Back to Journals » Journal of Inflammation Research » Volume 17
High Level of Serum Complement C3 Expression is Associated with Postoperative Vasculopathy Progression in Moyamoya Disease
Authors Wang MJ, Wang J, Zhang H, Hao FB, Gao G, Liu SM, Wang XP, Li JJ, Zou ZX, Guo QB, Fu HG, Han YQ, Han C, Duan L
Received 23 November 2023
Accepted for publication 27 February 2024
Published 18 March 2024 Volume 2024:17 Pages 1721—1733
DOI https://doi.org/10.2147/JIR.S451538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Min-Jie Wang,1– 3 Jiayu Wang,4 Houdi Zhang,2,3 Fang-Bin Hao,1– 3 Gan Gao,1– 3 Si-Meng Liu,1– 3 Xiao-Peng Wang,1– 3 Jing-Jie Li,1– 3 Zheng-Xing Zou,2,3 Qing-Bao Guo,1– 3 He-Guan Fu,2,3 Yi-Qin Han,2,3 Cong Han,2,3 Lian Duan2,3
1Chinese PLA Medical School, Beijing, People’s Republic of China; 2Department of Neurosurgery, the First Medical Centre, Chinese PLA General Hospital, Beijing, People’s Republic of China; 3Department of Neurosurgery, the Fifth Medical Centre, Chinese PLA General Hospital, Beijing, People’s Republic of China; 4Department of Immunology, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Lian Duan; Cong Han, Department of Neurosurgery, Department of Neurosurgery, the Fifth Medical Centre (South Campus), Chinese PLA General Hospital, No. 8 Dong-Da Street, Fengtai District, Beijing, 100071, People’s Republic of China, Tel +86-10-66947389 ; +86-13466346163 ; +86-10-66947388, Fax +86-10-62177976, Email [email protected]; [email protected]
Background: The immune system plays an important role in the onset and development of moyamoya disease (MMD), but the specific mechanisms remain unclear. This study aimed to explore the relationship between the expression of complements and immunoglobulin in serum and progression of MMD.
Methods: A total of 84 patients with MMD and 70 healthy individuals were enrolled. Serum immunoglobulin and complement C3 and C4 expression were compared between healthy individuals and MMD patients. Follow-up was performed at least 6 months post-operation. Univariate and multivariate analysis after adjusting different covariates were performed to explore predictive factors associated with vasculopathy progression. A nomogram basing on the results of multivariate analysis was established to predict vasculopathy progression.
Results: Compared to healthy individuals, MMD patients had significantly lower expression of serum complements C3 (P = 0.003*). Among MMD patients, C3 was significantly lower in those with late-stage disease (P = 0.001*). Of 84 patients, 27/84 (32.1%) patients presented with vasculopathy progression within a median follow-up time of 13.0 months. Age (P=0.006*), diastolic blood pressure (P=0.004*) and serum complement C3 expression (P=0.015*) were associated with vasculopathy progression after adjusting different covariables.
Conclusion: Complement C3 is downregulated in moyamoya disease and decreases even further in late–Suzuki stage disease. Age, diastolic blood pressure and serum complement C3 expression are associated with vasculopathy progression, suggesting that the complement might be involved in the development of moyamoya disease.
Keywords: moyamoya disease, complement, Suzuki stage, nomogram
Introduction
Moyamoya disease (MMD) is a chronic cerebrovascular disorder characterized by progressive bilateral stenosis or occlusion of the internal carotid arteries (ICA) and the development of abnormally thin and fragile collateral vessels (moyamoya vessels) at the base of the brain.1 Insufficient cerebral blood flow or rupture of the fragile collaterals may cause cerebral stroke or hemorrhage, resulting in death or severe neurological dysfunction.2
The molecular etiology and pathogenesis of moyamoya disease (MMD) are still controversial. In 2011, RNF213 was identified as a susceptibility gene for MMD,3 and a variant of RNF213—p.R4810K (c.14429G>A, rs112735431)—has since been shown to play an important role in the pathogenesis of moyamoya and other cerebrovascular and cardiovascular diseases.4 However, the population mutation rate of RNF213 is much higher than the incidence rate of MMD, suggesting that immunological, metabolic, and environmental factors trigger MMD in susceptible individuals.5 Histopathological analysis has demonstrated marked decrease in the outer diameters of carotid terminations, fibrocellular thickening of vascular intima, luminal thrombosis, and thinning of vascular media in MMD.6 Unlike atherosclerosis, MMD lesions do not generally show inflammatory infiltration, but there is accumulating evidence from recent studies of the existence of an immune response in angiopathy.7 Genome-wide association studies (GWAS)8 and transcriptome-wide analyses9 have shown that genes and pathways associated with MMD are principally enriched in immune tissue and proinflammatory cytokines. RNF213, the susceptibility gene, prominently expressed in immune system, is essential for antimicrobial host defense10 and might be inappropriately activated in autoimmune response, infection, and inflammation, leading to immune dysfunction.11 In vivo and in vitro experiments have identified RNF213 mutations that impair T cell response by dysregulating antigen uptake and processing.12 In addition, aberrant expression of IgG in intracranial vascular smooth muscle cells and upregulation of 165 autoantibodies have been identified in MMD.13 Clinical and biochemical studies support the idea that MMD may be an immune-related disease and, in part, an autoimmune condition.
The complement system, an essential part of the immune system for controlling certain bacterial infections and for promoting clearance of apoptotic cells.14 In healthy individuals, the complement system can be activated in three pathways (classical pathways, alternative pathways, and lectin pathways). Patients with abnormal complement activation tend to present with autoimmune disease.15 Complement C3, a protein participating in all three complement activation pathways, has been reported in vascular dysfunction disorders such as vasculitis, hypertension, and atherosclerosis.16–18 In the clinic, detection of complement overactivity, deficiency, or dysfunction is used for evaluating the patient’s immune status and for diagnosis and follow-up of various diseases. Previous studies suggest that dysfunction of the innate immune system plays a significant role in the onset and evolution of MMD.19 However, few studies have examined the association between complement system expression and MMD.20,21 The aim of this study was to detect the expression of complement system in different stages of MMD and to elucidate its value in assessing and predicting disease progression.
Materials and Methods
Study Setting and Participants
This prospective study was approved by the Ethical Review Board of the Chinese PLA General Hospital, Beijing, China (ky-2018-3-14). Written informed consent was obtained from every participant. All procedures involving human participants in this study were performed in accordance with the Declaration of Helsinki (1964).
The study was conducted at the Department of Neurosurgery, the Fifth Center of Chinese PLA General Hospital, Beijing, China. Blood samples for immunological molecules detection were collected from patients admitted to hospital between March 2018 and December 2018, with diagnosis of MMD. The inclusion criteria were 1) age <60 years; 2) at least 6 weeks out from any documented acute stroke or hemorrhage at the time of blood collection (to avoid immunological changes secondary to stroke); 3) digital subtraction angiography (DSA) findings suggestive of MMD, according to the criteria recommended by the 2021 Japanese Guidelines for the Management of Moyamoya Disease;22 and 4) both cerebral hemispheres classified as early-stage (II–III) or late-stage (IV–V) by the Suzuki staging system. The exclusion criteria were 1) Suzuki stage I and VI (to avoid unclear diagnosis of MMD); 2) concurrent autoimmune disease such as lupus erythematosus, Sjögren syndrome, or thyroid disease; 3) systemic infection, trauma in the past 4 weeks; 4) evidence of atherosclerosis on high-resolution MRI.23 A total of 84 patients met the eligibility criteria. Additionally, a comparison group comprising 70 randomly selected healthy individuals attending the hospital for routine yearly health checkups was recruited, meeting the following inclusion criteria: 1) Aged 18–60 years old; 2) Exclusion of any cerebrovascular diseases or cardiovascular diseases such as coronary heart disease, intracranial atherosclerosis, intracranial aneurysm, or arteriovenous malformation; 3) Absence of any of the aforementioned vascular risk factors; 4) No records of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, or any other inflammatory diseases, and no recent infectious diseases within the last 6 months; 5) No history of trauma, tumor, stress, or any other conditions that might influence immune function. Figure 1 shows the flowchart of the participant enrollment process.
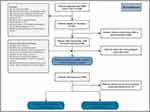 |
Figure 1 Flowchart showing patient selection process. Abbreviations: MMD, moyamoya disease; DSA, digital subtraction angiography. |
Study Design
Serum samples of MMD patients were obtained at least 1 day before DSA examination. Serum immunoglobulins (IgA, IgG, IgM, and IgE) and complement components (C3, C4) expression were assessed in the 84 patients and the 70 healthy individuals using enzyme-linked immunosorbent assay (ELISA).
Anterior circulation vasculopathy was classified by Suzuki stage system1 and divided into early- and late-stage according to whether the affected arteries occluded. The Mugikura four-stage classification system was used to grade posterior circulation vasculopathy.24 The modified Rankin Scale (mRS) was used to assess neurological status at baseline. The Matsushima scale was used to evaluate postoperative revascularization.25
Two experienced radiologists (CH and LD), blinded to the clinical and laboratory information, independently classified the moyamoya stage of each hemisphere on DSA images according to Suzuki criteria. Disagreements were resolved by open discussion.
Clinical Variables Collection and Follow-Up
Demographics and clinical variables (including symptom, comorbidity, angiography characteristics and probable risk factors associated with the progression of MMD) were collected. All patients accepted unilateral or bilateral encephalo-duro-arterio-synangiosis (EDAS). For patients with bilateral disease, the interval between operations on each hemisphere was at least 3 months. Patients routinely accepted DSA follow-up within 6–12 months after operation and annual MRA follow-up after 12 months. All suspected progressions were finally confirmed by DSA. The Matsushima grade was applied to evaluate the degree of cerebral revascularization on DSA,25 with grade C indicating poor revascularization and grade A or B indicating good revascularization.24 Vasculopathy progression was defined stringently as increase in average Suzuki stage by >1 stage (ie, upstaging of both hemispheres by >1 or upstaging of one hemisphere by >2) to mitigate observer bias.
Immunological Molecules Detection
The ELISA kits for C3, C4, IgA, IgE, IgG, and IgM were from Beckman Coulter, Inc., USA. The plate was coated with antibodies (IgA, IgE, IgG, IgM, C3, C4). Standard solutions and diluted patient serum were added. After incubation for 60 minutes at 37°C, each well was washed thrice with 200 μL of wash buffer. Then, 90 μL of 3’,5,5’-tetramethylbenzidine substrate solution was added to each well, and the plates were incubated at 37°C for 20 minutes, with protection from light. Finally, 50 μL of stop solution was added and thoroughly mixed. The absorbance optical density was measured at 450 nm and 570 nm.
Statistical Analysis
Categorical variables were summarized as frequencies and percentages, and differences between groups were compared using the chi-squared test. The distribution of the data was tested by Kolmogorov–Smirnov test. Continuous variables were summarized as means ± standard deviation or medians with interquartile range, and compared between groups using the two-tailed t-test or the Mann–Whitney test. The Wilcoxon signed rank test was used to compare differences between paired design variables. Linear regression was used to explore linear correlation between Suzuki stage with complement C3 expression and logistic regression analysis was used to identify the factors associated with vasculopathy progression. Covariates with P value <0.2 in univariate analysis or considered as clinically significant were selected for the multivariate analysis. Other covariates such as age, sex, comorbidities, and others were adjusted to ensure the accuracy and precision of the model. Tolerance and variance inflation factor (VIF) were calculated to evaluate the collinearity. Tolerance <0.1 or VIF >10 was considered with collinearity and variables with collinearity were removed.
A predictive nomogram model was constructed based on the multivariate analysis. The discrimination was measured by concordance index (C-index), and the calibration was assessed by Hosmer–Lemeshow test and calibration plot. The internal validation of the model was performed by calculating the corrected C-index generated from bootstrapping (1000 bootstrap samples). Decisive curve analysis (DCA) was used to evaluate the clinical benefits and utility. Statistical significance was set at p < 0.05. Statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA) and R 4.1.1 (https://cran.r-project.org/).
Results
Baseline Expression of Immunological Molecules in Different-Stage MMD Patients and Healthy Individuals
Baseline characteristics of all 84 MMD patients and 70 healthy individuals are shown in Supplement Table 1. Age and sex composition were not significantly different between MMD patients with healthy individuals. Table 1 shows the expression of immunological molecules in MMD patients and healthy individuals. Compared to healthy individuals, MMD patients had significantly lower expression of complement C3 (P = 0.003).
 |
Table 1 Expression of Serum Immunological Molecules in MMD Patients and Healthy Individuals |
According to the average Suzuki stage of bilateral hemispheres, the 84 MMD patients were divided into two groups as early-stage patients (n = 46, 54.8%) and late-stage patients (n = 38, 45.2%). Age and sex were comparable between the two groups. The expression of complement C3 (P=0.001) and C3-C4 ratio (P=0.026) were significantly higher in early stage. Expression of C3 was significantly lower in the late-stage group (P = 0.001), but expression of other molecules was comparable between the two groups. An inverse linear correlation between complement C3 with average Suzuki stage was observed in linear regression analysis (β=−0.373, P<0.001).
Subgroup analysis further confirmed some different expression of immunological molecules in patients grouped according to age, sex, symptom, comorbidities, mRS and posterior circulation involvement (Supplement Table 2). Expression of IgA (P<0.001) and IgG (P=0.005) were significantly higher in older patients (age≥40y) while expression of IgM (P=0.010) was lower. In male patients, IgM (P=0.001) expression was higher in comparison with female patients. The patients with ischemic symptoms (including TIA and infarction) had higher IgM (P<0.001) expression and lower IgA (P=0.036) and complement C4 (P=0.047) expression than non-ischemic symptom (including hemorrhage, headache and others) patients. Distribution of immunological molecules expression showed no difference in comorbidities, mRS and posterior circulation involvement.
Follow Up and Expression of Immunological Molecules
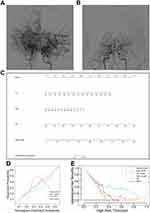
Clinical and imaging follow-up information was available for 84 patients while 12 patients had no blood sample for follow-up immunological molecules detection. Median follow-up time was 13.0 (IQR, 9.5–20.0) months. At follow-up, 27/84 (32.1%) patients in total suffered from vasculopathy progression, 6/84 (7.1%) of them experienced unilateral progression and 21/84 (25.0%) experienced bilateral progression. Among 27 patients with progression, 17/27 of them progressed from early stage to late stage, 10/27 of them progressed from late stage to an even later stage. 12/27 (44.4%) of patients progressed within 6–12 months post-operation, 6/27 (22.2%) progressed within 12–24 months post-operation, and 9/27 (33.3%) progressed within 24–36 months post-operation. The follow-up time had no difference in the two groups. Table 2 shows the baseline demographic, clinical characteristics and serum immunological molecules expression in patients with progressive disease and patients with stable disease. 17/27 (63.0%) patients with early-stage disease and 10/27 (37.0%) patients with late stage in progressive group, while 29/57 (50.9%) patients with early-stage disease and 28/57 (49.1%) patients with late-stage disease in non-progressive group. There were no significant differences between the two groups (P=0.299). Additionally, the distribution of the average Suzuki stage in the two groups showed no significant difference either (P=0.150). Age, sex, comorbidities, blood pressure, mRS and posterior circulation involvement were comparable in the two groups. Among several immunological molecules, expression of serum IgA (P=0.031) was lower while expression of IgE (P=0.038) and complement C3 (P=0.006) was significantly higher in patients with progressive disease (Figure 2). Expression of IgG, IgM, C4 and C3-C4 ratio showed no difference in two groups.
 |
Table 2 Clinical Characteristics and Immunological Molecules Expression in Progressive-Disease and Stable-Disease MMD Patients |
 |
Table 3 Risk Factors Association with Vasculopathy Progression in Multivariate Analysis |
Then, we analyzed how the levels of serum immunological molecules changed from baseline to follow-up (Supplement Table 3). The expression of C3 (P = 0.012) decreased in patients with progressive disease, whereas there was a significant increase in patients with stable disease (Figure 2D). In patients with progressive disease, the follow-up levels of C4 (Figure 2E) and IgE (Figure 2F) were significantly lower than the baseline levels (P < 0.001 and P = 0.002, respectively). In patients with stable disease, the follow-up levels were not significantly different from the baseline levels. Besides, expression of IgG had no difference at baseline and follow-up in progressive group. However, it increased at follow-up in stable group (P<0.001).
Finally, we estimated immunological molecules expression in revascularization. The Matsushima scale was used to evaluate the cerebral revascularization of 84 patients (60 accepted bilateral operation and 24 accepted unilateral operation, 144 hemispheres in total) who accepted EDAS. Good collateralization (Matsushima grade A and B) was found in 86/144 (59.7%) hemispheres. Expression of all immunological molecules showed no association with Matsushima grade (Supplement Table 4).
Risk Factors for Vasculopathy Progression
Univariate and multivariate logistic analyses were performed to explore correlation between immunological molecules and vasculopathy progression. In univariate analysis, age (OR, 0.968 [95% CI 0.939–0.997], P=0.031), symptom (OR, 4.324 [95% CI 1.158–16.151], P=0.029), diastolic blood pressure (OR, 1.044 [95% CI 1.001–1.089, P=0.045) and baseline expression of complement C3 (OR, 1.037 [95% CI 1.008–1.066], P=0.012) were associated with vasculopathy progression (Supplement Table 5). Then, these four variables were selected for multivariate analysis. After adjusting sex, smoking history, drinking history, family history, hypertension, diabetes, hyperlipidemia and hyperhomocysteinemia, we found that age (β=−0.066, OR, 0.922 [95% CI, 0.875–0.971], P=0.002) was protective factors associated with vasculopathy progression while DBP (β=0.100, OR, 1.106 [95% CI, 1.029–1.190], P=0.006) and baseline expression of complement C3 (β=0.045, OR, 1.049 [95% CI, 1.009–1.091], P=0.015) were independently risk factors (Table 3).
A Novel Nomogram Constructed for Predicting Vasculopathy Progression
A predictive nomogram was constructed basing on the results of multivariate analysis (Figure 3C). Three variables (including age, DBP and baseline expression of complement C3) were contained in the nomogram model finally. Besides, these 3 variables were also used to construct univariate models and compared with the nomogram, respectively. The VIF values were <2.0. The origin C-index value was 0.816 and was determined to be 0.794 after bootstrap. Hosmer–Lemeshow test was performed to exam the calibration of the model. The nomogram model (χ2 = 7.905, P = 0.443) and three univariate model (age model, χ2 = 14.635, P = 0.0673; DBP model, χ2 = 5.190, P = 0.6373 and C3 model, χ2 = 7.020, P = 0.534) presented with good calibration. The calibration plot (Figure 3D) showed that the predicted probability had a high consistency with the actually probability.
The clinical utility of nomogram and other 3 univariate models were estimated by DCA. In decision curve (Figure 3E), when the threshold probabilities >0.08, the nomogram model had more benefits for predicting MMD progression than intervention-for-all and intervention-for-none. Besides, the nomogram model presented with higher clinical benefits in comparison with other univariate models.
Discussion
In the present study, we firstly found differential expression of serum immunoglobulin and complement between patients with moyamoya disease and healthy individuals. The expression of complement C3 was lower in MMD patients compared to healthy individuals, and an inverse correlation between the average Suzuki stage and complement C3 was observed. Baseline expression of complement C3, age and diastolic blood pressure were identified as predictive factors for vasculopathy progression. No correlation between immunological molecules expression with revascularization was found. These findings indicated that dysfunction of immune system might participant in the onset and progression of MMD.
Up till now, rare studies about serum complement expression in MMD had been reported. We firstly investigated correlation between moyamoya vasculopathy progression with complement C3. In the present study, we observed lower serum level of complement C3 in late-stage moyamoya patients and inverse linearity between Suzuki stage and complement C3. In angiography, the most characteristic of late stage in comparison with early stage was the complete occlusion of arteries. Previous literature has also reported that inflammatory stimulation might induce proliferative response of smooth muscle cells. We hypothesized that complement-mediated immune dysfunction was involved in the formation of occlusive lesion and promoted progression from early- to late-stage. When the arteries occluded and the complement level decreased in the final period, this kind of stenosis-to-occlusion pathological changes appeared to be “completed”.
We also noticed other immunological molecules in different subtype of MMD patients. IgA and IgG expression in young (<40y) patients was lower than the older. Previous studies have reported that IgA is a potential regulator of immune complex formation, its reduced levels possibly promoting immune complex formation among other antibodies (like IgG-immune complex) and thus exacerbating the disease.26 In the absence of serum monomeric IgA, the IgA Fc receptor I (FcαRI) may not induce immune cell inhibition, which would favor the development of autoimmunity. An earlier study reported a higher incidence of autoimmune disease in IgA-deficient individuals than in healthy controls.27 The differential serum complement and immunoglobulin expression in MMD patients suggested the possibility of autoimmune involvement in the pathological process of young MMD.
Our multivariate analysis identified three independent predictive factors for vasculopathy progression: serum complement expression along with age and diastolic blood pressure. In our study, complement C3 expression was associated with Suzuki stage, therefore we adjusted the Suzuki stage to exam the predictive value of complement C3 expression in different disease stage. The result showed that complement C3 expression had good predictive value in all stages. Meanwhile, we also took other vascular risk factors such as sex, drinking history, smoking history and comorbidities into consideration and adjusted these variables to ensure the stability of the model. After adjusting these covariables, the predictive value of age, DBP and complement C3 expression remained significant. Finally, we constructed a nomogram model based on the multivariate logistic regression and conducted internal validation. The model presented with good discrimination, calibration and clinical utility to predict the vasculopathy progression.
Consistent with several previous studies, we found patients with lower age and higher DBP were more vulnerable to the vasculopathy progression. An analysis based on 394 consecutive adolescents unilateral moyamoya patients showed that an age at diagnosis <9 years old was an independent factor to predict contralateral progression.28 Several studies also showed that DBP reduction could significantly reduce the cardio- and cerebro-vascular risk and secondary stroke or cognitive impairment.29,30 In particular, after restricting inclusion of patients with high risk of atherosclerosis or other non-MMD artery diseases in our cohort, association between these risk factors and progression was still significant, indicating that these factors might not take part in the disease progression by mediating the occurrence of other stenosis-occlusion changes.
These findings have important clinical implications. The Suzuki staging system, to some extent, standardizes the description of MMD status and disease progression. In early-stage MMD, angiography reveals the initiation of steno-occlusive changes in the terminal internal carotid artery region and the development of abnormal moyamoya collateral vessels. As the disease progresses, there is occlusion of major intracranial arteries and the gradual disappearance of collateral vessels until, finally (at stage VI), there is disappearance of the intracranial internal carotid artery.1 However, angiography interpretation is relatively subjective and limited for long-term follow-up of chronic progressive diseases such as MMD. Our findings of progressive decrease in C3 in late-stage MMD could be useful for more accurate grading and stroke risk stratification. Generally, progressive-disease MMD patients tend to have a higher incidence of stroke and more postoperative complications due to the severe stenosis or occlusion and unstable blood flow.31 Furthermore, we implemented a more stringent criterion based on the Suzuki staging system to mitigate observer bias, which may have introduced an artificial ceiling effect on the potential progression of late-stage patients. Despite this, we found that the complement levels of patients in the progression group remained higher than those in the non-progression group, even when considering the similar proportions of early-stage/late-stage patients and the distribution of average Suzuki stage in the two groups. This indicates that the higher complement levels in the progressive group were not solely due to the fact that most patients in the progressive group were in the early-stage. In other words, even in the late-stage, patients with subsequent progressive MMD exhibited relatively higher complement C3 levels than patients with non-progressive MMD. Therefore, we concluded that the ceiling effect for our study might be limited. Our study suggested that patients with high level of complement C3, along with low age and high DBP had more risk of vasculopathy progression and should accepted earlier intervention such as operation.
The mechanism by which complement C3 participates in MMD is unclear. Complement C3 is at the center of complement system activation and has, therefore, been used for evaluating immune status and for diagnosis and follow-up of many diseases.32 In the onset and progression of vasculopathies, C3 has been shown to mediate both endothelium injury and vascular remodeling.33 Buono et al reported that the progression of atheromas depended partly on the complement system. Complement activation was found to be associated with plaque stability and maturation of the lesion.34 A recent study revealed that inhibition of vascular smooth muscle cells (VSMCs) migration induced by low-density lipoprotein could be reversed by C3a and iC3b (C3 active fragment) and thereby lead to vasculopathy progression.35 Based on the opposite expression of complement C3 in early-Suzuki and late-Suzuki stage, we suspect that complement plays a dual role in disease development. At early stage, a high level of complement might mainly participant in intima injury and media VSMCs migration by mediating the immune response which responsible for further stenosis of lumen. At the late stage, there was a decrease in complement expression, and the arteries became occluded. During this period, the main pathophysiological changes observed were compensation for ischemia and remodeling of collateral vessels. It is still not known how the moyamoya collateral vessel formed and disappeared. Some investigators believe that these collateral vessels are composed of both dilated penetrating arteries and neovasculature.36 Complement system has shown capacity of pro-angiogenesis by mediating immune cell polarization.37 It indicates that complement has the potential to regulate the formation of smoke-like collateral vessels and decrease to a relative low expression as the vessels gradually disappearing. This provided us a new insights to understand the pathogenesis of moyamoya vessels and therapy targets.
This study has some limitations. First, this is a single-center and small-sample study; only 84 patients met our strict inclusion criteria (similar bilateral stage). Only stage II–V MMD patients were included in this study; stage I and VI MMD patients were excluded as these patients were diagnosed clinically (it is difficult to distinguish these stages in DSA images). The results are mainly applicable to patients with bilateral disease and similar Suzuki stages in both hemispheres, especially for patients with early-stage disease. Further research is needed to clarify the predictive value of complement expression in patients with different Suzuki stages in the two hemispheres. Second, baseline levels of complements and fragments other than IgG, IgA, IgM, IgE, C3, and C4 were not measured, and so their effects were not elucidated. Third, due to the limited sample size, external validation was not conducted in this study. Further investigations are required to validate our nomogram model in future research.
Conclusion
To conclude, there are significant differences in complement expression between patients with moyamoya disease and healthy individuals, suggesting that autoimmune response plays an important role in the pathogenesis of the disease. Complement C3 expression could be used as a disease-specific biomarker for early diagnosis of moyamoya disease and prognosis prediction. A high baseline level of complement C3 expression, along with lower age and high DBP were risk factors associated with vasculopathy progression. Prospective studies of the complement system in moyamoya disease may be able to demonstrate the activation pathway. Such information might guide development new treatment strategies for moyamoya disease.
Abbreviations
C3, complement 3; C4, complement 4; C-index, concordance index; DCA, decision curve analysis; DSA, digital subtraction angiography; mRS, modified Rankin scale; MRI, magnetic resonance imaging; MMD, moyamoya disease; PCI, posterior cerebral involvement.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant numbers 82171280 and 82172021). Science and Technology Commission Project (2019-JCJQ-ZD-195-00).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Suzuki J, Takaku A, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20(3):288–299. doi:10.1001/archneur.1969.00480090076012
2. Lin R, Xie Z, Zhang J, et al. Clinical and immunopathological features of Moyamoya disease. PLoS One. 2012;7(4):e36386. doi:10.1371/journal.pone.0036386
3. Liu W, Morito D, Takashima S, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6(7):e22542. doi:10.1371/journal.pone.0022542
4. Okazaki S, Morimoto T, Kamatani Y, et al. Moyamoya disease susceptibility variant RNF213 p.R4810K increases the risk of ischemic stroke attributable to large-artery atherosclerosis. Circulation. 2019;139(2):295–298. doi:10.1161/CIRCULATIONAHA.118.038439
5. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. New Engl J Med. 2009;360(12):1226–1237. doi:10.1056/NEJMra0804622
6. Yamashita M, Oka K, Tanaka K. Histopathology of the brain vascular network in moyamoya disease. Stroke. 1983;14(1):50–58. doi:10.1161/01.STR.14.1.50
7. Masuda J, Ogata J, Yutani C. Smooth muscle cell proliferation and localization of macrophages and T cells in the occlusive intracranial major arteries in moyamoya disease. Stroke. 1993;24(12):1960–1967. doi:10.1161/01.STR.24.12.1960
8. Duan L, Wei L, Tian Y, et al. Novel susceptibility loci for moyamoya disease revealed by a Genome-Wide Association Study. Stroke. 2018;49(1):11–18. doi:10.1161/STROKEAHA.117.017430
9. Kanamori F, Yokoyama K, Ota A, et al. Transcriptome-wide analysis of intracranial artery in patients with moyamoya disease showing upregulation of immune response, and downregulation of oxidative phosphorylation and DNA repair. Neurosurgical Focus. 2021;51(3):E3. doi:10.3171/2021.6.FOCUS20870
10. Otten EG, Werner E, Crespillo-Casado A, et al. Ubiquitylation of lipopolysaccharide by RNF213 during bacterial infection. Nature. 2021;594(7861):111–116. doi:10.1038/s41586-021-03566-4
11. Ueno M, Oka A, Koeda T, Okamoto R, Takeshita K. Unilateral occlusion of the middle cerebral artery after varicella-zoster virus infection. Brain Dev. 2002;24(2):106–108. doi:10.1016/S0387-7604(02)00005-0
12. Tashiro R, Niizuma K, Kasamatsu J, et al. Dysregulation of Rnf 213 gene contributes to T cell response via antigen uptake, processing, and presentation. J Cell Physiol. 2021;236(11):7554–7564. doi:10.1002/jcp.30396
13. Sigdel TK, Shoemaker LD, Chen R, et al. Immune response profiling identifies autoantibodies specific to Moyamoya patients. Orphanet J Rare Dis. 2013;8(1):45. doi:10.1186/1750-1172-8-45
14. Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov. 2015;14(12):857–877. doi:10.1038/nrd4657
15. Truedsson L, Bengtsson AA, Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. 2007;40(8):560–566. doi:10.1080/08916930701510673
16. Chen M, Jayne DRW, Zhao MH. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol. 2017;13(6):359–367. doi:10.1038/nrneph.2017.37
17. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19(8):517–532. doi:10.1038/s41577-019-0160-5
18. Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost. 2011;9(3):428–440. doi:10.1111/j.1538-7836.2010.04172.x
19. Asselman C, Hemelsoet D, Eggermont D, Dermaut B, Impens F. Moyamoya disease emerging as an immune-related angiopathy. Trends Mol Med. 2022;28(11):939–950. doi:10.1016/j.molmed.2022.08.009
20. Kato M, Kudo Y, Hatase M, et al. Moyamoya Disease Associated with a Deficiency of Complement Component 6. J Stroke Cerebrovasc Dis. 2022;31(8):106601. doi:10.1016/j.jstrokecerebrovasdis.2022.106601
21. Troedson C, Wong M, Dalby-Payne J, et al. Systemic lupus erythematosus due to C1q deficiency with progressive encephalopathy, intracranial calcification and acquired moyamoya cerebral vasculopathy. Lupus. 2013;22(6):639–643. doi:10.1177/0961203313486950
22. Fujimura M, Tominaga T, Kuroda S, et al. 2021 Japanese Guidelines for the Management of Moyamoya Disease: guidelines from the Research Committee on Moyamoya Disease and Japan Stroke Society. Neurol Med Chir. 2022;62(4):165–170. doi:10.2176/jns-nmc.2021-0382
23. Ahn SH, Lee J, Kim YJ, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke. 2015;46(3):697–703. doi:10.1161/STROKEAHA.114.008181
24. Mugikura S, Takahashi S, Higano S, Shirane R, Sakurai Y, Yamada S. Predominant involvement of ipsilateral anterior and posterior circulations in moyamoya disease. Stroke. 2002;33(6):1497–1500. doi:10.1161/01.STR.0000016828.62708.21
25. Matsushima T, Inoue T, Suzuki SO, Fujii K, Fukui M, Hasuo K. Surgical treatment of moyamoya disease in pediatric patients--comparison between the results of indirect and direct revascularization procedures. Neurosurgery. 1992;31(3):401–405. doi:10.1227/00006123-199209000-00003
26. Leong KW, Ding JL. The unexplored roles of human serum IgA. DNA Cell Biol. 2014;33(12):823–829. doi:10.1089/dna.2014.2639
27. Mkaddem SB, Christou I, Rossato E, Berthelot L, Lehuen A, Monteiro RC. IgA, IgA receptors, and their anti-inflammatory properties. Curr Top Microbiol Immunol. 2014;382:221–235. doi:10.1007/978-3-319-07911-0_10
28. Yeon JY, Shin HJ, Kong DS, et al. The prediction of contralateral progression in children and adolescents with unilateral moyamoya disease. Stroke. 2011;42(10):2973–2976. doi:10.1161/STROKEAHA.111.622522
29. Rahimi K, Bidel Z, Nazarzadeh M. Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet. 2021;398(10305):1053–1064. doi:10.1016/S0140-6736(21)01921-8
30. Webb AJS, Werring DJ. New insights into cerebrovascular pathophysiology and hypertension. Stroke. 2022;53(4):1054–1064. doi:10.1161/STROKEAHA.121.035850
31. Sreenivasan SA, Suri A, Raheja A, et al. Effect of age, stage, and type of surgical revascularization on clinical and angiographic outcome in moyamoya disease - experience from a case series of 175 revascularization procedures. Neurol India. 2022;70(5):2072–2081. doi:10.4103/0028-3886.359200
32. Geisbrecht BV, Lambris JD, Gros P. Complement component C3: a structural perspective and potential therapeutic implications. Semin Immunopathol. 2022;59:101627. doi:10.1016/j.smim.2022.101627
33. Ma Y, Liu Y, Zhang Z, Yang GY. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis. 2019;10(2):429–462. doi:10.14336/AD.2019.0119
34. Buono C, Come CE, Witztum JL, et al. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105(25):3025–3031. doi:10.1161/01.CIR.0000019584.04929.83
35. Garcia-Arguinzonis M, Diaz-Riera E, Pena E, et al. Alternative C3 complement system: lipids and atherosclerosis. Int J Mol Sci. 2021;23(1):22. doi:10.3390/ijms23010022
36. Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR. Moyamoya disease: a summary. Neurosurgical Focus. 2009;26(4):E11. doi:10.3171/2009.1.FOCUS08310
37. Gotz P, Braumandl A, Kubler M, et al. C3 deficiency leads to increased angiogenesis and elevated pro-angiogenic leukocyte recruitment in ischemic muscle tissue. Int J Mol Sci. 2021;22(11):5800. doi:10.3390/ijms22115800
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.