BIC analysis. The causal relationship between genetically predicted OSA and HBP was largely consistent among the different methods (Figure 2), suggesting that OSA is a risk factor for HBP.
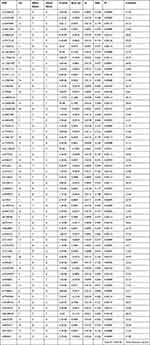 |
Table 3 SNPs Associated with Obstructive Sleep Apnea to High Blood Pressure |
Effect of Mediators on HBP
We further investigated the mechanisms by which OSA affects HBP and identified possible mediators that may play a role (Supplementary Figure 1). Using the analysis of sex hormone-binding globulin (SHBG) (female) from the EBI database,13 there was a negative correlation between OSA and SHBG (female) (β = −0.018, 95% CI: −0.029 to −0.007, P = 0.0012), and using the data reported by Hemani et al,14 there was also a negative correlation between OSA and SHBG (female) (β = −0.039, 95% CI: −0.065 to −0.013, P = 0.0029). This association was consistent across data from different sources and remained statistically significant after meta-analysis (β = −0.021, 95% CI: −0.031 to −0.011, P <0.0001). Similarly, SHBG (female) increased HBP risk (β = −0.022, 95% CI: −0.034 to −0.010, P = 0.0004). The Sobel test for the mediating effect of SHBG (female) on OSA-HBP yielded z = 2.65 and P = 0.0081, indicating a statistically significant mediating effect of SHBG (female). Analogously, there were also some mediating effects of SHBG (male), total testosterone (TT) (male), bioavailable testosterone (BioT) (female), high-density lipoprotein cholesterol (HDL-C), and apolipoprotein A1 (ApoA1). The above results were similar in the cML-MA-BIC analysis (Figure 2), suggesting that these factors may partially mediate OSA-causing HBP.
Additionally, we found mediating effects of C-C motif chemokine ligand 13 (CCL13) and endothelial monocyte-activating polypeptide II (EMAP-II) between OSA and HBP with the IVW-RE method; however, in the cML-MA-BIC method, the negative association between OSA and EMAP-II only showed a strong trend of statistical significance (P = 0.0510), and the negative association between OSA and CCL13 approached statistical significance (P = 0.0668). Therefore, the mediating effects of EMAP-II and CCL13 are debatable and require further validation.
Direct Effect on T2DM
The MR analysis of OSA to T2DM retained 37 independent genetic variants with genome-wide significance levels after significance screening (P < 5×10−6), removal of linkage disequilibrium (r2 < 0.001, kb = 10,000), and removal of outliers by MR-PRESSO, which explained approximately 4.0% of the total variance with heterogeneity and no pleiotropy (Table 4). All F-statistics of the included SNPs were higher than 20, which indicates that these SNPs were sufficient to predict OSA. Genetically predicted OSA also increased the risk of T2DM prevalence: OR was 1.140 (95% CI, 1.059–1.228, P = 0.0005) in the IVW-RE analysis and 1.133 (95% CI, 1.062–1.209, P = 0.0002) in the cML-MA-BIC analysis. The causal association between genetically predicted OSA and T2DM was broadly consistent across the methods (Figure 3), and the results strongly suggested that OSA is a risk factor for T2DM.
 |
Table 4 SNPs Associated with Obstructive Sleep Apnea to Type 2 Diabetes Mellitus |
Effect of Mediators on T2DM
We also identified certain mediating factors that might play a role in this process (Supplementary Figure 2). Analysis of data from UK Biobank15 showed that there was a negative correlation between OSA and omega-6 fatty acids (β = −0.043, 95% CI: −0.063 to −0.013, P = 0.0015). In contrast, using the data provided by Kettunen,16 there was no significant difference between OSA and omega-6 fatty acids (β = 0.026, 95% CI: −0.049 to 0.131, P = 0.5753). This association was inconsistent across data from different sources; however, there was a suggestive association after the meta-analysis (β = −0.038, 95% CI: −0.063 to −0.012, P = 0.0038). Low omega-6 fatty acid levels increased the risk of T2DM (β = −0.081, 95% CI: −0.127 to −0.035, P = 0.0005). The Sobel test for the mediating effect of omega-6 fatty acids in OSA-T2DM yielded z = 2.18 and P = 0.0296, indicating that the mediating effect of omega-6 fatty acids remained statistically significant after the data were combined. Similarly, there were some mediating effects of SHBG (female), SHBG (male), TT (male), BioT (female), HDL-C, and ApoA1. The above results were analogous to those of the cML-MA-BIC method (Figure 3), suggesting that these factors influence the process of OSA-causing T2DM.
Discussion
MR is a method used to assess whether an exposure has a causal effect on the development of a disease in which genetic variation is considered an IV. MR methods can overcome unmeasurable confounding factors and allow stronger causal inferences to be drawn. This two-step, two-sample MR study not only clarifies the relationship of OSA with HBP and T2DM, but may also contribute to revealing the specific mechanisms through which OSA causes HBP and T2DM.
Compared with previous MR studies that relied on less recent and precise GWAS data,17 our study used the most recent GWAS data on OSA. These data are based on a larger sample size and provide more accurate insight into the causal association of OSA with HBP and T2DM and facilitate the assessment of potential mediating effects. OSA increases the risk of developing HBP and T2DM, and our results indicated the role of specific mediators.
OSA is recognized to be associated with endocrine dysfunction and hypothalamic-pituitary-gonadal axis disorders,2 and OSA is more prone to occur in males or postmenopausal females, substantiating the probable association with sex hormones.18 Several previous studies have shown that males with OSA have lower serum testosterone levels3,19 and decreased SHBG levels,20 whereas females with OSA have elevated21 or equivalent19 serum testosterone levels and lower SHBG levels.22 Likewise, our research observed a decrease in SHBG, TT, and BioT in males, and a decrease in SHBG with an increase in BioT in females with OSA, which underscore the complex interplay of these endocrine mediators. Though a non-significant decrease in TT levels was observed, the potential biological significance and implications of these changes warrant further investigation.
Previous views on the relationship of blood pressure to testosterone and SHBG are not settled. While some studies suggest a negative correlation between TT and blood pressure,23–26 others fail to establish a significant relationship.23 Studies conducted in males have concluded that free testosterone and blood pressure are negatively correlated;24 and SHBG is not sure.23–26 There are few, but converging, conclusions about the effects of testosterone in females, with positive correlations between TT and blood pressure,27 as well as BioT and blood pressure,27 and negative correlations between SHBG and blood pressure.27 This discrepancy might be attributed to the varying methodologies, population demographics, and inherent biological variations. But our MR study, leveraging genetic variations to infer causal relationships, suggests a negative association between HBP and TT and SHBG in males, and a potential role for TT, BioT and SHBG in HBP prevalence in females. However, we acknowledge that these relationships are complex and may be influenced by multiple factors beyond the scope of this study.
Similar to hypertension, the role of SHBG in T2DM is also under debate, with studies reporting both negative and no associations.13,28–31 However, the trend we observed suggests a protective role of SHBG against T2DM, emphasizing the need for further exploration of its potential as a therapeutic target. Furthermore, the differential testosterone levels in male and female OSA patients highlight the complexity of this endocrine dysregulation and its potential impact on metabolic diseases such as T2DM.13,28,30 We propose that testosterone plays a mediating role in the development of HBP and T2DM induced by OSA. More specifically, we suggest that TT serve as a mediator in males, whereas BioT serve as a mediator in females for the development of HBP and T2DM due to OSA. However, this mediating mechanism may be related to the fact that OSA affects testosterone production at night via the disruption of sleep,32 further mechanistic studies are required to confirm and elucidate this relationship.
Notably, OSA patients tend to have lower levels of HDL-C and ApoA1, markers commonly associated with high cardiovascular risk,3,21,33–38 but high levels of HDL-C and low levels of ApoA1 have been associated with an increased risk of T2DM.39,40 Our MR analysis revealed a consistent trend amongst patients with OSA, in which they exhibited reduced levels of HDL-C and ApoA1. However, this predisposition to low levels of HDL-C and ApoA1, as indicated by our MR analysis, could potentially heighten the risk of HBP and T2DM in OSA patients.
We also examined other potential mediators such as EMAP-II and CCL13, known for their pro-inflammatory activities.41 Our study found OSA to result in lower levels of these markers, hinting at the possible role of inflammation in the HBP or T2DM via OSA. Finally, we analyzed the potential role of Omega-6 fatty acids, given their importance in inflammation regulation. Our MR analysis suggested a negative association between Omega-6 fatty acid levels and T2DM, indicating that OSA might predispose patients to a pro-inflammatory state, contributing to the increased prevalence of T2DM.
In conclusion, we selected these mediators based on their known associations with endocrine and metabolic functions, inflammation, and cardiovascular risk, all of which are pertinent to the HBP or T2DM via OSA. While there are some potential biases and uncertainties in these associations necessitate a cautious interpretation of our findings, further studies are warranted to validate these findings and shed light on additional mediators and mechanisms.
OSA is known to be an established risk factor and etiology of secondary hypertension.42,43 While the causal relationship has been previously investigated, the underlying mechanisms and mediators that play a role in this association remain unclear. Therefore, the novelty of our study lies not in reiterating the established causal link, but in employing an innovative methodological approach - two-step, two-sample MR - to delve deeper into the complex interplay between OSA, hypertension, and T2DM. This approach allows us to provide new insights into the potential mediating factors, which have been underexplored in previous research.
Our use of the most recent GWAS data on OSA, which is based on a larger sample size, not only provides more accurate insight into the causal association of OSA with hypertension and T2DM, but also facilitates the assessment of potential mediating effects. Furthermore, our findings on the role of specific mediators like testosterone, lipid levels, SHBG, and inflammatory markers like EMAP-II and CCL13 highlight novel aspects of how OSA could contribute to the development of hypertension and T2DM. Understanding these mediating factors and the mechanisms they influence can provide a more comprehensive picture of the intricate relationship between OSA, hypertension, and T2DM. This understanding has the potential to influence therapeutic strategies and contribute to personalized medicine, thereby bringing novelty to this field of research. Hence, we believe our study contributes significantly to the existing body of knowledge and paves the way for future investigations into the underlying mechanisms linking OSA with hypertension and T2DM.
This study had certain limitations. First, we could not find a potential nonlinear association between OSA severity and both HBP and T2DM risk because this study was based on summary-level data. Second, the causes of HBP and T2DM are complex, and many known and unknown clinical factors may have influenced their occurrence and development. We only screened some of the causes for MR validation and drew conclusions from the available data; however, there is a need to discover and verify more mediating factors. Furthermore, the generalizability of this study was limited, considering that all data were obtained from subjects of European ancestry. More detailed and convincing conclusions could have been drawn if data from other populations were available on a larger scale. Future studies should expand our findings.
Conclusions
OSA is an independent risk factor for both HBP and T2DM. Therefore, clinical attention and prompt management should be given to complications of HBP and T2DM in patients with OSA. This study suggests that OSA can also cause and exacerbate HBP and T2DM through mediators, such as SHBG, TT, BioT, HDL-C, ApoA1, and omega-6 fatty acids. Our findings provide a foundation for further experimental and clinical investigation of the mechanisms and usefulness of these mediators.
Abbreviations
ApoA1, apolipoprotein A1; BIC, Bayesian information component; BioT, bioavailable testosterone; CCL13, C-C motif chemokine ligand 13; cML-MA-BIC, constrained maximum likelihood-model average-Bayesian information component; CI, confidence interval; DP, data perturbation; EAMP-2, endothelial monocyte-activating polypeptide 2; HBP, high blood pressure; HDL-C, high-density lipoprotein cholesterol; ICD, International Classification of Diseases; IVs, instrumental variables; IVW, inverse variance weighted; IVW-RE, inverse variance weighted random effects; MA, model average; MAF, minor allele frequency; MR, Mendelian randomization; OR, odds ratio; OSA, obstructive sleep apnea; SHBG, sex hormone-binding globulin; SNPs, single-nucleotide polymorphisms; T2DM, type-2 diabetes mellitus; TT, total testosterone.
Data Sharing Statement
FinnGen data are available through the FinnGen research project (https://www.finngen.fi/en), and GWAS data are publicly available through the MRC Integrative Epidemiology Unit Open GWAS database (https://gwas.mrcieu.ac.uk/). The code used in this study is available upon request from the corresponding author.
Ethics Approval
The ethics committee of Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine strictly follows the Declaration of Helsinki and International Ethical Guidelines for Health-related Research Involving Humans, etc., to perform independent ethical review duties. This study utilizes publicly available data obtained legally, which complies with the conditions for exemption from review as stated in the “Ethical Review Measures for Life Sciences and Medical Research Involving Humans”.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81873423]. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504. doi:10.5664/jcsm.6506
2. Zhang XB, Jiang XT, Du YP, Yuan YT, Chen B, Atkin SL. Efficacy of continuous positive airway pressure on testosterone in men with obstructive sleep apnea: a meta-analysis. PLoS One. 2014;9(12):e115033. doi:10.1371/journal.pone.0115033
3. Alvarenga TA, Fernandes GL, Bittencourt LR, Tufik S, Andersen ML. The effects of sleep deprivation and obstructive sleep apnea syndrome on male reproductive function: a multi-arm randomised trial. J Sleep Res. 2022;32(1):e13664. doi:10.1111/jsr.13664
4. Zhang Y, Elgart M, Kurniansyah N, et al. Genetic determinants of cardiometabolic and pulmonary phenotypes and obstructive sleep apnoea in HCHS/SOL. EBioMedicine. 2022;84:104288. doi:10.1016/j.ebiom.2022.104288
5. Zhang J, Chen Z, Parna K, van Zon SKR, Snieder H, Thio CHL. Mediators of the association between educational attainment and type 2 diabetes mellitus: a two-step multivariable Mendelian randomisation study. Diabetologia. 2022;65(8):1364–1374. doi:10.1007/s00125-022-05705-6
6. Skrivankova VW, Richmond, RC, Woolf, BAR et al . (2021). Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. n2233 :10.1136/bmj.n2233
7. (FIMM) MMF. Finngen research project is an expedition to the frontier of genomics and medicine. Finngen. Available from: https://www.finngen.fi/en.
8. (IEU) MIEU. IEU OpenGwas project. Available from: https://gwas.mrcieu.ac.uk/.
9. Xue H, Shen X, Pan W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. Am J Hum Genet. 2021;108(7):1251–1269. doi:10.1016/j.ajhg.2021.05.014
10. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi:10.1093/ije/dyv080
11. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30–42. doi:10.1097/EDE.0000000000000559
12. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi:10.1007/s10654-017-0255-x
13. Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–258. doi:10.1038/s41591-020-0751-5
14. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. doi:10.7554/eLife.34408
15. Richardson TG, Sanderson E, Palmer TM, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med. 2020;17(3):e1003062. doi:10.1371/journal.pmed.1003062
16. Kettunen J, Demirkan A, Wurtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7(1):11122. doi:10.1038/ncomms11122
17. Chen W, Cai X, Yan H, Pan Y. Causal effect of obstructive sleep apnea on atrial fibrillation: a Mendelian randomization study. J Am Heart Assoc. 2021;10(23):e022560. doi:10.1161/JAHA.121.022560
18. Kruger M, Obst A, Ittermann T, et al. Menopause is associated with obstructive sleep apnea in a population-based sample from Mecklenburg-Western Pomerania, Germany. J Clin Med. 2023;12(6):2101. doi:10.3390/jcm12062101
19. Wang H, Lu J, Xu L, et al. Obstructive sleep apnea and serum total testosterone: a system review and meta-analysis. Sleep Breath. 2022. doi:10.1007/s11325-022-02655-6
20. Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, Sullivan CE. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. 1989;68(2):352–358. doi:10.1210/jcem-68-2-352
21. Yang R, Gao C, Yan Y, et al. Analysis of the proportion and clinical characteristics of obstructive sleep apnea in women with polycystic ovary syndrome. Sleep Breath. 2022;26(1):497–503. doi:10.1007/s11325-021-02376-2
22. Karadeniz Y, Onat A, Akbas T, Simsek B, Yuksel H, Can G. Determinants of obstructive sleep apnea syndrome: pro-inflammatory state and dysfunction of high-density lipoprotein. Nutrition. 2017;43–44:54–60. doi:10.1016/j.nut.2017.06.021
23. Svartberg J, von Muhlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol. 2004;150(1):65–71. doi:10.1530/eje.0.1500065
24. Firtser S, Juonala M, Magnussen CG, et al. Relation of total and free testosterone and sex hormone-binding globulin with cardiovascular risk factors in men aged 24–45 years. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2012;222(1):257–262. doi:10.1016/j.atherosclerosis.2012.02.020
25. Moon H, Choi I, Kim S, et al. Cross-sectional association between testosterone, sex hormone-binding globulin and metabolic syndrome: the Healthy Twin Study. Clin Endocrinol. 2017;87(5):523–531. doi:10.1111/cen.13390
26. Yang Q, Li Z, Li W, et al. Association of total testosterone, free testosterone, bioavailable testosterone, sex hormone-binding globulin, and hypertension. Medicine. 2019;98(20):e15628. doi:10.1097/MD.0000000000015628
27. Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224(1):228–234. doi:10.1016/j.atherosclerosis.2012.07.005
28. Rasmussen JJ, Selmer C, Frossing S, et al. Endogenous testosterone levels are associated with risk of type 2 diabetes in women without established comorbidity. J Endocr Soc. 2020;4(6):bvaa050. doi:10.1210/jendso/bvaa050
29. Simons P, Valkenburg O, van de Waarenburg MPH, et al. Serum sex hormone-binding globulin is a mediator of the association between intrahepatic lipid content and type 2 diabetes: the Maastricht Study. Diabetologia. 2022;66(1):213–222. doi:10.1007/s00125-022-05790-7
30. O’Reilly MW, Glisic M, Kumarendran B, et al. Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin Endocrinol. 2019;90(1):145–154. doi:10.1111/cen.13862
31. Joyce KE, Biggs ML, Djousse L, et al. Testosterone, dihydrotestosterone, sex hormone-binding globulin, and incident diabetes among older men: the cardiovascular health study. J Clin Endocrinol Metab. 2017;102(1):33–39. doi:10.1210/jc.2016-2623
32. Kim SD, Cho KS. Obstructive sleep apnea and testosterone deficiency. World J Mens Health. 2019;37(1):12–18. doi:10.5534/wjmh.180017
33. Adedayo AM, Olafiranye O, Smith D, et al. Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep Breath. 2014;18(1):13–18. doi:10.1007/s11325-012-0760-9
34. Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–116. doi:10.1038/nri3793
35. Li X, Fu Z, Xu H, et al. Influence of multiple apolipoprotein A-I and B genetic variations on insulin resistance and metabolic syndrome in obstructive sleep apnea. Nutr Metab. 2020;17(1):83. doi:10.1186/s12986-020-00501-8
36. Fadaei R, Mohassel Azadi S, Rheaume E, Khazaie H. High-density lipoprotein cholesterol efflux capacity in patients with obstructive sleep apnea and its relation with disease severity. Lipids Health Dis. 2022;21(1):116. doi:10.1186/s12944-022-01723-w
37. Yang G, Qian T, Sun H, et al. Adjustment for body mass index changes inverse associations of HDL-cholesterol with blood pressure and hypertension to positive associations. J Hum Hypertens. 2022;36(6):570–579. doi:10.1038/s41371-021-00548-x
38. Dong H, Zhang Y, Hu P, Wang J, Lu N. Serum apolipoprotein A1 rather than apolipoprotein B is associated with hypertension prevalence in Chinese people with coronary artery disease. Blood Press Monit. 2022;27(2):121–127. doi:10.1097/MBP.0000000000000576
39. Hwang YC, Ahn HY, Kim WJ, Park CY, Park SW. Increased apoB/A-I ratio independently associated with Type 2 diabetes mellitus: cross-sectional study in a Korean population. Diabet Med. 2012;29(9):1165–1170. doi:10.1111/j.1464-5491.2012.03622.x
40. Wu X, Yu Z, Su W, et al. Low levels of ApoA1 improve risk prediction of type 2 diabetes mellitus. J Clin Lipidol. 2017;11(2):362–368. doi:10.1016/j.jacl.2017.01.009
41. Schluesener HJ, Seid K, Zhao Y, Meyermann R. Localization of endothelial-monocyte-activating polypeptide II (EMAP II), a novel proinflammatory cytokine, to lesions of experimental autoimmune encephalomyelitis, neuritis and uveitis: expression by monocytes and activated microglial cells. Glia. 1997;20(4):365–372. doi:10.1002/(sici)1098-1136(199708)20:4<365::aid-glia8>3.0.co;2-4
42. Han B, Chen WZ, Li YC, Chen J, Zeng ZQ. Sleep and hypertension. Sleep Breath. 2020;24(1):351–356. doi:10.1007/s11325-019-01907-2
43. Yuan FJ, Zhang SS, Liu X, Liu YL. Correlation between obstructive sleep apnea hypopnea syndrome and hypertension: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(12):12251–12261. doi:10.21037/apm-21-3302
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





