Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Hepatectomy After Conversion Therapy with Hepatic Arterial Infusion Chemotherapy, Tyrosine Kinase Inhibitors and Anti-PD-1 Antibodies for Initially Unresectable Hepatocellular Carcinoma
Authors Yu B, Zhang N, Feng Y, Zhang Y , Zhang T, Wang L
Received 24 July 2023
Accepted for publication 27 September 2023
Published 4 October 2023 Volume 2023:10 Pages 1709—1721
DOI https://doi.org/10.2147/JHC.S432062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Bingran Yu, Ning Zhang, Yun Feng, Yongfa Zhang, Ti Zhang, Lu Wang
Department of Hepatic Surgery, Shanghai Cancer Center, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China
Correspondence: Lu Wang, Department of Hepatic Surgery, Shanghai Cancer Center, Shanghai Medical College, Fudan University, No. 270 Dongan Road, Shanghai, 200032, People’s Republic of China, Email [email protected]
Background: Most patients with hepatocellular carcinoma (HCC) are not candidates for liver resection. We investigated the clinicopathological characteristics and prognosis of patients with initially unresectable HCC who underwent hepatectomy after conversion therapy with hepatic arterial infusion chemotherapy (HAIC), tyrosine kinase inhibitors (TKIs), and anti-PD-1 antibodies.
Materials and Methods: Patients with initially unresectable HCC who received HAIC combined with TKIs and anti-PD-1 antibodies followed by hepatectomy between December 2020 and December 2022, were retrospectively analyzed. Patient characteristics, tumor characteristics, treatment efficacy, perioperative characteristics, pathological characteristics, and survival outcomes were summarized and analyzed.
Results: 67 patients were enrolled in this study. Patients were treated with 3 sessions (range:2– 6 sessions) of combination therapy and were performed with hepatectomy in 4 months (range:1.4– 17.8 months) after the initiation of the combination therapy. The median size of tumor shrinkage was 4.7 cm (range:0.9– 11.7 cm). A pathological complete response (pCR) was achieved in 34.3% of the patients (n = 23). The median recurrence-free survival (RFS) was 19.3 months and the median overall survival (OS) was 28.7 months. Patients who achieved pCR had a better RFS (P = 0.004) and those without microscopic vascular invasion (MVI) had a better prognosis (RFS, P = 0.011; OS, P = 0.023). Multivariable logistic analysis revealed that the tumor number was associated with pCR.
Conclusion: Hepatectomy after conversion therapy with HAIC, TKIs, and anti-PD-1 antibodies is a feasible treatment strategy for patients with unresectable HCC. This treatment strategy is associated with a promising prognosis.
Keywords: hepatectomy, conversion therapy, hepatocellular carcinoma, pathological complete response
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of cancer-related deaths worldwide.1 Radical treatments including liver resection, liver transplantation, and radiofrequency ablation have been considered for patients with early-stage HCC.2 However, most patients are diagnosed at intermediate or advanced stages when they are not candidates for curative resection.3,4 Despite various therapies for advanced HCC, including interventional therapies, tyrosine kinase inhibitors (TKIs), and anti-PD-1 antibodies, survival remains poor, with a 5-year survival rate of approximately 18%.1
Owing to the development of treatment strategies for HCC, conversion therapy, with the aim of achieving tumor downstaging and transforming initially unresectable tumors or borderline resectable tumors into resectable tumors, has become more common.5,6 The modalities of conversion therapy include transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiotherapy, TKIs, anti-PD-1 antibodies, and combined therapy based on locoregional and systemic treatments.6–9 The combination of TKIs and anti-PD-1 antibodies was reported to be a feasible conversion therapy for patients with unresectable HCC to become resectable, and salvage hepatectomy after downstaging unresectable HCC was associated with favorable long-term outcomes.9–12
Hepatic arterial infusion chemotherapy (HAIC) is widely utilized as an effective treatment for HCC in China, Japan, and South Korea.13,14 HAIC alone, the combination of HAIC with TKIs, and HAIC combined with TKIs and anti-PD-1 antibodies were reported to have an overall response rate (ORR) of 36.0%-70.6%.13–17 Furthermore, 4 of 36 patients and 9 of 71 patients were reported to undergo curative surgery due to tumor downstaging by the combination of HAIC with TKIs and anti-PD-1 antibodies in studies by Lai et al16 and He et al,17 respectively. These favorable results have prompted the use of this combination therapy in the conversion therapy for HCC. However, no study has reported detailed data including pretreatment, preoperative, operative, and pathological characteristics and prognosis of patients with initially unresectable HCC receiving hepatectomy after conversion therapy with HAIC, TKIs, and anti-PD-1 antibodies.
Here, we report 67 patients with initially unresectable HCC who were treated with a combination of HAIC with TKIs and anti-PD-1 antibodies followed by curative resection. We summarized the clinicopathological characteristics and analyzed the survival outcomes of these patients.
Materials and Methods
Study Design and Patients
This single-center retrospective study included patients with initially unresectable HCC receiving HAIC combined with TKIs and anti-PD-1 antibodies, followed by sequential curative resection due to tumor downstaging at Fudan University Shanghai Cancer Center (FUSCC) between December 2020 and December 2022. This study was conducted in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of FUSCC (Approval No. 1612167–18). The criteria of unresectable HCC were: (1) multiple tumors with distribution of bilateral lobe; (2) insufficient remnant liver volume after hepatectomy (<40% for patients with liver cirrhosis; <30% for patients with liver cirrhosis); (3) concomitant bilateral portal vein tumor thrombosis, or main portal vein tumor thrombosis, or inferior vena cava tumor thrombosis; (4) R0 resection is technically difficult; and (5) unresectable HCC judged by experienced multidisciplinary clinicians.
Combination Therapy
The Seldinger technique was used to puncture the femoral artery and a catheter was inserted into the feeding hepatic artery under digital subtraction angiography guidance. The HAIC chemotherapeutic regimen (oxaliplatin 85 mg/m2 from hour 0 to 2 on day 1, leucovorin 400 mg/m2 from hour 2 to 3 on day 1, 5-fluorouracil 400 mg/m2 bolus at hour 3, and 2400 mg/m2 over 46h on days 1 and 2) was infused via catheter. TKIs were administered above 1 week prior to the initial HAIC and discontinued one day before each session of HAIC until the time of catheter withdrawal. Anti-PD-1 antibodies were administered intravenously within 2 days of HAIC initiation. The TKIs used in this study were lenvatinib (12 mg/day for body weight ≥ 60 kg or 8 mg/day for body weight < 60 kg), donafenib (200 mg twice daily), and apatinib (250 mg/day), and the anti-PD-1 antibodies were tislelizumab (200 mg), sintilimab (200 mg), and camrelizumab (200 mg). The patients were treated with a combination of HAIC, TKIs and anti-PD-1 antibodies every 3 weeks. The combination of TKIs and anti-PD-1 antibodies used in the study were listed in Supplementary Table 1.
Clinical Assessment
Complete blood count, liver function, renal function, coagulation function, and tumor marker levels were monitored every three weeks before HAIC. Tumor response was evaluated using contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT) every 6 weeks and assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST (mRECIST).18,19 Treatment-related adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Liver Resection
After combination therapy, patients underwent curative surgery when meeting the following conditions: (1) R0 resection could be performed with sufficient remnant liver volume and function; (2) patients achieved complete response (CR), partial response (PR), or stable disease (SD); (3) no serious or persistent adverse events occurred due to combination therapy; and (4) no surgical contraindications were observed.
Major hepatectomy was defined as resection of three or more Couinaud segments or resection of the right posterior and anterior sections because of the unique techniques required.20 Posthepatectomy liver failure (PHLF) was diagnosed based on an increased international normalized ratio and concomitant hyperbilirubinemia on or after postoperative day 5 and graded based on its impact on clinical management.21 Postoperative complications were classified using the Clavien-Dindo classification.22 Pathological complete response (pCR) was defined as the absence of residual viable tumor cells on hematoxylin and eosin staining of slide sections from completely resected primary tumors, tumor thrombosis, and metastatic lesions.
Postoperative Management
Patients underwent 1 session of HAIC and TKIs for 3 months and 4 sessions of anti-PD-1 antibodies a month after surgery. Follow-up laboratory examinations (complete blood count, liver function, renal function, coagulation function, and tumor marker levels) and imaging examinations (contrast-enhanced CT/MRI) were performed every 2–3 months.
Statistical Analyses
Statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 9.4.1. Continuous variables are expressed as median and ranges. Disease-free survival (DFS) was defined as the interval from the date of surgery to the date of tumor recurrence or death from any cause. Overall survival (OS) was defined as the interval from the date of combination therapy initiation to the date of the patient’s death. DFS and OS were calculated using the Kaplan-Meier method and compared using the log-rank method. Clinicopathological characteristics between the groups were compared using the chi-square test or Fisher’s exact test. The Cox proportional hazards model was used to analyze the factors associated with survival. Binary logistic regression analysis was used to identify factors associated with pathological response. Statistically significant factors (P < 0.05) in the univariate analysis were candidates for entry into the multivariate Cox proportional hazards model and binary logistic regression analysis. All P values were two-sided, with P values <0.05 considered significant.
Results
Patient Characteristics
67 patients with initially unresectable HCC were treated with a combination of HAIC with TKIs and anti-PD-1 antibodies, and underwent curative resection due to tumor shrinkage and downstaging between December 2020 and December 2022. Patient demographics are listed in Table 1. Most of the patients (91.0%) were male. The median age was 52 years (range:26–74 years). 35 patients were graded as having an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0. Of these patients, 76.1% had hepatitis B virus (HBV). The Child-Pugh score of most patients (86.6%) was 5. Of the patients, 67.2% were classified as ALBI grade 1 and 22 patients were classified as ALBI grade 2.
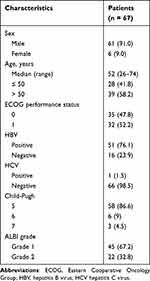 |
Table 1 Patient Characteristics |
Tumor Characteristics and Treatment-Related Characteristics
Tumor characteristics and treatment efficacy of the patients are summarized in Table 2. The median treatment session was 3 sessions (range:2–6 sessions), with a median interval between the date of the first session and the date of surgery of 4 months (range:1.4–17.8 months). Multiple tumors were observed in 62.7% of the patients, and 61.2% of the tumors were located in one lobe of the liver. Of the patients, 49.3% had portal vein tumor thrombosis (PVTT), including four with Vp2, 18 with Vp3 and 11 in Vp4. Only 2 patients had hepatic vein tumor thrombosis (HVTT). The median tumor size before treatment was 11.3 cm (range:3.8–23.0 cm) and the median preoperative tumor size was 7 cm (range:1.0–15.2 cm). The median tumor shrinkage was 4.7 cm after combination therapy (range:0.9–11.7 cm; Figure 1a and b). A total of 76.1% of the patients achieved PR based on the RECIST, while 16 patients achieved CR based on the mRECIST. Before combination therapy, 39 patients had AFP > 400 ng/mL, which reduced to 14 patients after combination therapy. A reduction in AFP was observed in all patients, with elevated APF reducing to normal in 29 patients after combination therapy (Figure 1c). The treatment-related AEs were listed in Supplementary Table 2. The most common AEs were elevated ALT (35.8%), elevated AST (34.3%), nausea (34.3%) and hypoalbuminemia (34.3%).
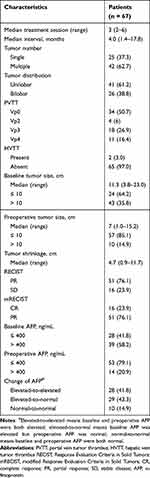 |
Table 2 Tumor Characteristics and Treatment Efficacy |
Figure 2 shows a representative case. The patient was diagnosed with HCC with a tumor size of 11.8 cm, invading the right and main branches of the portal vein (Figure 2a). The patient received three sessions of HAIC, lenvatinib, and sintilimab. The intrahepatic lesion and PVTT significantly decreased, and the AFP reduced from 2189 ng/mL to 2.98 ng/mL (Figure 2b). The patient underwent right hemihepatectomy, and pathological examination of the surgically resected specimen revealed a pCR (Figure 2c).
Perioperative Characteristics
The perioperative characteristics of patients are shown in Table 3. Major hepatectomy was performed in 52.5% of the patients. Portal triad clamping was performed in 52 patients with a median time of 15 minutes (range:0–40 minutes). The median intraoperative blood loss was 300 mL (range:50–3000 mL), and 11 patients required intraoperative blood transfusion. Postoperative complications were observed in 29 patients, including 24 patients with grade I, 3 patients with grade II, 1 patient with grade IV, and 1 patient with grade V. Besides, 35 patients present with PHLF: 33 patients with grade A, 1 patient with grade B, and 1 patient with grade C. One patient died of an abdominal infection and multiple organ failure after surgery. The median postoperative hospital stay was 6 days (range:4–21 days).
 |
Table 3 Surgical Characteristics |
Pathological Characteristics
The pathological characteristics of patients are shown in Table 4. Thirty patients had tumor capsules and only 13 patients had satellite lesions. In the assessment of microscopic vascular invasion (MVI), 45 patients were diagnosed with M0, and 22 patients were diagnosed with M1/M2. After extensive tumor tissue sampling, no viable tumor cells were found in 23 patients who were diagnosed with pCR.
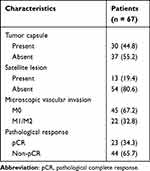 |
Table 4 Pathological Characteristics |
Survival Outcomes
After a median follow-up of 15.9 months (range:2.5–37.1 months), tumor recurrence was detected in 20 patients, and seven patients died from tumor progression. The median DFS was 19.3 months and the median OS was 28.7 months. Patients whose baseline and preoperative APF levels were both normal had the worst DFS (P = 0.024; Figure 3a). Compared to patients with PR based on mRECIST, those with CR had better DFS (not reached vs 13.7 months, P = 0.036; Figure 3c). A better DFS was also found in patients with M0 than in those with M1/M2 (not reached vs 11.6 months, P = 0.011; Figure 3e). Furthermore, patients with pCR had a better DFS than those without pCR (not reached vs 11.6 months, P = 0.004; Figure 3g). However, no difference of OS was observed between patients with different change of AFP (P = 0.711; Figure 3b), or with different treatment efficacy based on mRECIST (Not reached vs 28.7 months, P = 0.660; Figure 3d) or different pathological responses (not reached vs 28.7 months, P = 0.467; Figure 3h). Patients with M0 had better OS than those with M1/M2 (not reached vs 24.4 months, P = 0.023; Figure 3f). In multivariate analyses of DFS, pathological response was a prognostic factor (Table 5).
 |
Table 5 Univariate and Multivariate Analyses of Variables Associated with Recurrence-Free Survival |
Analyses of Factors Associated with pCR
The clinical characteristics of the patients with pCR or non-pCR are summarized in Supplementary Table 3. Patients with pCR had more single tumors (69.6% vs 20.5%, P < 0.001) and unilobar tumors (78.3% vs 52.3%, P = 0.038) than those without non-pCR. Furthermore, patients with pCR had more PVTT than those without pCR (65.2% vs 38.6%, P = 0.038). In multivariate analysis, only tumor number was identified as an independent risk factor associated with pCR (HR, 0.146; 95% CI, 0.044–0.490; P = 0.002; Supplementary Table 4).
Discussion
To our knowledge, this is the first and largest cohort study to report the clinicopathological characteristics and survival outcomes of patients with initially unresectable HCC who underwent curative surgery after conversion therapy with HAIC with TKIs and anti-PD-1 antibodies. The favorable survival rate of patients suggests that this treatment strategy is feasible for patients with initially unresectable HCC.
The combination of HAIC with TKIs and anti-PD-1 antibodies was first reported for the treatment of advanced HCC by He et al,17 with an ORR of 67.6%. A Phase II trial of HAIC combined with lenvatinib and toripalimab also confirmed the encouraging antitumor activity of this combination therapy in advanced HCC.16 Furthermore, HAIC, TKIs, and anti-PD-1 antibodies have a synergistic effect on the antitumor activity.23 These theories and the results of these studies support the application of HAIC combined with TKIs and anti-PD-1 antibodies for the treatment of HCC. At our center, this combination therapy has been widely used as a treatment modality for patients with advanced HCC. A total of 487 patients with initially unresectable HCC were treated with HAIC, TKIs, and anti-PD-1 antibodies, and 67 patients underwent surgery because of tumor shrinkage and downstaging after combination therapy. The conversion rate was 13.7%, which was better than 11.1% and 12.7% reported in previous studies.16,17 Furthermore, the tumor size and AFP levels significantly decreased, suggesting that the combination of HAIC with TKIs and anti-PD-1 antibodies is an effective treatment for initially unresectable HCC.
The current study supports the feasibility and potential benefit of hepatectomy after conversion therapy with HAIC, TKIs, and anti-PD-1 antibodies in patients with initially unresectable HCC who respond to combination therapy. In our study, the median OS was 28.7 months, which was better than 19.2 months reported in the IMbravel 15024 and 17.9 months reported in a phase II trial on lenvatinib and toripalimab plus HAIC.16 The difference in OS demonstrated the effect of hepatectomy after combination therapy on the survival of patients with advanced HCC. Resection of intrahepatic tumors following combination therapy was also safe, despite the fact that more than half of the patients underwent major hepatectomy in our study. Most patients recovered from postoperative complications following symptomatic treatment. Furthermore, we thought that the tumors had to be removed even when they were completely necrotic after the combination therapy because residual viable intrahepatic lesions could develop resistance to combination therapy, thus resulting in disease progression. There is a discrepancy between radiological and pathological characteristics.23,25 The pathological characteristics could help clinicians accurately evaluate the efficacy of combination therapy based on the proportion of tumor necrosis and develop appropriate postoperative treatment strategies for patients.26
We analyzed the factors influencing survival after surgical resection and obtained intriguing results. AFP-secreting HCC is more aggressive and has a worse prognosis than HCC without AFP production.27 Our study demonstrated that patients with both normal baseline and preoperative APF had the worst DFS, which has not been previously investigated. pCR was observed in 23 patients whose DFS was better than that of those without pCR. This not only indicated that HCC had been cured by combination therapy, but also showed that tumor response after combination therapy played a vital role in tumor recurrence. However, no difference in OS was observed between the patients with and without pCR. These results were also reported by Majno et al,28 in which patients with total tumor necrosis had better DFS, instead of OS, when compared to non-responders. Furthermore, MVI is a critical determinant of early recurrence and survival in HCC.29 In our study, better DFS was observed in patients without MVI, and MVI was the only prognostic factor for OS. Therefore, we hypothesized that patients with MVI should receive postoperative adjuvant treatment to prevent tumor recurrence and initiated a clinical trial on postoperative adjuvant treatment for patients with MVI (NCT05311319).
pCR has been reported to be associated with excellent survival outcomes in patients with HCC treated with TACE or TKIs combined with anti-PD-1 antibodies.30–32 Various factors influenced pCR, including HBV DNA load ≤ 1×102 IU/mL, AFP ≤ 20 ng/mL, maximum tumor size ≤ 5 cm, multiple preoperative TACE sessions, and CR based on mRECIST.33 In a study by Huang et al,31 AFP before surgery, AFP response (a change from a positive status at baseline to a negative status before resection), and radiographic response were predictors of pCR in patients with HCC who received TKIs plus anti-PD-1 antibodies. In the current study, more single tumors, unilobar tumors, and PVTT were observed in patients with pCR, and multivariable logistic regression revealed that tumor number was related to pCR. In fact, we found that effective treatment is usually observed in patients with single large tumors after combination therapy with HAIC, TKIs, and anti-PD-1 antibodies in clinical practice. We hypothesized that necrosis occurred in the large tumor because of the tumor blood supply before treatment, and more drugs could accumulate in a single tumor, thus leading to total tumor necrosis after the combination therapy. Further studies are required to confirm these results.
Our study had several limitations. First, this was a single-center retrospective study with a small sample size, which could have led to a selection bias. Second, combination therapy with HAIC, TKIs, and anti-PD-1 antibodies has not been officially approved, and different TKIs and anti-PD-1 antibodies have been used. Finally, the follow-up period was relatively short, and some important results will be observed after a long follow-up period. Considering these limitations, more multicenter and large-scale clinical trials are needed to explore the role of HAIC combined with TKIs and anti-PD-1 antibodies followed by hepatectomy in the treatment of initially unresectable HCC.
In conclusion, our study confirmed that the combination of HAIC with TKIs and anti-PD-1 antibodies could convert unresectable HCC into resectable HCC. Patients who underwent hepatectomy after combination therapy achieved favorable survival outcomes. Changes in AFP levels, microscopic vascular invasion, and pCR were prognostic factors for patients. The tumor number was associated with pCR after combination therapy with HAIC, TKIs, and anti-PD-1 antibodies in patients with HCC.
Abbreviations
HCC, Hepatocellular carcinoma; TKIs, tyrosine kinase inhibitor; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; HAIC, Hepatic arterial infusion chemotherapy; ORR, overall response rate; FUSCC, Fudan University Shanghai Cancer Center; MRI, magnetic resonance imaging; CT, computed tomography; RECIST, Response Evaluation Criteria in Solid Tumors; mRECIST, modified Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PHLF, posthepatectomy liver failure; pCR, pathological complete response; DFS, Disease-free survival; OS, Overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; PVTT, portal vein tumor thrombosis; HVTT, hepatic vein tumor thrombosis; MVI, microscopic vascular invasion.
Data Sharing Statement
Data from the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The patients in the FUSCC cohort included in the study signed informed consent forms. The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center (Approval No. 1612167-18).
Consent for Publication
Written informed consent was obtained from all patients for being included in the study.
Acknowledgments
We thank all patients included for their contributions to the present study.
Funding
This work was jointly supported by the National Natural Science Foundation of China (81874182), National Science and Technology Major Project (2018ZX10723204-007-004), Shanghai Municipal Health Bureau (201940043), and the Shanghai Hospital Development Center (SHDC12019X19).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
3. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
4. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2021. doi:10.1016/j.jhep.2021.11.018
5. Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15(3):155–160. doi:10.5582/bst.2021.01091
6. Sun HC, Zhu XD. Downstaging conversion therapy in patients with initially unresectable advanced hepatocellular carcinoma: an overview. Front Oncol. 2021;11:772195. doi:10.3389/fonc.2021.772195
7. Tang ZY, Yu YQ, Zhou XD, et al. Treatment of unresectable primary liver cancer: with reference to cytoreduction and sequential resection. World J Surg. 1995;19(1):47–52. doi:10.1007/BF00316979
8. Shi X-J, Jin X, Wang M-Q, et al. Effect of resection following downstaging of unresectable hepatocelluar carcinoma by transcatheter arterial chemoembolization. Chin Med J. 2012;125(2):197–202. doi:10.3760/cma.j.issn.0366-6999.2012.02.007
9. Zhang Y, Huang G, Wang Y, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist. 2016;21(12):1442–1449. doi:10.1634/theoncologist.2016-0094
10. Yi Y, Sun B-Y, Weng J-L, et al. Lenvatinib plus anti-PD-1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:1046584. doi:10.3389/fonc.2022.1046584
11. Tang H, Cao Y, Jian Y, et al. Conversion therapy with an immune checkpoint inhibitor and an antiangiogenic drug for advanced hepatocellular carcinoma: a review. Biosci Trends. 2022;16(2):130–141. doi:10.5582/bst.2022.01019
12. Zhu X-D, Huang C, Shen Y-H, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. 2021;10(4):320–329. doi:10.1159/000514313
13. Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, Phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–432. doi:10.1016/s2468-1253(18)30078-5
14. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
15. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
16. Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi:10.1016/j.ejca.2022.07.005
17. He M-K, Liang R-B, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
20. Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg. 2013;257(2):205–213. doi:10.1097/SLA.0b013e31827da7fe
21. Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–724. doi:10.1016/j.surg.2010.10.001
22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
23. Gan L, Lang M, Tian X, et al. A retrospective analysis of conversion therapy with lenvatinib, sintilimab, and arterially-directed therapy in patients with initially unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:673–686. doi:10.2147/JHC.S404675
24. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
25. Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007;14(12):3301–3309. doi:10.1245/s10434-007-9549-7
26. Fan J, Tang ZY, Yu YQ, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15(6):674–678. doi:10.1159/000018676
27. He H, Chen S, Fan Z, et al. Multi-dimensional single-cell characterization revealed suppressive immune microenvironment in AFP-positive hepatocellular carcinoma. Cell Discov. 2023;9(1):60. doi:10.1038/s41421-023-00563-x
28. Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226(6):688–701. doi:10.1097/00000658-199712000-00006
29. Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi:10.1245/s10434-019-07227-9
30. Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63(1):83–92. doi:10.1016/j.jhep.2015.01.023
31. Huang C, Zhu XD, Shen YH, et al. Radiographic and alpha-fetoprotein response predict pathologic complete response to immunotherapy plus a TKI in hepatocellular carcinoma: a multicenter study. BMC Cancer. 2023;23(1):416. doi:10.1186/s12885-023-10898-z
32. Habibollahi P, Shamchi SP, Choi JM, et al. Association of complete radiologic and pathologic response following locoregional therapy before liver transplantation with long-term outcomes of hepatocellular carcinoma: a retrospective study. J Vasc Interv Radiol. 2019;30(3):323–329. doi:10.1016/j.jvir.2018.11.037
33. Lin J, Li X, Shi X, et al. Nomogram for predicting pathologic complete response after transarterial chemoembolization in patients with hepatocellular carcinoma. Ann Transl Med. 2021;9(14):1130. doi:10.21037/atm-21-1120
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



