Back to Journals » Infection and Drug Resistance » Volume 16
High Levels of Multidrug-Resistant and Beta-Lactamase-Producing Bacteria in Meat and Meat Contact Surfaces, Debre Berhan Town, Ethiopia
Authors Asfaw T , Genetu D, Shenkute D , Shenkutie TT, Amare YE , Yitayew B
Received 28 January 2023
Accepted for publication 29 March 2023
Published 1 April 2023 Volume 2023:16 Pages 1965—1977
DOI https://doi.org/10.2147/IDR.S405582
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Tsegahun Asfaw,1 Deribew Genetu,1 Demissew Shenkute,1 Tassew Tefera Shenkutie,1 Yosef Eshetie Amare,2 Berhanu Yitayew1
1Department of Medical Laboratory Science, Debre Berhan University, Debre Berhan, Ethiopia; 2Department of Biomedical Science, Debre Berhan University, Debre Berhan, Ethiopia
Correspondence: Tsegahun Asfaw, Email [email protected]
Background: Over the years, microbial contamination caused by foodborne bacteria has led to a significant number of food recalls, particularly for meat items that have been related to outbreaks of deadly diseases. Animals often carry Salmonella and Escherichia coli bacteria in their intestines, and these bacteria contaminate raw meat during slaughter. In addition, pathogens such as Staphylococcus aureus can contaminate meat processing equipment and utensils and spread to raw meat.
Methods: A cross-sectional study was undertaken between 30 February and 15 March 2022. Sanitary conditions of abattoir and butchers and food handlers’ hygienic practices were assessed using a structured questionnaire. An equal number of meat, swabs from carcasses, knife, weighing balance and cutting board samples (24 each, 120 total) were collected from abattoir and butcher shops. The collected samples were processed for bacterial isolation, antimicrobial susceptibility testing, MDR screening and confirmation, and ESBL screening and confirmation. Finally, SPSS software version 25 was used to compile and analyze the data. Descriptive data from surveys and laboratory procedures were cross-tabulated and summarized using statistical tables and figure.
Results: A total of 76 bacteria were isolated from 120 samples. Of all bacteria isolated, S. aureus 16 (21.1%). E. coli 13 (17.1%), and S. epidermidis 12 (15.8%) were the most prevalent. The rate of bacterial contamination was high in meat 18 (23.7%), carcasses 15 (19.7%) and weighing balance 15 (19.7%), respectively. Among the isolates, 18 (23.7%) were resistant for eight and more antibiotics. While, 17 (22.4%), 7(9.2%) and 4(5.3%) of the isolates were resistant for two and three, four and five, and six and seven antibiotics, respectively. Of bacteria isolated, 51/76 (67.1%) were MDR, 23/48 (47.9%) were screened for ESBL production and 13/48 (27.1%) isolates were confirmed as ESBL producer.
Conclusion: Multidrug-resistant bacterial contamination was common in meat and meat contact surfaces, which was exacerbated by inadequate sanitation and hygiene practices.
Keywords: food-borne bacteria, multidrug-resistant, beta-lactamase, meat, meat contact surfaces
Introduction
Meat is a nutrient-dense diet that contains more protein, vitamins, and minerals than other food sources, is necessary for the growth, repair, and maintenance of body cells, and is a vital component of our everyday existence.1 It is, nevertheless, regarded to be a significant vector of food-borne infections to humans.2 The water activity and pH of fresh meat are crucial factors in microbial development. Pathogenic bacteria can infect meat and meat products during handling, processing, preparation, and distribution,3 with serious socioeconomic consequences.4 According to recent data from either in low-income countries or developed countries, at least 10% of the population is susceptible to food-borne disease. Consumers are more concerned about bacterial infections among biological threats.5 Eating contaminated food can result in hospitalization and even death in cases of mild to severe sickness.6 The situation in low-income countries is worse, with obvious economic consequences.7
Ethiopia is estimated to have 53.99 million cattle.8 At the same time, per capita annual meat consumption continues to increase as per capita income increases.9 Raw meat consumption in particular is becoming a status symbol. For example, over 33% of population in the town of Debre Berhan ate raw meat, a common practice in some parts of Ethiopia.10 In Ethiopia, on the other hand, the incidence of food-borne disease appears to be higher than in developed countries, although precise data are not available.11 Data on meat-borne diseases in Ethiopia are very scarce, but several studies conducted in different parts of the country show that pathogens such as Campylobacter spp., Salmonella spp., Mycobacterium spp., Brucella spp., and E. coli were identified as a cause of foodborne illness.12 Because of the introduction and fast spread of multidrug-resistant bacteria (MDR) in humans, animals, and the environment, these bacterial infections are considered a worldwide health threat.
Multidrug resistance is on the rise around the world and is considered a public health risk. Several recent studies13,14 have found bacterial MDR pathogens emerging from a variety of sources, including humans and animals. Routine antimicrobial susceptibility testing in patients is becoming increasingly important.15 With the evolution of MDR strains, it is critical to continuously monitor antibiotic susceptibility for drug selection.16 Several studies have contributed to the emergence of MDR bacteria.17 It is frequently linked to a poor prognosis and treatment failure.17
In Ethiopia, the entire meat supply chain is not adequately controlled to ensure microbial quality, safety, and hygiene, from slaughterhouses to distribution and butchers to the final consumer.18 There has also been little research into MDR and beta-lactamase-producing bacteria on meat and meat contact surfaces. Furthermore, there is a scarcity of data to assess food safety practices and the microbial load of meat contact surfaces in Ethiopian abattoirs and butcher shops. These factors may make it difficult for governments and other stakeholders to apply accurate measures to the public health impact of food contamination problems. End users also have little knowledge about the quality and safety of the meat they eat on a regular basis. Therefore, this study was designed to determine antimicrobial resistance patterns of foodborne pathogens in meat and meat contact surfaces in relation to abattoir and butcher shops hygiene practices and sanitary conditions.
Methods
Study Area and Period
A study was conducted in Debre Berhan town, 130 kilometres northeast of Addis Ababa. The altitude is 2840 meters, the latitude and longitude is (9041’N39032’E). According to 2012 national census conducted by the central statistical Agency the town has a total population of 160,408.19 There are only one abattoir in the town and over 50 butcher shops. The abattoir had the space to slaughter more than 400 cattle per week. In Debre Berhan, cattle, sheep, goats and poultry are often the animals that are slaughtered for human food. However, the abattoir mainly deals in cattle (beef). The number of animals slaughtered per day varied from time to time. Finally, meat and meat products are transported from abattoir to butchers, where consumers purchase meat.
Study Design
A cross-sectional study was conducted from 30 February to 15 March 2022. Three basic data collection methods (questionnaire, observation and laboratory examination) were used. Samples were collected from the abattoir and 10 butchers’ shops. Prior to the actual survey, a preliminary survey was conducted to select a butcher shop. Finally, the selection of butcher shops for this study was supported by personal observations and community-derived data (customer size and number).
Questionnaires and Observational Survey
Data on abattoir and butcher shops sanitary conditions, as well as food handler sanitary practices, were collected using a structured questionnaire, individual interviews and observations (Supplementary Material One). From 10 butcher shops, a total of 20 meat handlers and processor (two from each butcher shop with direct contact with meat) were provided for their response. All workers in abattoir were also interviewed for their response.
Sampling Technique
Meat, swabs from carcasses, knife, weighing balance and cutting board were collected. An equal number of samples (24 each, 120 total) were collected from abattoir and butcher shops. Risk assessment guideline co-established by the Food and Agriculture Organization and WHO was used to set microbiological criteria for meat samples.20 The samples were collected in the mid-morning (9–11AM) once in every two week over a period of one and a half months. Samples of all types; meat, swabs from carcasses, knife, weighing balance, and cutting board were collected from each abattoir and each butcher shops at each sampling visit.
Samples Collection
Meat sample weighing 25 gm was collected aseptically using sterile polyethylene bag. Environmental surface swab samples were collected from the area of covering 30 cm2 of meat-cutting tools such as knives, cutting boards, and weighing balance using sterile swabs soaked into 0.1% saline solution. Furthermore, carcass swab samples from the area of covering 100 cm2 of lean meat were collected from abattoir and butcher shops using sterile swabs soaked into 0.1% saline solution. Finally, the samples were transported to Debre Berhan University’s microbiology laboratory using an icebox (4°C) for immediate analysis.
Samples Preparation
Meat
A meat sample was weighed and transferred to a stomacher bag under aseptic conditions. The samples were then diluted 10−1 using 225 mL of buffered peptone water and homogenised for 1–2 minutes using a stomacher. Following homogenization, further serial dilutions were made using sterile buffered peptone water (3M company, St. Paul, MN 55144–1000, USA).21
Swab Sample
Each tubes containing swab samples was vortexed to ensure a mixture of sample. A tenfold serial solution was prepared by transferring 1mL of the homogenised sample to 9 mL of sterile buffered peptone water (3M company, St. Paul, MN 55144–1000, USA).21
Isolation and Identification of Bacteria
To generate high bacterial recovery, raw samples were pre-enriched with buffered peptone water.22 From appropriate serial dilutions, a volume of 0.1 mL aliquot was aseptically taken and inoculated onto solidified MacConkey Agar (Oxoid Ltd., Basingstoke and Hampshire, UK), and Mannitol Salt Agar (Oxoid Ltd., Basingstoke and Hampshire, UK) using the pour plate technique. After obtaining pure colonies and recording key characteristics, gram-negative bacteria (E. coli, Klebsiella spp., Enterobacter spp., Shigella spp., Salmonella spp., Citrobacter spp., Serratia spp., and Proteus spp.) were identified based on colonial morphology and pigmentation, oxidase test, carbohydrate fermentation, H2S production, citrate utilization, motility, indole formation, lysine decarboxylase and lysine deaminase production, and urea hydrolysis. Gram positive isolates (S. aureus and S. epidermidis) were also differentiated by colonial characteristics, catalase test coagulase tests, and novobiocin susceptibility test.
Antimicrobial Susceptibility Testing
The CLSI-recommended Kirby-Bauer disk diffusion technique was performed to evaluate the antibiotic susceptibility profiles of the isolates. The Clinical Laboratory Standard Institute (CLSI) susceptibility breakpoints were used for interpretation.23 All antibiotics were obtained from Oxoid Ltd, UK. The antibiotics listed below were selected because they are widely prescribed in Ethiopia (Table 1).
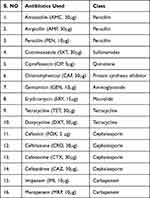 |
Table 1 List of Antibiotics Used in This Study |
Screening of Multidrug-Resistant Isolates
Multidrug-resistant isolates were those that were resistant to one or more drugs from three or more antimicrobial classes.24
Screening and Confirmation of ESBLs Producing Bacteria
Each Enterobacteriaceae isolates exhibiting decreased susceptibility to cefotaxime and/or ceftazidime was considered a possible ESBL producer. Potential ESBL producers were isolates with an inhibitory zone size of 22 mm with ceftazidime (30 g) and/or 27mm with cefotaxime (30 g).23 A disk of ceftazidime (30 g) and cefotaxime (30 g) alone, as well as their combination with clavulanic acid (30 g/10 g), were placed at a distance of 25 mm, centre to centre, on Muller Hinton agar plates that were seeded with a bacterial suspension of 0.5 McFarland turbidity standard and then incubated overnight (18–24 hrs.) at 37°C. Finally, a bacterial isolate that increased the inhibition zone diameter by more than 5mm for a combination disk over ceftazidime or cefotaxime disk alone was identified as an ESBL producer.23
Quality Assurance
Standard operating procedures were used to conduct laboratory analyses (SOPs). Reagents were tested for proper functioning and handled in accordance with standard protocols before to use. To assess the efficacy of the disk diffusion test, quality control strains such as E. coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were utilized as quality control organisms throughout the isolation, identification, and antimicrobial susceptibility testing. ESBLs positive K. pneumoniae ATCC 700603 and ESBLs negative E. coli ATCC 25922 quality control strains were also utilized for the ESBL confirmatory test.
Statistical Analysis
Data were analyzed using SPSS Statistics for Windows, Version 25.0 (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). Descriptive data from surveys and laboratory procedures were cross-tabulated. Finally, statistical table and figures were used to summarize and present the data.
Results
Abattoir Observations and Questionnaire Survey
Standard fasting period was not properly practiced. There were no stunning boxes in this abattoir. Instead, sharp knives were placed on the ground to stun the cattle. Butchers did most of the slaughtering work by hand, and gloves were not used. Potable water was used for washing carcasses prior to disposal. Most abattoir workers did not wear overcoat or head cover. Additionally, the “one man one job” principle was ignored. The abattoir was ventilated, but the production line lacked controlled air conditioning and refrigeration. The carcass was also transported with the offal (Table 2).
 |
Table 2 Abattoir Sanitary Conditions and Food Handler Sanitary Practices at Debre Berhan Town, Ethiopia, 2022 |
Butcher Shops Observation and Questionnaire Survey
Most meat handler’s 18 (90.0%) maintained the sanitary condition of the butcher shops. Not all meat handlers participated in sanitation training. Of the meat handlers, only 10 (50.0%) had a health certificate. Most were not used separate cutting board for cutting meat and abdominal organs. About 14 (70.0%) meat handlers practiced handling money while serving customers. The majority 14 (70.0%) of food handlers did not use easily washable cutting boards (Table 3).
 |
Table 3 Meat Handler Sanitary Practices at Debre Berhan Town, Ethiopia, 2022 |
Bacterial Contamination of Meat and Meat Contact Surfaces
A total of 76 bacteria were isolated from 120 samples. Among the isolates, S. aureus 16 (21.1%). E. coli 13 (17.1%), S. epidermidis 12 (15.8%), Serratia spp. 8 (10.5%), Klebsiella spp. 6 (7.9%), and Salmonella spp. 6(7.9%) were the most prevalent (Figure 1).
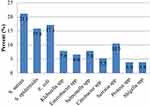 |
Figure 1 Prevalence of bacterial contamination in meat and meat contact surface at Debre Berhan Town, Ethiopia, 2022. |
Distribution of Bacterial Contamination in Meat and Meat Contact Surface
The rate of contamination was high in meat 18 (23.7%), carcasses 15 (19.7%) and Weighing Balance 15 (19.7%), respectively. S. aureus was predominantly isolated from weighing balance 4 (25.0%), meat 3 (18.8%), and cutting board 3 (18.8%). S. epidermidis was also dominantly isolated from knife swab 5 (41.7%) and cutting board 3 (25.0%). From enteric bacteria, E. coli was the most contaminant in meat 5 (38.5%), and cutting board 3 (23.1%). Similarly, Salmonella spp. was the most contaminant of carcasses 3 (50.0%). Other bacteria like Klebsiella spp., Enterobacter spp., Citrobacter spp., Serratia spp., Proteus spp., and Shigella spp., were also isolated from all types of samples (Table 4).
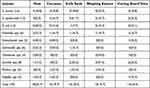 |
Table 4 Distribution of Bacterial Contamination in Meat and Meat Contact Surface at Debre Berhan Town, Ethiopia, 2022. N (%) |
Antibiotics Resistance Patterns of Bacterial Isolates
In this study, the isolates had the highest rates of resistance to amoxicillin 67 (88.2%), ampicillin 67 (88.2%), penicillin 25 (89.3%), erythromycin 43 (56.6%) and chloramphenicol 39 (51.3%). However, lower rates of resistance were observed for ciprofloxacin 27 (35.5%) and for newly introduced antibiotics in Ethiopia such as meropenem 6 (12.5%), imipenem 6 (12.5%), ceftazidime 18 (23.7%) and cefotaxime 21 (27.6%) (Table 5).
 |
Table 5 Antibiotics Resistance Patterns of Bacterial Contamination in Meat and Meat Contact Surface at Debre Berhan Town, Ethiopia, 2022. N (%) |
All isolates were resistant to at least one antibiotic tested. Among the isolates, 18 (23.7%) were resistant to eight or more antibiotics. While 17 (22.4%), 7 (9.2%), and 4 (5.3%) isolates were resistant to two and three, four and five, six and seven antibiotics, respectively (Table 6).
 |
Table 6 Multiple Drug Resistance Patterns of Bacterial Contamination in Meat and Meat Contact Surface Market at Debre Berhan Town, Ethiopia, 2022. N (%) |
Multidrug-Resistant and ESBL Producing Bacteria
Among 76 isolated bacteria, 51/76 (67.1%) were MDR, 23/48 (47.9%) were screened for ESBL production, and 13/48 (27.1%) isolates were confirmed as ESBL producer. Citrobacter spp. was 100% MDR followed by Klebsiella spp 5(83.3%), Enterobacter spp 4 (80.0%), E. coli 9 (69.2%), S. aureus 11 (68.8%), S. epidermidis 8 (66.7%), and Shigella spp. 2 (66.7%). Majority of the isolates like Citrobacter spp. 4 (80.0%), Shigella spp. 2(66.7%), Enterobacter spp. 3 (60.0%), Klebsiella spp. 3(50.0%), and E. coli 6 (46.1%) were screened for ESBL production. Citrobacter spp. 2 (50.0%), Enterobacter spp. 2 (40.0%), Klebsiella spp. 2 (33.3%), and E. coli 4 (30.8) were among the isolates confirmed for ESBL production (Table 7).
 |
Table 7 Distribution of MDR and ESBL Confirmed Bacterial Contamination in Meat and Meat Contact Surface, at Debre Berhan Town, Ethiopia, 2022. N (%) |
Discussion
In this study, the abattoir did not meet the standards recommended by the MoA.25 The abattoir must have a water supply with a reserve tank and its own power source as a backup that can reduce water pollution. Actual abattoir fasting periods differ from abattoir standards. A 12- to 24-hour fasting period prior to slaughter reduces intestinal content and thus bacteria, thereby reducing the risk of carcass contamination.26 There were no toilets or hand-washing facilities for abattoir workers, which might lead to contamination. All of the cutting, gluing, and bleeding was done on the ground. This stunning and homeostasis approach delays the bleeding process without instantly making the animal unconscious. This causes unneeded stress in butchered animals, which can result in glycogen depletion and poor meat quality.3 Furthermore, the bleeding time was not maintained at a typical level. The goal of bleeding out is to kill the animal as rapidly as possible while causing least harm to the carcass. This is due to the fact that blood is an optimal substrate for bacterial growth.26 The slaughter line did not have a clear separation between clean and dirty areas and did not restrict worker movement to specific locations. The principle of “one man, one job” did not apply. These slaughter practices increase the potential for cross-contamination. Delivery trucks did not have chilling facilities. A refrigeration system inhibits bacterial growth and increases shelf life if strict sanitation requirements are followed during slaughter and dressing.26 The loading and unloading personnel at the abattoir have inadequate hygiene. Furthermore, corpses and other offal were put on the same vehicle during distribution, which might lead to cross-contamination.
The general hygiene of the butcher shops meat handlers in this study was very poor. A separate cutting board was not used for cutting meat and abdominal organs. This approach allows sufficient time for microbial growth and cross-contamination. Consistent with this, another Ethiopian study27 reported significant bacterial growth when food was held at 15–45 °C for up to 4 hours. The majority of 14 (70.0%) meat handlers did not use easily washable cutting boards, which encourage bacterial growth leading to meat contamination during processing.
Regarding the training experience, not all meat handlers participated in sanitation training, even though, 18 (90.0%) meat handler’s maintained the sanitary condition of the butcher shops. The same study conducted in Addis Ababa,18 Mekelle28 and other countries29 reported that more than 50% of surveyed meat handlers had completed formal food hygiene training. On the contrary, there is a strong link between foodborne disease, poor hygiene practices and low levels of training. Additionally, correlations have been found between management attitudes towards training, levels of food hygiene knowledge, and standards of food handling practices.29
In this study, 11 (55.0%) meat handlers wore clean coats, but most 16 (80%) of the meat handlers did not wear clean hair cover. This result is much higher than the study done in Mekelle28 which showed that only 5/12 (41.7%) meat handlers did not wear clean coats and 7/12 (58.3%) of them did not put hair cover. This study showed comparable result to those of a study conducted in Addis Ababa18 which reported that 10/16 (65.5%) meat handlers did not wear clean coat and 12/16 (75%) meat handlers did not put hair cover. On the other hand, a study done in Jigjiga30 reported that none of the food handlers wore hair cover. Another study in Kenya found that 70% and 82% of abattoir operators in Nairobi and Isiolo respectively did not wear protective clothing when selling meat.31 In addition, the regulations of the WHO Food and Nutrition Division32 state that food handlers must wear clean, suitable clothing and wash their hands with soap and water after any activity potentially pose a hazard.
In this study about 14 (70.0%) meat handlers practiced handling money while serving customers. The same study conducted in Ethiopia18,28 indicated that many of the butcher man handled many while serving the meat. Study conducted in Kenya31 also suggested that 90% and 70% the butchery operators in Isiolo and Nairobi respectively handled many while handling of meat which can lead to meat contamination.
A total of 76 bacteria were recovered from 120 samples of meat and meat contact surfaces. The rate of contamination was high in meat 18 (23.7%), carcasses 15 (19.7%), and weighing balance 15 (19.7%), respectively. A high degree of bacterial contamination in the analyzed samples suggests poor meat quality and might be a source of foodborne illness and food deterioration. Twenty-eight (36.8%) of the isolates were gram-positive, whereas 48 (63.2%) were gram-negative. The presence of Enterobacteriaceae and Staphylococci in meat contact surface samples suggests that microbiological quality and safety might be considerably affected, potentially leading to pathogen infections.33,34 Another study in Ethiopia18,33 reported that the same species were dominantly isolated from minced meat. Similarly, a study conducted in Mekelle28 reported Staphylococci and Enterobacteriaceae from household meat samples. A similar study in Nigeria35 showed that Enterobacteriaceae (28.56%) and Staphylococci (21.4%) were dominantly isolated from beef samples. In addition, another study conducted in Ethiopia36 reported Enterobacteriaceae (85%) and Staphylococci spp. (12.2%). A comparable investigation in a small number of butcher shops in Addis Ababa, Ethiopia, discovered low meat quality and microbiological safety.18 Another study revealed that cooked meat and fish constitute a public health concern in Ethiopia.37
In this study, the isolates showed the highest rates of resistance to amoxicillin 67 (88.2%), ampicillin 67 (88.2%), penicillin 25 (89.3%), to erythromycin 43 (56.6%) and chloramphenicol 39 (51.3%). Many studies in Ethiopia13,38,39 and abroad40 have also reported high levels of resistance of some species to some antibiotics such as amoxicillin, ampicillin and penicillin. This high resistance to penicillin may be due to the continued use of penicillin derivatives in domesticated animals for slaughter.41 Overall, this study showed a high level of resistance to the most commonly prescribed antibiotics in Ethiopia. The observed variations in antibiotic susceptibility profiles can be attributed to inconsistencies in antibiotic prescribing policies, the use of antibiotics as veterinary medicines, and the mixing of antibiotics in livestock feed.
Of the 76 isolated bacteria, the prevalence of MDR was 51 (67.1%) in meat and meat contact surfaces. Another study conducted in Ethiopia38 also reported 60% of MDR isolates. The finding of this study was much higher than study conducted in Hawassa, Ethiopia13 indicating that multidrug resistance differs significantly among different regions. It is difficult to determine the MDR bacterial load isolated from meat in Ethiopia due to the restricted breadth of investigations and the lack of a coordinated epidemiological monitoring system. Infection rates with Salmonella spp. isolated from raw meat were found to be high in Jimma, south-western Ethiopia42 Gondar, Ethiopia43 and Wolaita Sodo, southern Ethiopia.44 A similar investigation in Jimma found a significant incidence of E. coli and multidrug-resistant (MDR) E. coli O157:H7 in slaughterhouse and butcher meat.45 S. aureus was also identified at a substantial frequency in another investigation done in slaughterhouses and butcheries in Addis Ababa, Ethiopia.46 This may be due to the result of over-prescription of antibiotics, patients not completing the full course of antibiotics, overuse of antibiotics in animal husbandry and farm land, poor infection control in health care, poor hygiene and sanitation.
From meat and meat contact surfaces, 19 isolates (25.0%) were identified as ESBL producers. The frequency of multidrug-resistant Enterobacteriaceae is growing globally due to the extensive use of third- and fourth-generation cephalosporin ESBL production is a prominent antibiotic resistance mechanism in Enterobacteriaceae, and ESBL-producing isolates are common in Ethiopia and other countries. As a result, ESBL-induced MDR and antibiotic resistance are significant public health problems. Although ESBL-producing isolates in food are uncommon, several instances of ESBL-producing isolates in raw meat have been recorded in Ethiopia.39,47
Limitation of the Study
The identification diarrheagenic strains of E. coli, enterotoxigenic strains of S. aureus and species of Salmonella, Shigella, Klebsiella, Enterobacter, Citrobacter and Serratia was not performed. Molecular detection of the virulence and antibiotic resistance genes of the isolates was not performed due to lack of laboratory settings. In addition, the clonal relatedness of the isolates and the molecular characterization of the MDR and ESBL-positive isolates were not performed. Because of the small sample size, this study did not show any statistical association describing the relationship between MDR and ESBL-positive isolates and the hygienic practices of meat handlers.
Conclusions
The findings of this investigation revealed that multidrug resistant and beta-lactamase producing bacteria contaminated meat and meat contact surfaces in both abattoir and butcher shops. Many reasons contributed to this, including low sophistication, inadequate sanitation and hygienic processes in abattoirs and butcher shops, a lack of training, and low levels of worker education. According to these findings, contamination exists from the slaughterhouse to the butcher shop, and the meat is contaminated before reaching the customer. As a result, it is critical to enhance hygiene and meat hygiene knowledge at both abattoirs and butcher shops, and effective controls on the issue should be devised and implemented.
Ethical Approval and Informed Consent
Ethical approval for this study was obtained from the Debre Brehan university Institutional Review Board [protocol number: IRB-003] and the head department of Debre Brehan Town’s North Shoa Zonal Office provided formal permission. All participants were made aware of the study’s goal. Finally, each food handler provided their signed informed consent.
Funding
Debre Berhan University provided funding for data collection. There was no funding for the study’s design, interpretation of results, manuscript writing, or publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McAfee AJ, McSorley EM, Cuskelly GJ, et al. Red meat consumption: an overview of the risks and benefits. Meat Sci. 2010;84(1):1–3. doi:10.1016/j.meatsci.2009.08.029
2. European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonosis, zoonotic agents and food‐borne outbreaks in 2012. EFSA J. 2014;12(2):3547.
3. Dave D, Ghaly AE. Meat spoilage mechanisms and preservation techniques: a critical review. Am J Agric Biol Sci. 2011;6(4):486–510. doi:10.3844/ajabssp.2011.486.510
4. Frenzen PD, Buzby JC, Rasco B. Product liability and microbial foodborne illness; 2001.
5. Sofos JN. Challenges to meat safety in the 21st century. Meat Sci. 2008;78(1–2):3–13. doi:10.1016/j.meatsci.2007.07.027
6. Tessema AG, Gelaye KA, Chercos DH. Factors affecting food handling Practices among food handlers of Dangila town food and drink establishments, North West Ethiopia. BMC Public Health. 2014;14(1):1–5. doi:10.1186/1471-2458-14-571
7. Nel S, Lues JF, Buys EM, Venter P. Bacterial populations associated with meat from the deboning room of a high throughput red meat abattoir. Meat Sci. 2004;66(3):667–674. doi:10.1016/S0309-1740(03)00187-6
8. CSA /Central Statistics Agency. Ethiopia- Livestock sample survey 2012–2013 (2005 E.C). Minister of finance and Economic Development; 2013.
9. Bongaarts J. Food and Agriculture Organization of the United Nations: the state of food and agriculture: agricultural trade and poverty: can trade work for the poor? Popul Dev Rev. 2007;33(1):197–198.
10. Craddock HA, Maring EF, Grutzmacher SK. Foodborne illness prevention in Debre Berhan, Ethiopia: preliminary efforts to understand household agricultural practices. African J Food Agric Nutr Dev. 2020;20(1):15194–15204. doi:10.18697/ajfand.89.17810
11. Tavakoli HR, Riazipour M. Microbial quality of cooked meat foods in Tehran University’s Restaurants. Pak J Med Sci. 2008;24(4):595–599.
12. Kebede T, Afera B, Taddele H, Bsrat A. Assessment of bacteriological quality of sold meat in the butcher shops of Adigrat, Tigray, Ethiopia. Appl J Hyg. 2014;3(3):38–44.
13. Worku W, Desta M, Menjetta T. High prevalence and antimicrobial susceptibility pattern of salmonella species and extended-spectrum β-lactamase producing Escherichia coli from raw cattle meat at butcher houses in Hawassa city, Sidama regional state, Ethiopia. PLoS One. 2022;17(1):e0262308. doi:10.1371/journal.pone.0262308
14. Algammal AM, Hashem HR, Al-Otaibi AS. Emerging MDR-Mycobacterium avium subsp. avium in house-reared domestic birds as the first report in Egypt. BMC Microbiol. 2021;21:1. doi:10.1186/s12866-021-02287-y
15. Tola MA, Abera NA, Gebeyehu YM, Dinku SF, Tullu KD. High prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae fecal carriage among children under five years in Addis Ababa, Ethiopia. PLoS One. 2021;16(10):e0258117. doi:10.1371/journal.pone.0258117
16. Makharita RR, El-Kholy I, Hetta HF, et al. Antibiogram and genetic characterization of carbapenem-resistant gram-negative pathogens incriminated in healthcare-associated infections. Infect Drug Resist. 2020;4:3991–4002. doi:10.2147/IDR.S276975
17. Algammal AM, Hetta HF, Batiha GE, et al. Virulence-determinants and antibiotic-resistance genes of MDR-E. coli isolated from secondary infections following FMD-outbreak in cattle. Sci Rep. 2020;10(1):1–3. doi:10.1038/s41598-020-75914-9
18. Zerabruk K, Retta N, Muleta D, Tefera AT. Assessment of microbiological safety and quality of minced meat and meat contact surfaces in selected butcher shops of Addis Ababa, Ethiopia. J Food Qual. 2019;2019:1–9. doi:10.1155/2019/3902690
19. Federal Democratic Republic of Ethiopia Central Statistical Agency. Statistical Report on Urban Employment Unemployment Survey; 2012.
20. Risk A. Joint FAO/WHO expert consultation on risk assessment of microbiological hazards in foods; 2001.
21. Atlabachew T, Mamo J. Microbiological quality of meat and swabs from contact surface in butcher shops in Debre Berhan, Ethiopia. J Food Qual. 2021;2021:1. doi:10.1155/2021/7520882
22. El Shamy HA, Bakr WI, Gomaa NF, Barheem OH. Evaluation of two enrichment broths, three plating media and ELISA technique for the isolation of Salmonella from dairy products. J Egypt Public Health Assoc. 2008;83:133–137.
23. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
24. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
25. MoA. Constriction Guideline 2. Ministry of Agriculture and Rural Development Animal and Plant Health Regulatory Directorate. Addis Ababa, Ethiopia: MoA; 2008.
26. FAO. Techniques and hygiene practices in slaughtering and meat handling; 2010. Available from: http://www.fao.org/docrep/004/T0279E/T0279E04.htm.
27. Muleta D, Ashenafi M. Some street vended food from Addis Ababa: microbiological and socio-Economical descriptions. Ethiop J Health Sci. 2000;10(2):89–100.
28. Gurmu EB, Gebretinsae H. Assessment of bacteriological quality of meat contact surfaces in selected butcher shops of Mekelle city, Ethiopia. J Environ Occup Health. 2013;2(2):61–66.
29. Walker E, Pritchard C, Forsythe S. Food handlers’ hygiene knowledge in small food businesses. Food Control. 2003;14(5):339–343. doi:10.1016/S0956-7135(02)00101-9
30. Tafesse F, Desse G, Bacha K, Alemayehu H. Microbiological quality and safety of street vended raw meat in Jijiga town of Somali Regional State, southeast Ethiopia. Afr J Microbiol Res. 2014;8(48):3867–3874.
31. Sharon C, Peter OL, George OA, Joseph M. Sanitation and hygiene meat handling practices in small and medium enterprise butcheries in Kenya-case study of Nairobi and Isiolo Counties. Int J Food Saf. 2015;17:64–74.
32. World Health Organization. Essential Safety Requirements for Street-Vended Foods. World Health Organization; 1996.
33. Dabassa A. Evaluation of home slaughtered meat quality used for human consumption at household and food seller house in Jimma. J Med Sci. 2013;13(8):779–784. doi:10.3923/jms.2013.779.784
34. Jay JM, Loessner MJ, Golden DA. Modern Food Microbiology. Springer Science & Business Media; 2008.
35. Odey MO, Mboso EO, Ujong UP, Johnson JT, Gauje B, Ategwu MA. Microflora analysis of selected meat and meat products from Calabar, Cross River State-Nigeria. Arch Appl Sci Res. 2013;5(3):50–56.
36. Tassew H, Abdissa A, Beyene G, Gebre-Selassie S. Microbial flora and food borne pathogens on minced meat and their susceptibility to antimicrobial agents. Ethiop J Health Sci. 2010;20(3):137–143. doi:10.4314/ejhs.v20i3.69442
37. Bedada TL, Feto TK, Awoke KS, et al. Microbiological and public health status of cooked meat and fish in Ethiopia. Open Microbiol J. 2020;14:1. doi:10.2174/1874285802014010123
38. Tonjo T, Manilal A, Seid M. Bacteriological quality and antimicrobial susceptibility profiles of isolates of ready-to-eat raw minced meat from hotels and restaurants in Arba Minch, Ethiopia. PLoS One. 2022;17(9):e0273790. doi:10.1371/journal.pone.0273790
39. Garedew L, Hagos Z, Zegeye B, Addis Z. The detection and antimicrobial susceptibility profile of Shigella isolates from meat and swab samples at butchers’ shops in Gondar town, Northwest Ethiopia. J Infect Public Health. 2016;9:348–355. doi:10.1016/j.jiph.2015.10.015
40. Dsani E, Afari EA, Danso-Appiah A, Kenu E, Kaburi BB, Egyir B. Antimicrobial resistance and molecular detection of extended spectrum β-lactamase producing Escherichia coli isolates from raw meat in Greater Accra region, Ghana. BMC Microbiol. 2020;20(1):1–8. doi:10.1186/s12866-020-01935-z
41. Rahman MA, Rahman AK, Islam MA, Alam MM. Detection of multi–drug resistant Salmonella from milk and meat in Bangladesh. Bangladesh J Vet Med. 2018;16(1):115–120. doi:10.3329/bjvm.v16i1.37388
42. Geresu MA, Desta WZ. Carriage, risk factors, and antimicrobial resistance patterns of Salmonella isolates from raw beef in Jimma, Southwestern Ethiopia. Infect Drug Resist. 2021;24:2349–2360. doi:10.2147/IDR.S313485
43. Ejo M, Garedew L, Alebachew Z, Worku W. Prevalence and Antimicrobial Resistance of Salmonella Isolated from animal-origin food items in Gondar, Ethiopia. Biomed Res Int. 2016;2016:1–8. doi:10.1155/2016/4290506
44. Wabeto W, Abraham Y, Anjulo AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J Health Popul Nutr. 2017;36(1):1–7. doi:10.1186/s41043-017-0131-z
45. Sebsibe MA, Asfaw ET. Occurrence of multi-drug resistant Escherichia coli and Escherichia coli O157: H7 in meat and swab samples of various contact surfaces at abattoir and butcher shops in Jimma town, Southwest district of Ethiopia. Infect Drug Resist. 2020;13:3853. doi:10.2147/IDR.S277890
46. Adugna F, Pal M, Girmay G. Prevalence and antibiogram assessment of Staphylococcus aureus in beef at municipal abattoir and butcher shops in Addis Ababa, Ethiopia. Biomed Res Int. 2018; 2018:1.
47. Abayneh M, Tesfaw G, Woldemichael K, Yohannis M, Abdissa A. Assessment of extended-spectrum β-lactamase (ESBLs)–producing Escherichia coli from minced meat of cattle and swab samples and hygienic status of meat retailer shops in Jimma town, Southwest Ethiopia. BMC Infect Dis. 2019;19(1):1–8. doi:10.1186/s12879-019-4554-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
