Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
High Platelet Count is a Potential Prognostic Factor of the Early Recurrence of Hepatocellular Carcinoma in the Presence of Circulating Tumor Cells
Authors Lu Z , Huang Y , Huang J, Ni HH, Luo T , Wei X, Bai X, Qi L , Xiang B
Received 22 November 2022
Accepted for publication 4 January 2023
Published 14 January 2023 Volume 2023:10 Pages 57—68
DOI https://doi.org/10.2147/JHC.S398591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mohamed Shaker
Zhan Lu,1,2,* Yiyue Huang,1,2,* Juntao Huang,1,2,* Hang-Hang Ni,1,2 Tai Luo,1 Xingyu Wei,1 Xue Bai,1 Lunnan Qi,1– 3 Bangde Xiang1– 3
1Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, People’s Republic of China; 2Key Laboratory of Early Prevention and Treatment for Regional High-Frequency Tumors, Ministry of Education, Nanning, People’s Republic of China; 3Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bangde Xiang; Lunnan Qi, Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, 71# Hedi Road, Qingxiu District, Nanning, Guangxi, 530021, People’s Republic of China, Tel +86-7715301253 ; +86-135-1788-6990, Email [email protected]; [email protected]; [email protected]
Purpose: Recent studies indicated the vital role of platelet in enhancing the survival of circulating tumor cells (CTCs) in the blood, thereby stimulating the metastasis of tumors. CTCs have been considered an indicator of early tumor recurrence. Therefore, this study evaluated the prognostic potential of platelet count in predicting the early recurrence of hepatocellular carcinoma (HCC) in the presence of CTCs.
Patients and Methods: 127 patients, whose preoperative CTCs were detected, were enrolled in this study. Univariate analysis was performed to identify the significant association of factors with the early recurrence of HCC, followed by multivariate analysis to determine the independent prognostic indicators. The prediction potential was evaluated using receiver operating characteristic (ROC) curves.
Results: A total of 81 (63.7%) patients showed early HCC recurrence. The platelet count ≥ 225× 109/L (hazard ratio, HR: 1.679, P = 0.041), CTCs > 5/5 mL (HR: 2.467, P = 0.001), and presence of microvascular invasion (MVI) (HR: 2.580, P = 0.002) were independent factors correlated with the early recurrence of HCC in multivariate analysis. The prognostic potential of the combined CTCs-platelet count (0.738) was better than that of CTCs (0.703) and platelet (0.604) alone. The subgroup analysis, excluding 23 patients with pathological cirrhosis and splenomegaly, showed that the platelet count ≥ 225× 109/L and CTCs > 5/5 mL were also independent factors of early HCC recurrence. The prediction potential of the combined CTCs-platelet count was 0.753, which was better than that of the whole cohort. Kaplan–Meier survival curve analysis indicated that the HCC patients with high platelet or CTCs had the worse recurrence-free survival (RFS).
Conclusion: The high platelet count was an independent factor of early HCC recurrence in the presence of CTCs. The combination of preoperative CTCs and platelet count could effectively predict the early recurrence of HCC. The subgroup analysis also showed similar results.
Keywords: hepatocellular carcinoma, liver resection, early recurrence, platelet count, circulating tumor cells
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and a leading cause of cancer-related mortality worldwide.1,2 It has a poor overall prognosis after diagnosis with a median survival time of fewer than two years. Patients with early-stage HCC can undergo surgery or liver transplantation to obtain a radical cure; however, the 5-year recurrence rate is still high up to 50–75%.3 Its prognosis is limited due to the early tumor recurrence, which might be correlated with micro-metastases formed by the circulating tumor cells (CTCs).4,5 Tumor invasion and metastasis are the key reasons for over 90% of cancer-related mortality.6,7
Predicting the early recurrence of HCC is critical for the patient’s survival after radical treatment. The CTCs, which escape the primary tumor site and enter the blood circulation, are an effective marker for the metastasis and recurrence of HCC.8–10 The CTCs analysis can be considered a non-invasive biomarker and real-time “liquid biopsy” for cancer patients, which can be performed before surgery.11,12 The CTCs were detected in advanced tumors as well as in the early and intermediate stage HCC.13,14 The prognostic value of CTCs has been demonstrated before. The preoperative detection of CTCs can indicate tumor micro-metastases, which might lead to early recurrence.4,5,15
The high shear stress exerted by blood flow or the body’s immune response can destroy the CTCs present in the blood.16–18 However, the platelets can adhere to CTCs, thereby reducing their destruction by blood flow and altering the immunogenicity.16,18 The immune cells cannot recognize CTCs, which leads to an increase in their survival rate.17,19,20 Furthermore, the platelets stimulate the shedding and metastasis of cancer cells by releasing a large number of cytokines and interact with the tumor microenvironment by enhancing inflammatory response and forming new blood vessels.21,22 Therefore, the protective effects of platelets on the survival of CTCs should be considered even in the presence of a small number of CTCs. The high platelet count might be a prognostic marker for cancers in the presence of CTCs; it has been demonstrated in some cancer types, such as prostate cancer and colorectal cancer.23,24 Consequently, in order to better evaluate the early recurrence risk of HCC, the interaction between platelets and CTCs should be investigated. However, the related studies are limited.
The current study aimed to explore the importance of preoperative platelet count in combination with CTCs in order to assess the early recurrence in HCC patients who underwent liver resection (LR).
Materials and Methods
Patients
A total of 226 HCC patients, who underwent LR at the Affiliated Cancer Hospital of Guangxi Medical University, Nanning, China, between November 2013 and September 2019, were recruited. The criteria for including the patients in this study were as follow: (1) the patients with Barcelona Clinic Liver Cancer (BCLC) 0-B stage; (2) the patients who achieved R0 resection (complete elimination of the tumor according to the naked eye observation, negative resection margins, and no intra- or extra-hepatic metastatic lesions); (3) the patients whose blood CTCs counts were identified; (4) the patients who had no prior history of antitumor therapy; (5) the patients who had no hematological system diseases and other malignant tumors; (6) the patients diagnosed with HCC using postoperative pathology; (7) the patients with performance status test (PST) score of 0–1 and Child-Pugh A or B stage.
Among the 226 HCC patients, a total of 127 patients, who met the inclusion criteria, were included in the final analysis. The endpoint of the follow-up was August 10, 2022. The study was performed in accordance with the Declaration of Helsinki guidelines and approved by the Ethics Committee of Cancer Hospital of Guangxi Medical University, Nanning, China. All the selected patients provided written informed consent.
Blood Element Counts and Serological Markers
The blood samples were collected and assayed within one week before surgery. The following laboratory data were collected from the blood samples after their respective tests: platelet count, hepatitis B surface antigen (HBsAg) levels, serum α-fetoprotein (AFP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total bilirubin levels, and prothrombin time (PT). Histological cirrhosis was defined as an Ishak fibrosis score of 5 to 6,25 and the tumor grading was based on Edmondson-Steiner (E-S) grade.26 The presence of splenomegaly was defined as the spleen length, exceeding 10 cm or pedicle rib unit >5 in preoperative computed tomography (CT).27,28
Collection of Blood Samples and Isolation of CTCs
The peripheral blood samples (5mL, mixed with ethylenediaminetetraacetic (EDTA) as an anticoagulant) were collected and assayed within one or two days before surgery. The CTCs were isolated using the CanPatrolTM system. Before filtration, erythrocytes were lysed by adding red blood cell lysis buffer. The cells were resuspended in phosphate-buffered saline (PBS) with 4% formaldehyde (all the reagents were purchased from Sigma, St. Louis, MO, USA) and then transferred to the specialized filtration system. The samples were filtered through a system to capture tumor cells. The filtration system contained a membrane with 8-μm diameter pores (Sur Exam, Guangzhou, China), a manifold vacuum plate with valve settings (SurExam, Guangzhou, China), an E-Z96 vacuum manifold (Omega, Norcross, GA, USA), and a vacuum pump (Auto Science, Tianjin, China). The vacuum pumping pressure was 0.08 MPa.
The expression levels of target genes in CTCs were assessed using the RNA-ISH (in-situ hybridization) method. The target marker included leukocyte biomarker (CD45), epithelial biomarkers (Epithelial cell adhesion molecule, EpCAM; Cytokeratin 8/18/19, CK8/18/19), and mesenchymal biomarkers (vimentin and Twist).
Follow-Up and Endpoint
The patients were followed up once every 1–2 months for the first year after surgery and then every 3 months. In each follow-up, all patients underwent AFP level tests, liver biochemistry tests, and at least one image examination, such as abdominal ultrasound or contrast-enhanced CT/magnetic resonance imaging (MRI). The diagnosis of HCC recurrence was based on comprehensive evidence from medical history, AFP assays, and imaging characteristics. The postoperative adjuvant therapy included adjuvant trans-arterial chemoembolization (A-TACE), incisal margin radiotherapy, adjuvant targeted therapy, and immunotherapy.
The endpoint of this study was an early recurrence, which was defined as recurrence within 2 years after LR.
Statistical Analyses
All statistical analyses were performed using SPSS statistical software version 25.0 (IBM, Chicago, IL, USA) and GraphPad Prism software version 8.0 (San Diego, CA, USA). The characteristics of the patient were analyzed using descriptive statistics. The data were expressed as numbers (%). The correlation between platelets and CTCs was assessed using Spearman correlation coefficient. Receiver operating characteristic (ROC) curve analysis with maximal Youden index values was used to identify the best cut-off values of preoperative platelets and CTCs for the early recurrence of HCC using MedCalc statistical software version 20.1.0. Then, the area under the ROC curve (AUC) was compared. The larger the AUC value, the higher the accuracy of the prediction. Kaplan–Meier curve analysis with a Log rank test was used for the analysis of patient survival. The analysis of prognostic factors to detect the early recurrence of HCC was performed using the Cox proportional hazards model. A P-value of <0.05 was considered statistically significant.
Results
Clinical Characteristics of Patients
A total of 127 patients, who met the screening criteria, were included in the final analysis. None of the patients died within 1 month after surgery. A total of 81 (63.8%) patients experienced early postoperative recurrence, and 59 (37.8%) patients suffered from histological cirrhosis. Among the 59 patients, 23 HCC patients had splenomegaly. In order to eliminate the negative effects of hypersplenism on the platelet counts, a subgroup analysis was performed after excluding the patients with cirrhosis and splenomegaly.
The baseline characteristics of the patients are listed in Table 1. The age range of the included patients was 20–76 years, including 108 (85.0%) males. A total of 80.6% patients suffered from hepatitis B viral (HBV) infection, among which, 89 (70.1%) patients had HBV-DNA levels ≥5.0×102. Based on the Child-Pugh class, 95.3% of the patients were classified as grade A, while 4.7% of the patients were classified as grade B. Moreover, 37 (29.1%) patients presented multiple tumors, 100 (78.7%) patients had complete tumor capsules, 79 (62.2%) patients presented microvascular invasion (MVI), 27 (21.3%) patients showed satellite nodules, and 55 (43.3%) patients underwent postoperative adjuvant therapy. All the patients were followed up for more than 2 years.
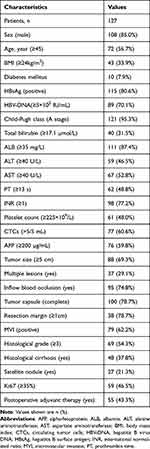 |
Table 1 Clinical Characteristics of the Hepatocellular Carcinoma Patients in the Study |
The platelet count showed a weak positive correlation with CTCs in the whole cohort (r = 0.230, P <0.01, Figure 1A) as well as in the subgroup analysis after excluding the patients with cirrhosis and splenomegaly (r = 0.266, P <0.01, Figure 1B).
Cut-off Point for Platelet and CTC Counts
The optimal cutoff values of platelet and CTC counts to predict the early recurrence of HCC were ≥225×109/L and >5/5 mL, respectively. Kaplan–Meier survival curve analysis showed that the patients with high platelet counts (≥225×109/L) or CTCs (>5/5 mL) before surgery were more likely to have shorter recurrence-free survival (RFS) as compared to those with the lower ones (Figure 2), and these differences were more significant in the subgroup analyses (Figure 3).
 |
Figure 2 Kaplan–Meier survival curves of early recurrence-free survival (RFS) after liver resection in the patients stratified by preoperative (A) circulating tumor cells (CTCs) and (B) Platelet. |
 |
Figure 3 Kaplan–Meier survival curves of early RFS for the subgroup patients stratified by preoperative (A) CTCs and (B) Platelet. |
Factors Associated with Early Recurrences
The univariate analysis indicated that body mass index (BMI), PT, platelet count, CTCs, AFP, tumor size, multiple lesions, tumor capsule, MVI, satellite nodule, and Ki67 were associated with the early recurrence of HCC. These factors significantly associated with a higher early recurrence risk of HCC were introduced into the multivariate analysis. The independent risk factors of RFS included platelet count ≥225×109/L (hazard ratio, HR = 1.679, P = 0.041), CTCs >5/5 mL (HR = 2.467, P = 0.001), and presence of MVI (HR = 2.580, P = 0.002) (Table 2).
 |
Table 2 Univariate and Multivariate Analyses of Prognostic Factors Associated with the Early Recurrence of HCC |
In the subgroup multivariate analysis, the platelet count (HR = 1.976, P = 0.024), CTCs >5/5 mL (HR = 2.751, P = 0.001), presence of MVI (HR = 2.074, P = 0.027), and presence of satellite nodule (HR = 1.972, P = 0.025) were the independent factors associated with the early recurrence of HCC (Table 3).
 |
Table 3 Subgroup Univariate and Multivariate Analyses of the Prognostic Factors Associated with the Early Recurrence of HCC |
Predictive Performance for Early Recurrences
In the whole cohort, the AUC values of CTCs and platelet count in assessing the early recurrence risk of HCC were 0.703 (Figure 4A) and 0.604 (Figure 4B), respectively. Once the CTCs were combined with the platelet count, the CTCs-platelet count (AUC 0.738) showed stronger prediction performance as compared to the CTCs or platelet count alone (P = 0.001, Figure 4C). The AUC value of CTCs-platelet count in the sub-group analysis (AUC 0.753) showed superior predictive accuracy for the early recurrence risk of HCC and improved the prediction accuracy as compared to the whole cohort (Figure 5).
 |
Figure 4 ROC curves for preoperative (A) CTCs, (B) Platelet, and (C) CTCs-platelet to predict the early recurrence of HCC. |
 |
Figure 5 ROC curves of the subgroup patients for the preoperative (A) CTCs, (B) Platelet, and (C) CTCs-platelet to predict the early recurrence of HCC. |
Discussion
Although LR is considered a curative treatment option for early- and mid-stage HCC, it is still unsatisfactory due to the high recurrence rate of HCC after surgery.2,3 Recent studies have more focused on the characteristics of primary tumor-related factors, including MVI, multiple nodules, tumor size, etc., to evaluate the high recurrence-associated risk factors.29,30 In addition to being a major blood component, numerous studies showed that platelets have numerous interactions with HCC and the tumor microenvironment, which not only promote the growth of HCC but also cause the shedding and metastasis of HCC cells.7,9,10,20 The anti-platelet therapy can inhibit related tumor pathways, attenuate hepatic inflammatory response, delay tumor progression, and improve the prognosis of HCC patients.31,32 Moreover, platelets are the targets of signaling molecules for the treatment of HCC.33,34 Platelets are routinely measured before surgery. As an inexpensive and easily measurable indicator, platelet count has important clinical significance in predicting the prognosis of HCC patients. Increasing studies have demonstrated that platelet counts are associated with the HCC prognosis.17–19,23,35
Platelets can serve as a prognostic marker; however, their activation can also affect the outcomes of predicting HCC prognosis. Therefore, Establishing a stable situation and making platelets a credible cancer biomarker might be a better option.34,36 CTCs had been confirmed associating with HCC recurrence; however, in order to improve the accuracy of using CTCs as a prognostic biomarker, the clinical characteristics must be evaluated together. Although there was a weak positive correlation between the preoperative CTCs and platelets, the interference of other clinical factors, which affect the platelet counts, such as liver cirrhosis and thrombosis, should be considered. The accurate identification and detection of CTCs are also important limitations.4,5 Previous studies have reported the interactions between CTCs and platelets.16–23 Platelets can promote the formation of CTCs, and their interaction with CTCs can increase the survival and metastasis possibility of tumor cells.16,17,19–22 Previous studies detected the platelet-derived RNA in a variety of cancer cells, including HCC (even in its early stages).16,36 Therefore, combining the platelet and CTC counts to evaluate the postoperative recurrence might increase the feasibility and reduce the limitations. CTCs can also be easily detected in preoperative blood samples. The effective preoperative estimation of recurrence assessment might play a key role in predicting the prognosis of HCC. Evaluating the platelet and CTC counts to predict the prognosis of HCC patients might have important clinical significance.
In this study, the early recurrence of HCC was associated with BMI, PT, platelet count, CTCs, AFP, tumor size, multiple lesions, tumor capsule, MVI, satellite nodule, and Ki67. The higher cutoff values of platelet count (≥225×109/L) and CTC count (>5/5mL) were indicated as independent risk factors for postoperative early recurrence in HCC patients. In this study, the higher platelet count and CTCs indicated a poor prognosis. The early and intermediate stages HCC patients also showed higher platelet and CTC counts; meanwhile, this indicated that there might be interactions between CTCs and platelets.10,16 Furthermore, the results also indicated that the patients with a high platelet count (≥225×109/L) had a significantly lower early RFS as compared to those with a low platelet count, suggesting an interaction between the platelet and HCC recurrence. These findings were consistent with those of previous studies. Wang et al36 conducted a prospective study to evaluate the prediction potential of platelet activation status in HCC patients and showed that the activated platelets were an independent risk factor for the poor prognosis of HCC. A retrospective study by Bihari et al37 concluded that platelets could significantly contribute to the development, growth, invasion, and metastasis of HCC as compared to cirrhosis. In terms of cut-off values, there is no consensus in previous studies. The current study calculated the cut-off value according to the cohort under study and consider it a “high platelet”. Lou et al19 proposed that due to the adherence of platelets to CTCs, the immune cells cannot recognize them, thereby increasing their survival rate. However, contrary to the conclusions in the current study, some studies suggested that lower platelets were associated with poor prognosis.38,39 These studies might have included patients with a more severe hepatic background. Although cirrhosis was not a risk factor in the current study, liver cirrhosis and splenomegaly might affect the prognosis potential of platelet count. Currently, severe cirrhosis is the main reason, affecting the survival and prognosis of patients. This might have affected the results in the current study;40 therefore, a subgroup analysis was performed. The results showed that the platelet and CTC counts could predict the early recurrence in HCC patients after excluding those with liver cirrhosis and splenomegaly.
ROC curve was used to further analyze the clinical potential of platelet and CTC counts alone and in combination to predict the early recurrence of HCC patients after LR. The ROC curve analyses revealed that the AUC value of the combined CTCs-platelet counts was significantly greater than those of the CTC and platelet counts alone, respectively. The results suggested that the combined detection of these indicators could significantly improve the AUC values, sensitivity, and specificity to predict the early recurrence of HCC before surgery.
There were several limitations to this study. First, this retrospective and small sample study only enrolled the patients treated in a single hospital; therefore, multi-center and prospective research studies are needed to confirm these results. Second, tumor cells are often present in a wide range of sizes, and detecting the CTC count was based on filtration; therefore, the detection efficiency might be prone to false negative results due to the passing of small CTCs through the barriers. Third, most of the HCC patients enrolled in this study mainly suffered from HBV, which might have affected the outcome. Moreover, the applications of these results to the HCC patients with other liver diseases require further investigation.
Conclusions
In summary, the high platelet count was an independent prognostic factor for the early recurrence of HCC. The present study suggested a possible interaction of CTCs with platelets. The combined detection of platelet and CTC counts might effectively overcome the shortcomings in the individual detection of CTC and platelet count. Monitoring these parameters before surgery or during follow-up might be clinically useful for improving the treatment of cancer or cancer recurrence in high-risk patients. However, these results should be validated in large cohorts.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant no. 81960450), the National Major Special Science and Technology Project (grant no. 2017ZX10203207), the High-Level Innovation Team and Outstanding Scholar Program in Guangxi Colleges and Universities, “139” Projects for Training of High-Level Medical Science Talents from Guangxi, The Key Research and Development Project of Guangxi (grant nos. AA18221001, AB18050020, and 2020AB34006), The Key Laboratory of Early Prevention and Treatment for Regional High-Frequency Tumors of the Ministry of Education, Guangxi Independent Research Project (grant nos. GKE2017-ZZ02, GKE2018-KF02, and GKE2019-ZZ07), and Development and Application of Medical and Health Appropriate Technology in Guangxi (grant no. S2019039).
Disclosure
The authors declare no conflicts of interest in this study.
References
1. Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi:10.1001/jamaoncol.2017.3055
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi:10.1097/sla.0000000000000710
4. Ahn JC, Teng PC, Chen PJ, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology. 2021;73(1):422–436. doi:10.1002/hep.31165
5. Qi LN, Xiang BD, Wu FX, et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 2018;78(16):4731–4744. doi:10.1158/0008-5472.Can-17-2459
6. Yu XQ, Dasgupta P, Baade P. Quantifying the absolute number of cancer deaths that would be avoided if cancers were diagnosed prior to progressing to distant metastasis, New South Wales, Australia 1985–2014. Int J Cancer. 2022;150(11):1760–1769. doi:10.1002/ijc.33931
7. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi:10.1038/nm1469
8. Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi:10.1016/j.cell.2014.07.013
9. Wei C, Yang C, Wang S, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18(1):64. doi:10.1186/s12943-019-0976-4
10. Wang PX, Xu Y, Sun YF, et al. Detection of circulating tumour cells enables early recurrence prediction in hepatocellular carcinoma patients undergoing liver transplantation. Liver Int. 2021;41(3):562–573. doi:10.1111/liv.14734
11. Mocan T, Simão AL, Castro RE, et al. Liquid biopsies in hepatocellular carcinoma: are we winning? J Clin Med. 2020;9(5):1541. doi:10.3390/jcm9051541
12. von Felden J, Garcia-Lezana T, Schulze K, Losic B, Villanueva A. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut. 2020;69(11):2025–2034. doi:10.1136/gutjnl-2019-320282
13. Ha Y, Kim TH, Shim JE, et al. Circulating tumor cells are associated with poor outcomes in early-stage hepatocellular carcinoma: a prospective study. Hepatol Int. 2019;13(6):726–735. doi:10.1007/s12072-019-09994-9
14. Chen VL, Xu D, Wicha MS, Lok AS, Parikh ND. Utility of liquid biopsy analysis in detection of hepatocellular carcinoma, determination of prognosis, and disease monitoring: a systematic review. Clin Gas Hepatol. 2020;18(13):2879–2902.e2879. doi:10.1016/j.cgh.2020.04.019
15. Lim SB, Yeo T, Lee WD, et al. Addressing cellular heterogeneity in tumor and circulation for refined prognostication. Proc Natl Acad Sci USA. 2019;116(36):17957–17962. doi:10.1073/pnas.1907904116
16. Anvari S, Osei E, Maftoon N. Interactions of platelets with circulating tumor cells contribute to cancer metastasis. Sci Rep. 2021;11(1):15477. doi:10.1038/s41598-021-94735-y
17. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Throm Haemost. 2011;9(2):237–249. doi:10.1111/j.1538-7836.2010.04131.x
18. Bu J, Jeong WJ, Jafari R, et al. Bimodal liquid biopsy for cancer immunotherapy based on peptide engineering and nanoscale analysis. Biosens Bioelectron. 2022;213:114445. doi:10.1016/j.bios.2022.114445
19. Lou XL, Sun J, Gong SQ, Yu XF, Gong R, Deng H. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res. 2015;27(5):450–460. doi:10.3978/j.issn.1000-9604.2015.04.10
20. Pereira-Veiga T, Schneegans S, Pantel K, Wikman H. Circulating tumor cell-blood cell crosstalk: biology and clinical relevance. Cell Rep. 2022;40(9):111298. doi:10.1016/j.celrep.2022.111298
21. Wang H, Lu Z, Zhao X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol. 2019;12(1):133. doi:10.1186/s13045-019-0806-6
22. Tesfamariam B, Wood SC. Targeting glycoprotein VI to disrupt platelet-mediated tumor cell extravasation. Pharmacol Res. 2022;182:106301. doi:10.1016/j.phrs.2022.106301
23. Chai S, Matsumoto N, Storgard R, et al. Platelet-coated circulating tumor cells are a predictive biomarker in patients with metastatic castrate-resistant prostate cancer. Mol Cancer Res. 2021;19(12):2036–2045. doi:10.1158/1541-7786.Mcr-21-0383
24. Abdallah EA, Souza ESV, Braun AC, et al. A higher platelet-to-lymphocyte ratio is prevalent in the presence of circulating tumor microemboli and is a potential prognostic factor for non-metastatic colon cancer. Transl Oncol. 2021;14(1):100932. doi:10.1016/j.tranon.2020.100932
25. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi:10.1016/0168-8278(95)80226-6
26. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi:10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e
27. Bezerra AS, D’Ippolito G, Faintuch S, Szejnfeld J, Ahmed M. Determination of splenomegaly by CT: is there a place for a single measurement? Am J Roentgenol. 2005;184(5):1510–1513. doi:10.2214/ajr.184.5.01841510
28. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43(1):167–176. doi:10.1016/j.jhep.2005.05.009
29. Sheng X, Ji Y, Ren GP, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14(6):1034–1047. doi:10.1007/s12072-020-10111-4
30. Goh BK, Teo JY, Chan CY, et al. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: implications on the current AJCC staging system. J Surg Oncol. 2016;113(1):89–93. doi:10.1002/jso.24099
31. Hayashi T, Shibata M, Oe S, Miyagawa K, Honma Y, Harada M. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers. 2020;12:11. doi:10.3390/cancers12113215
32. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Meta Rev. 2017;36(2):249–262. doi:10.1007/s10555-017-9673-1
33. Papa AL, Jiang A, Korin N, et al. Platelet decoys inhibit thrombosis and prevent metastatic tumor formation in preclinical models. Sci Transl Med. 2019;11:479. doi:10.1126/scitranslmed.aau5898
34. Malehmir M, Pfister D, Gallage S, et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25(4):641–655. doi:10.1038/s41591-019-0379-5
35. Ma C, Fu Q, Diggs LP, et al. Platelets control liver tumor growth through P2Y12-dependent CD40L release in NAFLD. Cancer Cell. 2022;40(9):986–998.e985. doi:10.1016/j.ccell.2022.08.004
36. Wang B, Zhu J, Ma X, et al. Platelet activation status in the diagnosis and postoperative prognosis of hepatocellular carcinoma. Clin Chimica Acta. 2019;495:191–197. doi:10.1016/j.cca.2019.03.1634
37. Bihari C, Rastogi A, Shasthry SM, et al. Platelets contribute to growth and metastasis in hepatocellular carcinoma. APMIS. 2016;124(9):776–786. doi:10.1111/apm.12574
38. Wu CJ, Chau GY, Lee IC, et al. Early and late recurrence of surgically resected hepatitis B virus-related hepatocellular carcinoma on nucleos(t)ide analogues therapy. J Formos Med Assoc. 2021;120(8):1563–1571. doi:10.1016/j.jfma.2020.11.019
39. Asesio N, Pollo-Flores P, Caliez O, et al. Baveno VI criteria as a prognostic factor for clinical complications in patients with compensated cirrhosis. Digest Liver Dis. 2022;54(5):645–653. doi:10.1016/j.dld.2021.09.004
40. Midorikawa Y, Takayama T, Higaki T, et al. High platelet count as a poor prognostic factor for liver cancer patients without cirrhosis. Biosci Trends. 2020;14(5):368–375. doi:10.5582/bst.2020.03230
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

