Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Healthcare Resource Utilization, Exacerbations, and Readmissions Among Medicare Patients with Chronic Obstructive Pulmonary Disease After Long-Acting Muscarinic Antagonist Therapy Initiation with Soft Mist versus Dry Powder Inhalers
Authors Singer D, Bengtson LG, Elliott C, Buikema AR, Franchino-Elder J
Received 1 October 2020
Accepted for publication 16 November 2020
Published 7 December 2020 Volume 2020:15 Pages 3239—3250
DOI https://doi.org/10.2147/COPD.S284678
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
David Singer,1 Lindsay GS Bengtson,2 Caitlin Elliott,2 Ami R Buikema,2 Jessica Franchino-Elder1
1Health Economics and Outcomes Research, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA; 2Health Economics and Outcomes Research, Optum, Eden Prairie, MN, USA
Correspondence: David Singer
Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA
Tel +1-203-791-6409
Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is often managed with inhaled long-acting muscarinic antagonists (LAMAs), yet real-world data on healthcare resource utilization (HRU) by inhaler type are lacking. This study compared HRU after LAMA initiation with a soft mist inhaler (SMI) versus a dry powder inhaler (DPI).
Patients and Methods: Inclusion criteria were COPD diagnosis, age ≥ 40 years, LAMA initiation (index date = first LAMA SMI or DPI claim 9/1/14— 6/30/18), and Medicare Advantage enrollment 1 year pre-index (baseline) to ≥ 30 days post-index (follow-up). Patients were followed to the earliest of discontinuation, switch, disenrollment, 1 year, or study end (7/31/18). Exclusion criteria were asthma, cystic fibrosis, or lung cancer diagnoses, unavailable demographics, multiple index LAMAs, or baseline LAMA use. Cohorts (SMI or DPI) were balanced on baseline characteristics using inverse probability of treatment weighting. Outcomes included per patient per month (PPPM) COPD-related HRU encounters, and exacerbations (defined as moderate [ambulatory visit with corticosteroid or antibiotic within ± 7 days] or severe [emergency visit or inpatient admission]); and 30-day readmissions following COPD-related hospitalizations.
Results: After weighting, cohorts (SMI [n=5360] and DPI [n=22,880]) were similar in age (72 and 73 years, respectively), gender (both 52% female), and COPD severity score (31.3 and 31.5, respectively). Cohorts had similar counts of follow-up HRU encounters. However, the SMI cohort had fewer (mean ± standard deviation) COPD-related exacerbations (0.054± 0.082 vs DPI cohort 0.059± 0.088 PPPM, p< 0.001) overall. Moreover, the SMI cohort had fewer severe exacerbations (0.030± 0.058 vs DPI: 0.034± 0.065 PPPM, p< 0.001). Hospitalizations among SMI patients had a lower adjusted odds of readmission versus hospitalizations among DPI patients (odds ratio: 0.656, 95% confidence interval= 0.460, 0.937; p=0.020).
Conclusion: SMI initiators had significantly fewer COPD-related exacerbations than DPI initiators. In addition, lower odds of readmissions were observed following COPD-related hospitalizations among the SMI cohort, as compared with the DPI cohort.
Keywords: chronic obstructive pulmonary disease, COPD, long-acting muscarinic antagonist, LAMA, soft mist inhaler, SMI, dry powder inhaler, DPI, healthcare resource utilization, readmissions, exacerbations
Introduction
Patients with moderate or severe chronic obstructive pulmonary disease (COPD) are often prescribed one or more long-acting bronchodilators, which are central to optimal symptom management and prevention of exacerbations.1 Long-acting muscarinic antagonists (LAMAs) are one class of bronchodilators, which are commonly administered using a soft mist inhaler (SMI) or dry powder inhaler (DPI).2 LAMAs delivered using each inhaler type have been found to be safe and effective in clinical trials, as compared with placebo. The key differences in an SMI inhaler, as compared with DPIs, include higher fine particle fraction and deposition in the lungs, and longer plume duration.3 These differences may be particularly important for patients with insufficient inspiratory flow or difficulties coordinating actuation and inspiration.2,4 In fact, SMIs have been shown to deliver a higher proportion of drug to the lungs than DPIs, independent of inspiratory flow rate.5,6 However, few studies have examined real-world outcomes directly comparing the two inhaler types.
Tiotropium is a LAMA that is available in both SMI and DPI inhaler types, branded as Respimat® and HandiHaler®, respectively. These inhalers have each been studied extensively, with both Respimat and HandiHaler tiotropium associated with improvements in lung function, symptoms, and quality of life, each as compared with placebo.7–10 The TIOSPIR® study, the only randomized controlled clinical trial directly comparing the two inhalers for administration of tiotropium, found no statistically significant differences in the primary endpoint measures: mortality and time to first exacerbation.11 Scarce data are published which compare patients and outcomes in clinical practice settings with an SMI versus DPI, and none to date include a US patient sample.12,13 Relative to clinical trials, real-world studies fill an evidence gap, given their different study design, setting, sample, and often, outcomes. The results of real-world studies also may be generalizable to a larger population of patients with COPD, as compared with randomized controlled clinical trials, and may be more reflective of actual clinical practice. As COPD is a leading cause of death worldwide, affecting at least 170 million people,14 understanding treatment outcomes for a broad spectrum of affected patients is critical to optimizing appropriate therapeutic approaches.
Furthermore, COPD-related exacerbations—acute worsening of COPD symptoms resulting in costly hospitalization—are the major driver of continuing increases in COPD healthcare costs; yet, exacerbations can be reduced in frequency with appropriate use of maintenance therapy, which includes inhaled long-acting bronchodilators.15 To date, healthcare resource utilization (HRU) outcomes, including exacerbations and 30-day readmission following an inpatient stay, have not been compared in a real-world sample of patients initiating LAMA with SMI versus DPI inhalers. The objective of this study was to describe patients treated in routine clinical practice who initiated a LAMA SMI or a LAMA DPI, and to compare the following outcomes: HRU encounters, COPD-related exacerbations, and 30-day all-cause readmission after COPD-related hospitalization.
Methods
Data Sources
Administrative healthcare data were accessed via a proprietary database, the Optum Research Database (ORD). The ORD contains medical and pharmacy claims data (including linked enrollment data) from 1993 to the present, covering more than 73 million lives. Medical and pharmacy claims data are available for approximately 6.4 million enrollees in the Medicare Advantage with Part D program (MAPD) dating from 2006 to the present. The claims history includes all medical encounters occurring at all available sites and outpatient prescription pharmacy claims. Data obtained for this study were accessed using techniques compliant with the Health Insurance Portability and Accountability Act.
Study Design and Sample
This was a non-interventional retrospective database study using de-identified administrative claims data from 01 September 2013 through 31 July 2018 (Figure 1). Patients diagnosed with COPD who initiated LAMA treatment using an SMI or DPI inhaler, between 01 September 2014 and 30 June 2018 (identification period) were eligible for inclusion. An index date was set as the date of the first pharmacy claim for a LAMA SMI or DPI (aclidinium bromide; glycopyrrolate; tiotropium bromide; umeclidinium bromide) that met study criteria during the identification period. For inclusion, patients were required to be MAPD beneficiaries aged ≥40 years as of the index year; to have a COPD diagnosis code (Appendix Table A1) in any position on a medical claim during the identification period; and to have continuous enrollment with medical and pharmacy coverage for at least 12 months before and at least 30 days after the index date. Patients were excluded if they had at least one non-diagnostic medical claim with a diagnosis code for asthma, cystic fibrosis, or lung cancer (Appendix Table A2); incomplete demographic information; pharmacy claims for multiple index medications on the index date; a pharmacy claim for a LAMA/LABA single inhaler device on the index date; use of any LAMA (single or combination form) during the baseline period, excluding the index date; or <30 days use of index medication. COPD medications other than LAMA used at the index date did not disqualify a patient; in fact, non-LAMA medications could be used at any time during the study period. The 12-month baseline period, including the index date, was used to assess patient characteristics. Outcomes were observed from the day after LAMA initiation until the earliest of the following: index LAMA medication discontinuation; switch to another LAMA medication; disenrollment from the health plan; 12 months following the index date; or the end of the study period (31 July 2018). Discontinuation was defined as a gap in therapy of ≥90 days following the runout of days supply (discontinuation date = days supply + 90 days). Switch was defined as a pharmacy fill for at least a 30-day supply for a non-index LAMA medication. However, a pharmacy fill for a nebulized LAMA medication was not used to identify a switch, because administration of nebulized medications is observed on medical, not pharmacy, claims. The switch date was the first claim date of the non-index LAMA medication in the follow-up period.
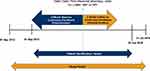 |
Figure 1 Study design. Abbreviations: LAMA, long-acting muscarinic antagonist; SMI, soft mist inhaler; DPI, dry powder inhaler. |
Measures
Baseline Patient Characteristics
Baseline demographic characteristics included patient age as of the index year, gender, and geographic region in accordance with the US Census Bureau’s designations.16
Baseline comorbidity burden was estimated using the Charlson Comorbidity Index (CCI) which calculates a score based upon diagnosis codes for comorbid conditions.17 Severity of COPD was estimated using the COPD severity score, derived from a validated algorithm accounting for current symptoms and therapies used.18 Diagnosis codes were observed during the baseline period to describe key comorbidities, such as congestive heart failure, diabetes, dyspnea, hypertension, ischemic heart disease, metabolic syndrome, myocardial infarction, stroke, pneumonia, or acute bronchitis/bronchiolitis (full list in Appendix Table A6). Evidence of current or prior tobacco use was identified with diagnosis codes for tobacco use disorder, smoking cessation procedure codes, and smoking cessation medications.
In addition, the prescribing provider specialty for the index LAMA medication was obtained hierarchically from the pharmacy claim or, if not available, from the baseline medical claim immediately preceding the index date. The provider specialty was classified as one of the following: pulmonology, primary care (family practice, general practice, and geriatrics), internal medicine, allied health professional, cardiology, other specialty, unknown specialty, and multiple. The number of patients who received services from a pulmonologist within 30 days pre-index date was noted. Baseline (not including the index date) COPD-related medication (Appendix Table A3) use was described based on pharmacy (for non-nebulized formulations) and medical claims (for administration of nebulized medications) for all COPD-related medications used during the baseline period: long-acting beta-agonist (LABA) monotherapy, inhaled corticosteroid (ICS) monotherapy, ICS/LABA combined in a single device, short-acting muscarinic antagonist (SAMA) monotherapy, short-acting beta-agonist (SABA) monotherapy, SAMA/SABA combined in a single device, methylxanthines, phosphodiesterase-4 inhibitors, oral corticosteroids (OCS), and guideline-recommended respiratory antibiotics.19 The proportion of patients having at least one pharmacy or medical claim for each medication during baseline was reported by for each cohort.
HRU Encounters and Costs
Baseline COPD-related HRU encounters and costs were calculated as count of events or $US, respectively, per patient per month (PPPM) for the following categories: emergency department (ED), inpatient hospitalization, and ambulatory (physician office and hospital outpatient) visits, and outpatient pharmacy fills. HRU and costs were defined as COPD-related if the claim had a diagnosis for COPD or pneumonia in any position or was a pharmacy claim for a medication used to treat COPD. Healthcare costs (sum of health plan-paid and patient-paid) were adjusted to 2018 $US using the medical care component of the Consumer Price Index.20 Baseline HRU and costs were included as covariates for the weighting procedure. During the follow-up period, COPD-related HRU was also reported as an outcome event count PPPM.
COPD-related exacerbations were calculated as PPPM for baseline and follow-up periods. A severe COPD-related exacerbation was defined by an inpatient admission or an ED visit with a COPD or pneumonia diagnosis code in any position. A moderate COPD-related exacerbation was defined by an ambulatory (office or outpatient) visit with a COPD or pneumonia diagnosis code in any position, plus a pharmacy claim for an OCS or COPD guideline-recommended antibiotic19 prescription within ±7 days of the noted visit. All COPD-related encounters meeting either definition of an exacerbation occurring within 14 days of each other were considered a single exacerbation, and were classified according to the highest severity contributing utilization. Baseline exacerbations were included as covariates for the weighting procedure.
Hospital Readmissions
Any (all-cause) hospital readmission within 30 days of a COPD-related acute inpatient hospital discharge was captured according to the Centers for Medicare and Medicaid Services (CMS) Hospital Readmission Reduction Program (HRRP) readmission definition.21 For each qualifying COPD-related hospitalization, an indicator variable was created to identify an all-cause readmission that started within 30 days of the discharge of the COPD-related hospitalization. Hospitalizations were classified as COPD-related if they met either of the following claims criteria for the acute portion of an inpatient stay: 1) at least one diagnosis code for COPD in the primary position any time; or 2) at least one diagnosis of respiratory failure in the primary position and a diagnosis of acute exacerbation of COPD in a later position on the same claim. COPD-related hospitalizations that ended because the patient discharged themselves against medical advice were excluded from the analysis, and all-cause hospitalizations that included scheduled procedures were not eligible to be a readmission. COPD-related hospitalizations that ended within 30 days of the end of follow-up were not included, because readmission status could not accurately be determined.
Statistical Analyses
Weighting
Patient demographics and baseline clinical characteristics were analyzed descriptively, stratified by SMI or DPI, before and after inverse probability of treatment weighting (IPTW). IPTW methodology has been used widely in observational studies to adjust for differences between patient study populations.22–24 IPTW weights were estimated by logistic regression models that incorporated potential predictors of treatment initiation as independent variables with cohort (SMI vs DPI) as the outcome. Patient weights were computed as the inverse fitted probability of being in that cohort. The performance of the weighting procedure was examined by comparing baseline characteristics (Appendix Table A4) between cohorts using standardized differences. A standardized difference of less than 10% between study cohorts was considered adequately balanced.
Comparisons by Inhaler Type
Before and after the weighting procedure, measures compared between cohorts were expressed as frequencies and percentages for categorical variables, and means with standard deviations for continuous variables. After IPTW, z-tests using robust standard errors were used to test for differences in binary measures. Rao–Scott chi-square tests were used for categorical measures. z-Tests using robust standard errors in an ordinary least squares regression were used for continuous measures. The analysis accounted for variable lengths of follow-up observation time using PPPM calculations.
Readmissions
The number and percentage of follow-up COPD-related hospitalizations that resulted in an all-cause readmission were recorded. Unweighted descriptive analysis was conducted because the unit of analysis was hospitalizations and the weights from IPTW were calculated at the patient level. Logistic regression was conducted to determine if the odds of all-cause readmission were different for COPD-related hospitalizations among the SMI cohort as compared with COPD-related hospitalizations among the DPI cohort. The logistic model was implemented including covariates that were included in the IPTW model, plus an additional covariate that captured whether or not a patient had a baseline readmission (patients categorized as having ≥1 baseline COPD hospitalization that resulted in a readmission; having ≥1 baseline COPD hospitalization with no readmissions; or having no baseline COPD-related hospitalizations). The logistic regression model accounted for clustering using robust standard errors (sandwich estimators), since patients could contribute multiple qualifying COPD hospitalizations.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Study Sample Identification
Among 216,331 MAPD enrollees who filled a prescription for a LAMA during the identification period, 28,240 patients (SMI cohort: n=5360; DPI cohort: n=22,880) met all study selection criteria (Figure 2). The SMI cohort included only users of tiotropium bromide as index LAMA. The DPI cohort had tiotropium bromide (n=19,644 [86%]); umeclidinium bromide (n=2893 [13%]); aclidinium bromide (n=337 [1%]); and glycopyrrolate (n=6 [<1%] as their index LAMA). Baseline characteristics and measures of HRU and exacerbations, as follows, were compared for the SMI versus DPI cohorts and were used as variables in the weighting procedure.
Baseline Demographic and Clinical Characteristics
Baseline patient characteristics are shown in Table 1. Prior to IPTW, most demographic characteristics were similar (standardized difference <10%) between the SMI and DPI cohorts, including mean age (SMI=72 years; DPI=73 years) and gender distribution (52% female). Before weighting, the only demographic characteristic with a standardized difference ≥10% was geographic region.
 |
Table 1 Baseline Patient Characteristics, by Inhaler Type, Before and After IPTW |
Prior to weighting, the majority of clinical characteristics were also similar (standardized difference <10%) between the SMI and DPI cohorts, including CCI (mean SMI=2.5; DPI=2.6) and COPD Severity Score (mean 31.5). However, a greater proportion of the SMI cohort had a mail order index pharmacy fill, pulmonologist index prescriber, used an ICS/LABA single inhaler combination, SABA, systemic corticosteroids, and guideline-recommended antibiotics, compared to DPI patients (Table 1).
After applying the weighting procedure to control for such differences, all categories of patient characteristics, comorbid conditions, and medication use were balanced between cohorts (standardized differences <10%; Appendix Table A6). More than 50% of the patients in each cohort had metabolic syndrome, hypertension, and dyspnea.
Baseline COPD-Related HRU and Exacerbations
A larger proportion of SMI patients had a pulmonologist visit within the 30 days prior to the index date (28% vs 21%) than DPI patients (no difference after weighting). Without weighting, the SMI cohort had a larger number of COPD-related ambulatory visits (including office visits and outpatient hospital visits) and pharmacy fills, and fewer ED and inpatient visits PPPM (Table 2). After IPTW, all categories of HRU were balanced (standardized differences <10%). During the baseline period, the mean PPPM counts of both moderate and severe COPD-related exacerbations differed between cohorts before the weighting procedure was applied: SMI vs DPI, 0.05 ± 0.08 vs 0.06 ± 0.09, respectively, for severe; SMI vs DPI, 0.05 ± 0.08 vs 0.04 ± 0.07, respectively, for moderate. With the weighting procedure, the cohorts were balanced (standardized differences <10%) in counts for both severe and moderate exacerbations.
 |
Table 2 Baseline COPD-Related HRU and Acute COPD-Related Exacerbations |
The IPTW procedure was successful in balancing cohorts for these characteristics, to enhance the ability to detect an effect of the type of inhaler on outcomes after initiating LAMA.
Follow-Up Outcomes
COPD-Related HRU Encounters
The mean follow-up duration was similar between the SMI and DPI cohorts, 186 ± 104 days and 185 ± 103 days, respectively; p=0.786. The weighted counts of COPD-related visits/fills among all categories of HRU were not significantly different between the SMI and DPI cohorts (Figure 3).
COPD-Related Exacerbations
The weighted mean number of COPD-related exacerbations was significantly lower among the SMI cohort compared with the DPI cohort (0.054 ± 0.082 PPPM versus 0.059 ± 0.088, p<0.001). Additionally, the weighted mean number of severe exacerbations was significantly lower among the SMI cohort compared with the DPI cohort (0.030 ± 0.058 PPPM versus 0.034 ± 0.065 PPPM, p<0.001). The number of moderate exacerbations was not statistically significantly different between cohorts. The SMI cohort had fewer inpatient-based (SMI: 0.020 ± 0.043 vs DPI: 0.022 ± 0.048 PPPM; p=0.055) and ED-based (SMI: 0.010 ± 0.035 vs DPI: 0.013 ± 0.038 PPPM; p<0.001) severe exacerbations than the DPI cohort [Figure 4].
COPD-Related Readmissions
During the follow-up period, among the SMI cohort, there were 369 COPD-related hospitalizations, 47 (12.7%) of which resulted in an all-cause readmission within 30 days of discharge. Among the DPI cohort, there were 1786 COPD-related hospitalizations, 359 (20.1%) of which resulted in an all-cause readmission within 30 days of discharge. Readmissions were less likely to occur after hospitalizations among SMI initiators than DPI initiators (12.7% vs 20.1%; p=0.001). After controlling for baseline covariates, hospitalizations among SMI patients had lower adjusted odds of readmission compared to hospitalizations among DPI patients (odds ratio = 0.656, 95% CI = 0.460–0.937, p=0.020) (Appendix Table A5).
Discussion
This observational cohort study compared HRU outcomes among US patients with COPD who initiated a LAMA SMI inhaler versus a LAMA DPI inhaler. In this study, no significant differences in the number of HRU encounters PPPM were observed between the SMI and DPI cohorts. However, the SMI cohort had significantly fewer COPD-related exacerbations, in total and severe, than the DPI cohort. This finding is noteworthy, as the main driver of cost increases in COPD-related healthcare over recent years is treatment for moderate to severe exacerbations.25 Additionally, after controlling for baseline covariates, the proportion of COPD-related hospitalizations that resulted in readmission within 30 days was significantly lower among SMI patients compared to DPI patients. The occurrence of all-cause readmissions is an outcome used by the CMS HRRP to identify areas that need attention by linking payment with quality of care;26–28 hospitals with excess readmissions receive reduced payments. Thus, readmission rates reflect an important measure of healthcare burden and quality of care.
Studies of patients treated in routine clinical practice provide an important perspective to add to clinical trial findings. To date, the TIOSPIR trial is the only randomized clinical study that has performed a head-to-head comparison of outcomes for people using DPI (Handihaler 18 µg) versus SMI (Respimat 2.5 µg) inhalers.29 TIOSPIR found no significant differences in time to COPD exacerbation or hospitalization rates for COPD exacerbations among more than 17,000 patients assigned to the two groups. However, only a few patients in TIOSPIR were naïve to tiotropium prior to the trial, and therefore the results may be most applicable to patients who are known to tolerate LAMAs. Moreover, the generalizability of the TIOSPIR study results to the broader COPD patient population has been called into question as the trial excluded patients with severe respiratory disorders and recent COPD exacerbation.12 A comparison of the TIOSPIR trial population and COPD patients treated with tiotropium in routine practice in Germany revealed that the vast majority of patients treated with tiotropium would have been excluded from the TIOSPIR trial.12 Patients with COPD most often treated in routine clinical practice are more likely to have multiple comorbid conditions and exacerbations leading to costly hospitalizations, and subsequently are more likely to die, but may be excluded from trials.30,31 These observations underscore the importance of including real-world evidence in addition to data obtained from RCTs in comparing COPD treatment outcomes.12,32
Only one other published observational study, from Italy, compared outcomes among patients who initiated LAMA therapy, specifically examining tiotropium DPI (Handihaler) or SMI (Respimat) inhalers.13 The team used propensity score matching (PSM) to control for differences in patient characteristics and found no significant differences between groups in follow-up occurrence of hospitalization for COPD exacerbation, respiratory failure, hypoxemia, or pneumonia. Although both studies balanced cohorts in baseline characteristics, differences between their findings and those of our study may result from differing methods for characterizing baseline comorbidities, or defining drug exposure and outcomes. For example, in the current study, comorbid conditions were identified by ICD codes evident on any type of claim during the baseline period, whereas in the Italian study, comorbid conditions were identified by ICD codes evident for inpatient hospitalizations only. Such an approach may result in an incomplete measure of comorbid conditions. Even with balancing cohorts within studies, significant differences in the health status of the cohorts could influence the results, based on disease severity of the overall patient sample. In addition, the medication exposure periods and outcomes were defined differently. Thus, even among large observational studies, key differences in methods and study settings can limit comparisons of findings across studies. It should also be noted that TIOSPIR and the comparator study included only tiotropium SMI and DPIs, while this study included patients initiating any LAMA DPI, although 86% of the patients indexed on tiotropium.
Despite the large real-world sample and analytic strengths of this study, limitations must be considered. First, inherent in any retrospective cohort study using administrative claims, the possibility of coding errors and the inability to measure appropriate use of inhalers may influence results. In addition, COPD severity based on lung function, and clinical characteristics such as smoking status, symptomology, and peak inspiratory flow (PIF) or forced expiratory volume (FEV1), are unavailable in claims data, preventing comparison and adjustment of these characteristics between cohorts in this study. However, the COPD severity score is a validated claims-based measure of disease severity18 and the cohorts were balanced on measured baseline characteristics prior to comparative analyses. Furthermore, these results were from a sample of MAPD enrollees in the US and may not be generalizable to other populations, such as the uninsured, Medicaid beneficiaries, and those with commercial insurance plans. Finally, the mean follow-up was 185 days for both the SMI and DPI cohorts and 25% of the patients had <120 days of follow-up on index medication for both cohorts. The relatively short follow-up duration may have been insufficient to observe the full impact of treatment with the inhaler initiated. It is also possible that there would be differences observed in the number of HRU encounters PPPM between SMI and DPI patients who remained on treatment for a longer period of time. Further analyses, including a more recent sample, stratified by duration of follow-up may provide helpful insights about the subgroup of patients who remain on index medication treatment for a longer period of time.
Despite some limitations, the current study represents a large (>28,000) real-world sample of patients, who might not be included in RCTs.12 A strong analytic approach balanced cohorts on a wide range of baseline characteristics using probability of treatment, allowing practical comparisons of outcomes. Such a design can be seen as simulating the randomization used in RCTs. In addition, timing-specific claims-based definitions for exacerbations are unique in this study, which contributes an important perspective toward optimizing treatment for COPD.
Conclusions
The study evaluated, within a real-world setting in the US, outcomes of LAMA use specifically comparing cohorts of COPD patients initiating SMI and DPI inhalers. After controlling for baseline differences, patients who initiated treatment with a LAMA SMI had significantly fewer acute COPD-related exacerbations, and a lower proportion of these patients’ COPD-related hospitalization resulted in 30-day readmissions, as compared with those initiating a LAMA DPI. Further research including detailed clinical information such as FEV1, symptoms of dyspnea, and PIF, as well as in different patient populations may help elucidate reasons for these observed differences.
Ethics Approval and Informed Consent
Data obtained for this study were accessed using techniques compliant with the Health Insurance Portability and Accountability Act. The study involved only de-identified retrospective claims. As such, it did not require institutional review or approval or any type of informed consent.
Acknowledgments
Medical writing assistance was provided by Caroline Jennermann of Optum, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). Portions of this work were presented at AMCP Nexus 2019, October 31, 2019, National Harbor, MD, USA; and ISPOR Europe, November 5, 2019, Copenhagen, Denmark.
Author Contributions
All authors made a significant contribution to the work reported, that is, in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored and funded by BIPI and conducted by Optum. The sponsor participated in study design, review, and approval, as well as manuscript review and approval for submission. The authors received no direct compensation related to the development of the manuscript.
Disclosure
LGSB, CE, and ARB are employees of Optum, Eden Prairie, MN; employment is not contingent upon this work. DS and JF-E are employees of BIPI. The authors report no other conflicts of interest in this work.
References
1. Vogelmeier C, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2019 report. Available from: www.goldcopd.org.
2. Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–472. doi:10.2147/TCRM.S160365
3. Dalby RN, Eicher J, Zierenberg B. Development of respimat® soft mist™ inhaler and its clinical utility in respiratory disorders. Med Devices. 2011;4:145–155.
4. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry power inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. doi:10.1089/jamp.2017.1416
5. Anderson P. Use of respimat® soft mist™ inhaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2006;1:251–259.
6. Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by respimat soft mist™ inhaler compared to deposition by metered dose inhaler or by turbuhaler dry powder inhaler. J Aerosol Med. 2005;18:264–272. doi:10.1089/jam.2005.18.264
7. Hohlfeld JM, Sharma A, van Noord JA, et al. Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease. J Clin Pharmacol. 2014;54:405–414. doi:10.1002/jcph.215
8. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;7:CD009285.
9. Keating GM. Tiotropium bromide inhalation powder: a review of its use in the management of chronic obstructive pulmonary disease. Drugs. 2012;72:273–300. doi:10.2165/11208620-000000000-00000
10. Keating GM. Tiotropium respimat® soft mist™ inhaler: a review of its use in chronic obstructive pulmonary disease. Drugs. 2014;74(15):1801–1816. doi:10.1007/s40265-014-0307-4
11. Wise RA, Anzueto A, Calverley P, et al. The Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial—design and rationale. Respir Res. 2013;14:40. doi:10.1186/1465-9921-14-40
12. Schmiedl S, Fischer R, Ibanez L, et al. Tiotropium Respimat® vs. HandiHaler®: real-life usage and TIOSPIR trial generalizability. Br J Clin Pharmacol. 2016;81(2):379–388. doi:10.1111/bcp.12808
13. Spila-Aligiani S, Trotta F, Da Cas R, Rossi M, Venegoni M, Traversa G. Comparative effectiveness of two tiotropium formulations: a retrospective cohort study. COPD. 2018;15(5):418–423. doi:10.1080/15412555.2018.1554032
14. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global burden of Disease Study 2015. Lancet. 2017;5(9):691–706.
15. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benefits. 2014;7(2):98–106.
16. US Department of Commerce. US census bureau. Census regions and divisions of the United States. Available from: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf.
17. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi:10.1093/aje/kwq433
18. Wu EQ, Birnbaum HG, Cifaldi M, et al. Development of a COPD severity score. Curr Med Res Opin. 2006;22(9):1679–1687. doi:10.1185/030079906X115621
19. Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of chronic obstructive pulmonary disease. 2014. Available from: https://www.healthquality.va.gov/guidelines/CD/copd/.
20. U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Medical Care. Series ID: CUUR0000SAM. Washington, DC: U.S. Dept. of Labor, Bureau of Labor Statistics, 2018. Available from: http://data.bls.gov/cgi-bin/surveymost?cu.
21. Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation. 2013 measure updates and specifications report: hospital-level 30-day readmission following admission for an acute exacerbation of chronic obstructive pulmonary disease (version 2.0). March, 2013. Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.
22. Robins JM. Marginal Structural Models. In ASA Proceedings in the Section on Bayesian Statistical Science. Alexandria, VA: American Statistical Association; 1997:1–10.
23. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol. 2000;11(5):550–560. doi:10.1097/00001648-200009000-00011
24. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi:10.1002/sim.6607
25. Bollmeier SG, Hartmann AP. Management of chronic obstructive pulmonary disease: a review focusing on exacerbations. Am J Health Syst Pharm. 2020;77(4):259–268. doi:10.1093/ajhp/zxz306
26. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. doi:10.1378/chest.14-2181
27. Rinne ST, Castaneda J, Lindenauer PK, Cleary PD, Paz HL, Gomez JL. Chronic obstructive pulmonary disease readmissions and other measures of hospital quality. Am J Respir Crit Care Med. 2017;196(1):47–55. doi:10.1164/rccm.201609-1944OC
28. CMS.gov. Quality Net: Hospital Readmissions Reduction Program (HRRP). Available from: https://www.qualitynet.org/inpatient/hrrp.
29. Wise R, Calverley PM, Dahl R, et al. Safety and efficacy of tiotropium respimat versus HandiHaler in patients naïve to treatment with inhaled anticholinergics: a post hoc analysis of the TIOSPIR trial. NPJ Prim Care Respir Med. 2015;25:15067. doi:10.1038/npjpcrm.2015.67
30. Pahus L, Burgel P-R, Roch N, et al. Randomized controlled trials of pharmacological treatments to prevent COPD exacerbations: applicability to real-life patients. BMC Pulm Med. 2019;19(1):127. doi:10.1186/s12890-019-0882-y
31. Schichilone N, Basile M, Battaglia S, Bellia V. What proportion of chronic obstructive pulmonary disease outpatients is eligible for inclusion in randomized clinical trials? Respiration. 2014;87(1):11–17. doi:10.1159/000355082
32. Trotta F, Spila Alegiani S, Da Cas R, et al. Cardiovascular safety of tiotropium respimat vs HandiHaler in the routine clinical practice: a population-based cohort study. PLoS One. 2017;2(4):e0176276. doi:10.1371/journal.pone.0176276
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



