Back to Journals » Pathology and Laboratory Medicine International » Volume 8
Higher frequency of isolated PMS2 loss in colorectal tumors in Colombian population: preliminary results
Authors Shamekh R, Cives M, Mejia J, Coppola D
Received 19 August 2015
Accepted for publication 7 April 2016
Published 2 August 2016 Volume 2016:8 Pages 37—41
DOI https://doi.org/10.2147/PLMI.S94771
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Paul Zhang
Rania Shamekh,1 Mauro Cives,2 Jaime Mejia,3 Domenico Coppola,4
1Department of Pathology, University of South Florida, 2Department of Gastrointestinal Oncology, Moffitt Cancer Center, Tampa, FL, USA; 3Department of Pathology, Institutode Patologia Mejia Jimenez, Cali, Colombia; 4Department of Anatomic Pathology, Moffitt Cancer Center, Tampa, FL, USA
Abstract: Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of death worldwide. It accounts for >9% of all cancers. One of the pathogenic factors of CRC is germline mutation, leading to alteration and inactivation in the mismatch repair (MMR) genes. The aim of the study is to compare the frequency of alterations in MMR protein expression in Caucasian CRC patients with Colombian CRC patients.
A total of 45 Colombians and 48 Caucasians with CRC were studied. The microsatellite instability status of tumors was determined in primary CRC by immunohistochemistry using the automated Ventana Ultra. The combined loss of MLH1 and PMS2 was the most common alteration in both Colombian (11%, five out of 45) and Caucasian (12%, six out of 48) CRC patients. Interestingly, the loss of PMS2 expression in the presence of intact MLH1 was the second most common alteration in Colombians (8%, four out of 45), which was never seen in the Caucasian cohort (P=0.05). The loss of MLH1 alone and the combined loss of MSH6 and PMS2 expression were only observed in one out of 45 (2%) Colombians but not in Caucasians. The combined loss of MSH2 and MSH6 was not observed in any of the patients studied. The preliminary findings support a significant difference in alterations of MMR protein expression in Colombian CRC patients compared with Caucasian CRC patients. These findings are novel and warrant further studies in larger cohorts.
Keywords: colon cancer, MSI, MMR, immunohistochemistry
Introduction
Colorectal cancer (CRC) is a leading cause of cancer death in both males and females in the US.1–3 The incidence and mortality rates of CRC vary among major racial/ethnic groups. The African Americans have the highest incidence of CRC (males: 63.8/100,000; females: 47.6/100,000) and highest mortality (males: 29.4/100,000; females: 19.4/100,000).4 The non-Hispanic Whites have the lowest incidence of CRC (males: 50.9/100,000 and females 38.6/100,000) and lowest mortality (males: 19.2/100,000; females: 13.6/100,000).4 Compared to the African Americans and the Non-Hispanic Whites, the Hispanic/Latino population tends to have the lowest incidence of CRC (males: 47.3/100,000; females: 32.6/100,000) and lowest mortality (males: 16.1/100,000; females: 10.2/100,000).4
A subset of the CRC is due to the alteration in the mismatch repair (MMR) genes. These tumors are characterized by molecular alteration in the “serrated pathway”, including mutation of the proto-oncogene BRAF in sporadic microsatellite instability (MSI) CRC. Conversely, familial MSI CRC is associated with KRAS mutation.5
The MMR system is responsible for correcting mismatches generated during DNA replication.6 Mutations in one of the MMR genes (MLH1, MSH2, MSH6, and PMS1) are referred to as MSI, which in turn results in failure to repair errors that occur during DNA replication.5 MSH3 and PMS1 genes are not found to be mutated in CRC with MSI.
MSI is the hallmark of hereditary nonpolyposis colorectal cancer, a condition responsible for 10%–15% of sporadic colon, gastric, and endometrial cancers. In sporadic primary CRC with MSI, hypermethylation of the 5′ CpG of the MLH1 gene was the most observed genetic alteration, which was often associated with the loss of hMLH1 protein expression.7
Comparison between populations with different ethnical or cultural background is a common strategy in cancer research to detect epidemiologic variables that can be traced back to molecular basics.
Studies have suggested a low prevalence of sporadic MSI CRC among Hispanics (individuals whose primary language is Spanish or whose family is from Mexico, Central America, South America, or the Caribbean where the primary language is Spanish) and that the molecular aberration associated with MSI CRC was most likely due to Lynch syndrome.8
The aim of this study is to compare the frequency of alterations in MMR protein expression in primary, sporadic CRC in Caucasian patients with that of Colombian patients.
Material and methods
Forty-five Colombian CRC patients (21 females:24 males; age: 27–91 years; median: 63 years) who visited Instituto de Patologia Mejia Jimenez (Cali, Colombia) and 48 Caucasian CRC patients (21 females:27 males; age: 35–92 years; median: 65 years) who visited Moffitt Cancer Center (Tampa, FL, USA) were studied retrospectively. Individuals were not prescreened by laboratory methods to identify hereditary CRC, but the selection process was based on the evaluation of familial clinical history. Individuals with hereditary CRC were not included in this study. The tumor tissues were formalin fixed, paraffin embedded, cut into slices of 4 μm thickness, and stained with hematoxylin and eosin for evaluation. The hematoxylin and eosin-stained tumor tissues were examined by gastrointestinal pathologists (JM and DC) at both institutions to assess adequacy and confirm the diagnosis. The tumor tissues were further examined for the MMR protein expression MLH1 (Clone ES05; Dako Denmark A/S, Glostrup, Denmark), MSH2 (Clone FE11; Dako Denmark A/S), MSH6 (Clone EP49; Dako Denmark A/S), and PMS2 (Clone EP51; Dako Denmark A/S) by immunohistochemistry using the automated Ventana Ultra. The staining was evaluated using the Allred score system.9
This study was approved by the Moffitt Cancer Center Institutional Review Board. All patients provided written informed consent to participate in this study.
Statistical analysis
A paired t-test was used to evaluate the difference in the expressions of MLH1, MSH2, MSH6, and PMS2 in Colombians and Caucasians. A P-value of <0.05 was considered to be statistically significant.
Results
This study showed higher frequency of MMR alterations in Colombian CRC patients (24%, eleven out of 45) compared with Caucasian CRC patients (12%, six out of 48) (Table 1). Age, sex, cancer location, tumor grade, and tumor stage in MSI colon cancer in Colombians and Caucasians are shown in Tables 2 and 3, respectively.
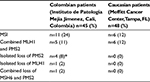  | Table 1 MMR Alterations in Colombian patients compared to Caucasians Note: *P=0.05. Abbreviation: MSI, microsatellite instability. |
The combined loss of MLH1 and PMS2 was the most common alteration in both Colombian (11%, five out of 45) and Caucasian (12%, six out of 48) CRC patients (Figure 1). Interestingly, the loss of PMS2 expression in the presence of intact MLH1 was the second most common alteration in Colombians (8%, four out of 45; Figure 2), which was never seen in the Caucasian cohort (P=0.05). The clinical data for the four cases with isolated PMS2 loss are reviewed (Table 2). The loss of MLH1 alone and the combined loss of MSH6 and PMS2 expression were only observed in one out of 45 (2%) Colombians but not in Caucasians. The combined loss of MSH2 and MSH6 was not observed in any of the patients studied. These findings in this small cohort highlight the significant difference in alterations in the expression of MMR protein in CRC patients from Colombia compared with Caucasians.
Discussion
In this study, we compared the frequency of MSI in CRC patients from Colombian and Caucasian CRC patients. The Colombian CRC patients showed a higher frequency of MMR alterations (24%) when compared with Caucasian CRC patients (12%). The combined loss of MLH1 and PMS2 was the most common alteration in both Colombian and Caucasian CRC patients. We found the frequency of such alteration to be almost identical in Colombian (11%) and Caucasian (12%) CRC patients. Interestingly, we observed the isolated loss of PMS2 protein expression with normal MLH1, MSH2, and MSH6 expression only in Colombian CRC patients and not in the Caucasian cohort.
The function of MMR genes is to correct mismatches during DNA replication, thus maintaining genetic stability. Many tumors such as gastric cancer, ovarian cancer, bladder cancer, endometrial cancer, and CRC harbor mutation in the MMR, resulting in what is called MSI tumor.10–15
MSI CRCs were first described by Thibodeau et al.16 They showed that MSI colon cancers usually occur in the proximal colon with dense lymphocytic infiltrate. They are poorly differentiated with mucinous or signet ring appearance. MSI colon cancer tends to have increased survival and better response to chemotherapy when compared with microsatellite stable colon cancer.16–18 Thus, the microsatellite status of colon cancer is essential for proper clinical management.
As a result, the National Cancer Institute proposed international guidelines for mutation analyses of the MMR to determine the appropriate clinical management of MSI tumors.17,18 The level of MSI was determined as 1) MSI-high (MSI-H, >30% of tested loci are unstable), 2) MSI-low (MSI-L, <30% of tested loci are unstable), and 3) microsatellite stability (unstable loci are absent).
MMR genes include MLH1, MSH2, MSH6, and PMS2. Studies have shown that the majority of colorectal tumors with MSI have combined loss of MLH1 and PMS2 protein expression.17,18 These findings were similar to the findings of this study.
Similarly, Gill et al19 showed that the majority of MSI-H colon cancers (499 of 535, 93%) had loss of expression for at least one of the proteins for MLH1, MSH2, and/or MSH6. However, the selective loss of PMS2 (with normal expression of MLH1, MSH2, and/or MSH6) was detected in 4.3% (23 of 529) of all MSI-H colon cancer.19
According to Gill et al19, PMS2 negative cancer occurs mostly in males (64%) aged 28–67 years and is more common in the right colon (52%). In our study, PMS2 negative colon cancers were more common in males (75%) aged 29–83 years as well. However, PMS2 negative colon cancers were diagnosed in the left colon (50%), transverse colon (25%), and the right colon (25%).
Another study by Nakagawa et al was conducted on 103 patients with MSI CRC. The combined loss of MLH1 and PMS2 was seen in 19% of the MSI CRC (20 of 103). The isolated PMS2 loss, with intact MLH1, was seen in 6% of the MSI CRC (seven out of 103). The isolated PMS2 loss in these seven patients was due to either frameshift mutation or deletion of the PMS2 gene. Other types of germline mutation or somatic mutation could not be ruled out.20
In another study, MMR loss was detected in 13.2% (139 out of 1,048 patients) of unselected CRC cases.21 The combined loss of MLH1 and PMS2 was the most frequent mutation and was seen in 10% (109 out of 1,048) of all CRC cases. Isolated PMS2 loss was detected in 1.5% of all CRC cases.21 They reported that these patients with isolated PMS2 loss were in the fifth and sixth decades of life and showed several extracolonic PMS2-deficient tumors. Their data excluded the methylation of the PMS2 promoter, which is frequently associated with sporadic CRC and rather suggested germline mutation of the PMS2.21
Conclusion
Our preliminary findings on PMS2 in Colombian CRC patients suggest PMS2 inactivation in the development of MSI-H CRCs in Colombia. PMS2-deficient CRCs may be associated with germline alterations. Molecular evaluation is warranted to detect specific of somatic and/or germline mutation of the PMS2 gene in the presence of wild-type MLH1.
Disclosure
The authors report no conflicts of interest in this work.
References
American Society of Clinical Oncology. The state of cancer care in America, 2015: a report by the American Society of Clinical Oncology. J Oncol Pract. 2015;11(2):79–113. | ||
Masters GA, Krilov L, Bailey HH, et al. Clinical cancer advances 2015: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2015;33(7):786–809. | ||
Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. | ||
The American Cancer Society. Colorectal Cancer Facts & Figures 2014-2016. Atlanta, GA: The American Cancer Society; 2014. | ||
Pavicic W, Perkiö E, Kaur S, Peltomäki P. Altered methylation at microRNA-associated CpG islands in hereditary and sporadic carcinomas: a methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA)-based approach. Mol Med. 2011;17(7–8):726–735. | ||
Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18(1):85–98. | ||
Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95(12):6870–6875. | ||
Gupta S, Ashfaq R, Kapur P, et al. Microsatellite instability among individuals of Hispanic origin with colorectal cancer. Cancer. 2010;116(21):4965–4972. | ||
Allred DC, Clark GM, Elledge R, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85(3):200–206. | ||
Diaz-Padilla I, Romero N, Amir E, et al. Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2013;88(1):154–167. | ||
Mongiat-Artus P, Miquel C, Van der Aa M, et al. Microsatellite instability and mutation analysis of candidate genes in urothelial cell carcinomas of upper urinary tract. Oncogene. 2006;25(14):2113–2118. | ||
V S, Bhagat R, C S P, V R P, Krishnamoorthy L. Microsatellite instability, promoter methylation and protein expression of the DNA mismatch repair genes in epithelial ovarian cancer. Genomics. 2014;104(4):257–263. | ||
Abe Y, Masuda H. Genetic alterations of sporadic colorectal cancer with microsatellite instability, especially characteristics of primary multiple colorectal cancers. J Surg Oncol. 2000;74(4):249–256. | ||
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–618. | ||
Lee HJ, Jang YJ, Lee EJ, et al. The significance of mismatch repair genes in gastric cancer. J Cancer Res Ther. 2013;9(1):80–83. | ||
Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. | ||
Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–2087.e2073. | ||
Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. | ||
Gill S, Lindor NM, Burgart LJ, et al. Isolated loss of PMS2 expression in colorectal cancers: frequency, patient age, and familial aggregation. Clin Cancer Res. 2005;11(18):6466–6471. | ||
Nakagawa H, Lockman JC, Frankel WL, et al. Mismatch repair gene PMS2: disease-causing germline mutations are frequent in patients whose tumors stain negative for PMS2 protein, but paralogous genes obscure mutation detection and interpretation. Cancer Res. 2004;64(14):4721–4727. | ||
Truninger K, Menigatti M, Luz J, et al. Immunohistochemical analysis reveals high frequency of PMS2 defects in colorectal cancer. Gastroenterology. 2005;128(5):1160–1171. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




