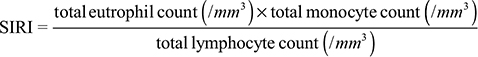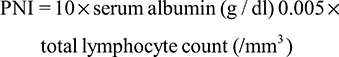Back to Journals » Cancer Management and Research » Volume 10
Health-related quality of life for gemcitabine and nab-paclitaxel plus radiotherapy versus gemcitabine and S-1 plus radiotherapy in patients with metastatic pancreatic cancer
Authors Zhu X, Li F, Shi D, Ju X, Cao Y, Shen Y, Cao F , Qing S, Fang F, Jia Z, Zhang H
Received 27 February 2018
Accepted for publication 14 June 2018
Published 23 October 2018 Volume 2018:10 Pages 4805—4815
DOI https://doi.org/10.2147/CMAR.S166713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Xiaofei Zhu,* Fuqi Li,* Dongchen Shi,* Xiaoping Ju,* Yangsen Cao, Yuxin Shen, Fei Cao, Shuiwang Qing, Fang Fang, Zhen Jia, Huojun Zhang
Department of Radiation Oncology, Changhai Hospital Affiliated to Navy Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Purpose: To compare the effects of gemcitabine and nab-paclitaxel (GT) plus stereotactic body radiation therapy (SBRT) or gemcitabine and S-1 (GS) plus SBRT on health-related quality of life (HRQOL) of metastatic pancreatic cancer.
Methods: Patients with biopsy-proven and radiographically metastatic pancreatic cancer were included. HRQOL was assessed using the Chinese version of Brief Pain Inventory (BPI) and 5-level European quality of life 5-dimensions (EQ-5D-5L). Data were analyzed with Spearman’s rank correlation, ordinal regression, and propensity score-matched analysis.
Results: A total of 75 and 89 patients received GT and GS, respectively. The median biological effective dose of GT group and GS group was 59.5 Gy (range 48–85.5 Gy) and 64.4 Gy (range 52.48–85.5 Gy) in 5–8 fractions, respectively. More patients in the GS group had improvement in BPI and EQ-5D-5L compared with those in the GT group (n=38 vs n=15, P<0.001; n=42 vs n=20, P<0.001). No differences of BPI scores were found between pre- and post-treatment in each group, while only the post-treatment EQ-5D-5L score was higher than that at baseline in GS the group (P<0.001). Compared with GS group, it was unlikely for patients receiving GT to have better BPI and EQ-5D-5L. After propensity-matched analysis, more patients in GS group had improvement in BPI and EQ-5D-5L (n=24 vs n=12, P=0.002; n=28 vs n=16, P=0.002). Furthermore, patients with GS had a superior overall survival than those with GT (11.1 months [95% CI: 10.6–11.6 months] vs 9.9 months [95% CI: 8.8–11.0 months]; P=0.005). Both incidences of grade 3 hematological (P=0.024) and gastrointestinal (P=0.049) toxicities were higher in the GT group.
Conclusion: GS may achieve better HRQOL than GT. Therefore, GS may be an alternative of GT for metastatic pancreatic cancer, especially for Asians.
Keywords: quality of life, stereotactic body radiation therapy, chemotherapy, gemcitabine, pancreatic cancer
Introduction
Pancreatic cancer has been the fourth leading cause of cancer mortality in the USA with a dismal 5-year survival rate of 7%.1 The latest findings also showed that in contrast to the declining trends for the four major cancers, the mortality of pancreatic cancer continues to increase slightly (by 0.3% per year) in men but have leveled off in women.2 Similar trends were found in China with increasing incidences and cancer deaths.3
Moreover, ~50% of patients had metastatic pancreatic cancer at initial diagnosis.2 Therefore, priority may be given to the quality of life regarding advanced pancreatic cancer, which required improvement in survival without simultaneous compromise of health-related quality of life (HRQOL) during treatment. Though multiagent chemotherapy regimens have afforded gains in survival, attendant treatment-emergent toxicities might counteract the efficacy and result in the deterioration of HRQOL. As a result, influences on the HRQOL of different chemotherapy regimens were pivotal factors, which should be taken into consideration in clinical decision making.
Additionally, local symptoms, including abdominal pain, loss of appetite and weight, biliary tract obstruction, and pancreatic insufficiency, would lead to nutritional depletion and negatively affect HRQOL. Furthermore, chemotherapy may be less beneficial to remit local symptoms than local treatment. Therefore, radiotherapy could be a better option for the amelioration of abdominal pain, probably contributing to the remission of other symptoms and improvement in HRQOL. Due to precise treatment delivery with sharp dose fall-off within adjacent organs at risk, acceptable toxicity, on-line image verifications, and without delay of sequential chemotherapy, stereotactic body radiation therapy (SBRT) may be a more promising modality to alleviate the pain compared with conventional radiotherapy. Hence, we sought to evaluate the HRQOL after palliative SBRT and different chemotherapy regimens in the management of metastatic pancreatic cancer.
Methods
The Institutional Review Board of Changhai Hospital has approved this study. Individual written informed consent was mandatory before treatment. The study population was prospectively followed up from 2013 to 2017. A prospectively collected database was used to identify patients receiving gemcitabine and nab-paclitaxel (GT) or gemcitabine and S-1 (GS).
Eligibility
Pathological examinations with fine needle aspiration guided by endoscopic ultrasound were preferred for all patients. Comprehensive clinical and radiographic stagings, including abdominal computed tomography (CT) or MRI scan were mandatory before treatment. Usually positron emission tomography-CT may also be performed if it was deemed necessary by radiation oncologists. Patients who were treated with SBRT and GT or SBRT and GS were assessed for eligibility. The detailed exclusion criteria are listed in Figure 1.
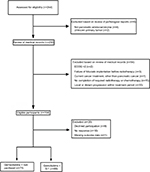  | Figure 1 Flowchart detailing inclusion and exclusion criteria for the study population (n=164). Abbreviation: ECOG, Eastern Cooperative Oncology Group. |
SBRT delivery
Radiotherapy was delivered by SBRT. The protocol of SBRT was similar to our previous studies.4,5 SBRT was delivered via CyberKnife® (Accuray Incorporated, Sunnyvale, CA, USA), an image-guided frameless stereotactic robotic radiosurgery system. All patients underwent endoscopic ultrasound-guided implantation of 3–5 gold fiducials within or adjacent to the pancreatic tumor. Patients underwent CT simulation supine in custom-fit immobilization devices with intravenous contrast. Gross tumor volume (GTV) was delineated as a radiographically evident gross disease by contrast CT. Clinical target volume (CTV) encompassing areas of the potential subclinical disease spread was also designated. In most cases, the CTV equaled GTV. Planning target volume (PTV) included a 2–5 mm margin on GTV. When the tumor abutted critical organs, the expansion of PTV outside of CTV in this direction should be avoided. Therefore, the margin expansion was allowed to be nonuniform.6 At least 90% of PTV should be covered by the prescription dose. Normal tissue constraints were referred to the American Association of Physicists in Medicine guidelines in Task Group-101.7
Chemotherapy
Treatment decisions were made at the discretion of the institutional multidisciplinary pancreatic cancer board, which generally followed National Comprehensive Cancer Network guidelines. S-1, the prodrug of 5-fluorouracil comprising tegafur, gimeracil, and oteracil, was an option as the regimen. Previous studies have proven that S-1 was not inferior to gemcitabine in terms of overall survival (OS) rates and progression-free survival (PFS) rates with tolerable effects.8–11 Due to the low tolerance of FOLFIRINOX in Chinese, GT was used as the option in addition to GS.
Chemotherapy was delivered after SBRT with an interval of 3 weeks. The regimens were GT group or GS group. Gemcitabine (1000 mg/m2) and nab-paclitaxel (125 mg/m2) were administered on days 1, 8, and 15 every 28 days for 4 cycles. S-1 was orally given at a dose of 80 mg/m2 for 14 days followed by a 14-day rest for 4 cycles.
Definitions and collection of data
The definition of disease recurrence was based on the review of the medical records and imaging studies. A new low density mass or growth of the tumor on CT or MRI consistent with recurrent local, regional, or new metastatic lesions was considered as such, and tumor biopsy was rarely performed.12 Differential diagnosis of tumor necrosis induced by SBRT, which may be mistaken for progression, would be performed by three radiologists based on MRI scan. OS was defined from the initial date of treatment to death. PFS was determined from the initial date of treatment to the date of the first recurrence or death. Adverse effects induced by chemotherapy were evaluated by Common Terminology Criteria for Adverse Events Version 4.0. Radiation-induced acute toxicities were determined by “Acute radiation morbidity scoring criteria” from Radiation Therapy Oncology Group, while late toxicities were evaluated by “Late radiation morbidity scoring schema” from Radiation Therapy Oncology Group/European Organization for Research on the Treatment of Cancer.13
A systemic inflammation response index (SIRI) might correlate with the survival of patients with pancreatic cancer.14 The value was calculated as
|
The prognostic nutritional index (PNI) represented patient’s nutritional status, which might also associate with the survival of pancreatic cancer.15,16 The formula was as follows:
|
Charlson age-comorbidity index (CACI) was originally designed to classify prognostic comorbidity.17 It was identified that CACI was associated with the prognosis of patients with pancreatic cancer.18 Pain was quantified by visual analog scale (VAS).
The recommended upper limit of normal for carbohydrate antigen 19-9 (CA19-9) is 37 U/mL.19 Additionally, a Phase I/II study of nab-paclitaxel + gemcitabine that preceded advanced pancreatic cancer reported a significant correlation between decreases in CA19-9 levels of ≥50 vs <50% from baseline and improved survival.20 Therefore, CA19-9 response was defined as the level of CA19-9 decrease by 50% from baseline levels of ≥74 U/mL. Hence, three CA19-9 groups were formed for univariate analysis: CA19-9 levels ≥74 U/mL with response vs CA19-9 levels ≥74 U/mL with no response (including CA19-9 levels within the normal range before SBRT while increased after treatment) vs CA19-9 levels <74 U/mL (before and after treatments). The value of CA19-9 level after the treatment was utilized for the estimation of CA19-9 decrease. Additionally, it was demonstrated that CA19-9 level <200 U/mL was associated with major response for localized pancreatic cancer treated with preoperative therapy.21 Therefore, the serum level of CA19-9 before SBRT was stratified as <200 and ≥200 U/mL.
Evaluation of HRQOL
Patients with advanced cancer may experience moderate-to-severe pain. Cancer pain was suggested to impact psychiatric symptoms, quality of life, and social functioning. Hence, pain alleviation was favorable for the improvement of HRQOL. Although there were many scales assessing HRQOL, only scales validated with local language were employed. Therefore, the Chinese version of Brief Pain Inventory (BPI)22 and 5-level European quality of life 5-dimensions (EQ-5D-5L)23 were used in this study. Questionnaires were completed at baseline (before SBRT) and right after the whole treatment. For EQ-5D-5L, a high score represents a high level of quality of life, while a high symptom scale score represents a high burden of symptoms regarding BPI.
BPI
The BPI was first validated in cancer population with an 11-item pain measure. It measures both the intensity of pain (a 4-item sensory dimension) and interference of pain in the patient’s life (a 7-item reactive dimension). The severity and interference items were scaled from 0 to 10, with response options rating point 0 being “no pain” and 10 being “pain as bad as you can imagine” for the severity item and being “does not interfere” and “interferes completely” for the interference items, respectively.
EQ-5D-5L
At first, the 3-level European quality of life 5-dimensions was an instrument for valuing health, compromising five items, such as mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, with three levels in each dimension, such as no problems, some problems, and extreme problems. Due to its insensitivity and ceiling effects, EQ-5D-5L was developed. It retained the original items but expanded the number of levels of severity in each dimension from three to five, including “no problems”, “slight problems”, “moderate problems”, “severe problems” and “extreme problems/unable to” for all five items.
Patient-reported global changes
Due to the limited life expectancy, patient-reported global changes were assessed at baseline and right after the completion of the whole treatment. A single item24 was used as the reference standard for responsiveness. Patients were asked “How would you describe your pain (or other items of HRQOL) now, compared to how you were when you started in our study?” based on the item. It was scaled from 1 to 7, with response options “much better”, “moderately better”, “a little better”, “no change”, “a little worse”, “moderately worse”, and “much worse”, respectively. To be simplified, all changes were stratified into “better”, “no change”, and “worse” in the analysis.
Statistical analyses
Patient characteristics and demographic data were summarized by descriptive statistics. Quantitative outcomes were compared by chi-squared test. Next, demographic and clinical factors were investigated for their association with patient-reported global changes in BPI and EQ-5D-5L by Mann–Whitney U-test or Kruskal–Wallis H-test and, then, by multinomial logistic regression because both of them were dichotomized variables. The comparisons of the pretreatment and post-treatment BPI and EQ-5D-5L scores between GT group and GS group were performed by Mann–Whitney U-test. Furthermore, the comparisons of pretreatment and post-treatment BPI and EQ-5D-5L scores in the GT group and in the GS group were performed by Wilcoxon signed-rank test.
To correct for potential imbalances in treatment assignments, propensity score matching was performed to decrease the differences between groups. A logistic regression model was built with treatment modality as the dependent variable and all other variables that could potentially influence its impact as independent variables, including those with statistical significance after univariate analyses and those probably counteracting the effects though without statistical significance. As a result, the independent variables may include pain evaluations before treatment, nutritional status, or medical conditions before treatment.
Two-sided P-values <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient’s characteristics
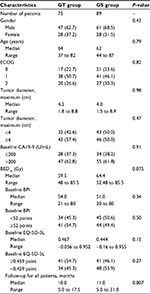
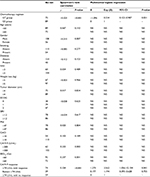
A total of 244 patients were identified with metastatic pancreatic cancer receiving SBRT and chemotherapy in our center. Thirty-three patients were excluded due to disease progression within the treatment period. A total of 75 and 89 patients received GT and GS, respectively. The median prescription dose and biological effective dose, α/β=10 (BED10), of GT group and GS group were 35 Gy (range 30–46.8 Gy) and 59.5 Gy (range 48–85.5 Gy) in 5–8 fractions and 37 Gy (range 30–45.5 Gy) and 64.4 Gy (range 52.48–85.5 Gy) in 5–8 fractions, respectively. Patients in the GS group tended to receive higher BED10 than those in the GT group (P=0.075), as well as longer follow-up (11 vs 10 months, P=0.007). Tumors were similarly sized in both GS and GT groups (4.0 vs 4.2 cm median maximum diameter, P=0.98). Additionally, there was no difference of baseline BPI and EQ-5D-5L between GT and GS groups (baseline BPI: 52.35±18.27 points vs 49.74±16.60 points, P=0.34; baseline EQ-5D-5L: 0.48±0.25 point vs 0.43±0.24 point, P=0.15). Details are shown in Table 1.
BPI
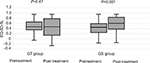
Pre- and post-treatment BPI scores of each group are shown in Figure 2. No significant difference was found between baseline scores and those after treatment in each group. After treatment, the mean BPI of the GT and GS groups was 51.60±18.44 and 45.35±19.14 points, respectively. Regarding patient-reported global changes, a better response was found in 15 and 38 patients in GT and GS groups, respectively (P<0.001) (Table 2). Additionally, different chemotherapy regimens (P<0.001), PNI (P=0.04), BED10 (P=0.015), and CA19-9 response (P=0.003) correlated with global changes in BPI (Table 3). On multinomial logistic regression, compared with GS group, PNI ≥47, BED10 <60 Gy and CA19−9≥74 U/mL without response, the probability of GT group, patients with PNI <47, BED10 ≥60 Gy, and CA19-9 response achieving better global change was 0.299 (P=0.015), 0.348 (P=0.019), 3.083 (P=0.041), and 3.272 (P=0.042), respectively (Table 3).
EQ-5D-5L
Pre- and post-treatment EQ-5D-5L scores of each group were shown in Figure 3. There was no difference between pre- and post-treatment EQ-5D-5L scores in GT group, while the post-treatment EQ-5D-5L score was higher than that at baseline in GS group (P<0.001). After SBRT and chemotherapy, the mean EQ-5D-5L of the GT and GS group was 0.46±0.35 point and 0.60±0.30 point, respectively. The better change was found in 20 and 42 patients in GT and GS group, respectively (P<0.001) (Table 2). Furthermore, different chemotherapy regimens (P<0.001), gender (P=0.007), baseline CA19-9 level (P=0.003), BED10 (P=0.001), and CA19-9 response (P<0.001) associated with global changes of EQ-5D-5L (Table 4). On multinomial logistic regression, compared with GS group and CA19−9≥74 U/mL without response, the probability of GT group and patients with CA19-9 response gaining better change was 0.334 (P=0.031) and 3.562 (P=0.041), respectively (Table 4).
Propensity score-matched analysis of BPI and EQ-5D-5L
Based on the previous results, BED10, PNI, and VAS were included as the independent variables for propensity-matched analysis of BPI. Sixty-three patients in each group were matched. The mean BPI of GT and GS groups was 52.25±17.77 and 46.38±19.41 points, respectively. A total of 12, 23, and 28 patients responded better, no change, and worse, respectively, in the GT group, while there were 24, 26, and 13 patients with better, no change, and worse response in the GS group, respectively. More patients had the remission of BPI in the GS group (P=0.002).
Furthermore, for the analysis of EQ-5D-5L, gender, baseline CA19-9, and BED10 were included for propensity score matching. A total of 62 patients of each group were matched. The mean EQ-5D-5L of GT and GS groups was 0.47±0.35 and 0.60±0.31 point, respectively. A total of 16, 21, and 25 patients responded better, no change, and worse, respectively, in the GT group, while there were 28, 25, and nine patients with better, no change, and worse response in the GS group, respectively. More patients had improvement in EQ-5D-5L in the GS group (P=0.002).
Overall survival (OS)
The OS of GT and GS groups was 9.9 months (95% CI: 8.7–11.0 months) and 11.1 months (95% CI: 10.5–11.7 months) (P=0.002). Additionally, after propensity score-matched analysis, a superior OS was found in the GS group (GT group: 9.9 months [95% CI: 8.8–11.0 months] vs GS group: 11.1 months [95% CI: 10.6–11.6 months]; P=0.005).
Adverse effects and response of radiation therapy and chemotherapy
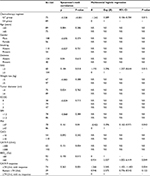
There were no grade ³3 acute and late radiation-induced toxicities in the GT and GS groups. Only 17 (22.7%) and 21 (23.6%) patients had grade 1 or 2 radiation-induced gastrointestinal toxicities in the GT and GS groups, respectively (P=0.89). However, regarding chemotherapy, more patients experienced grade ³3 hematological toxicities, including leukopenia, thrombocytopenia, and anemia, in the GT group (n=25, 33.3%) than those in the GS group (n=16, 18.0%) (P=0.024). Additionally, the incidence of grade ³3 gastrointestinal toxicities tended to be higher in the GT group than in the GS group (n=17, 22.7% vs n=10, 11.2%; P=0.049).
Regarding CA19-9 response, 59 patients had a significant decrease in CA19-9 level (CA19-9 levels ≥74 U/mL with response) and 13 patients had no decrease in CA19-9 level (CA19-9 levels ≥74 U/mL without response) in the GS group, while there were 15 and 38 patients with and without decrease in CA19-9 level in the GT group, respectively (P<0.001).
Discussion
In this study, the median profile of BPI and EQ-5D-5L was significantly better in the GS group than in the GT group after SBRT and chemotherapy, indicating that patients receiving GS were more likely to have improvement in BPI and EQ-5D-5L. Furthermore, adjusted analyses showed that more patients in the GS group reported decline in BPI and increase in EQ-5D-5L compared with those in the GT group. Hence, patient-reported global changes were consistent with changes in BPI and EQ-5D-5L scores.
The goals of treating metastatic pancreatic cancer were to mitigate symptoms and prolong survival.25 The finding in our study that HRQOL scores remained stable with GT regimen agrees with a recent systematic review, which clarified that of the 14 included studies on metastatic pancreatic cancer, five studies reported improved HRQOL scores, six studies reported stable scores and three with worsening scores.26 Additionally, previous studies, almost in Japan, elucidated that S-1 could be an option in chemotherapy for pancreatic cancer, which provided similar survival benefits compared with gemcitabine.8–11 These results may reflect that in addition to recommended regimens in guidelines, S-1 could also be employed in clinical practice for Asians. However, in all the previous randomized trials, only clinical outcomes were reported, while no HRQOL data were shown.
This is the first study to validate the use of BPI and EQ-5D-5L in patients with metastatic pancreatic cancer receiving S-1-based chemotherapy. Compared with baseline BPI and EQ-5D-5L in each group, only significant increase in EQ-5D-5L was found in the GS group after treatment. Moreover, more patients achieved better HRQOL in the GS group. The reason for the improved HRQOL associated with GS may be attributable to better response to the treatment. Because better tumor response may be hardly found in metastatic pancreatic cancer even though after aggressive treatment, CA19-9 response was considered as an alternative in this study. In the GS group, a significant decrease in CA19-9 level was found in 59 patients (66.3%), while only 15 patients had CA19-9 response in the GT group (20%). Correlations between response to the treatment and HRQOL have been clarified in other types of cancer based on Phase III randomized study.27,28
Another reason for the better HRQOL may be ascribed to lower incidences of toxicity in GS group. Fewer patients tended to experience grade ³3 hematological and gastrointestinal toxicities. Hence, the potential HRQOL benefit from GT regimen may have been compromised by adverse effects such as leukopenia, anemia, thrombocytopenia, fatigue, nausea, and vomiting. Likewise, though FOLFIRINOX was effective for pancreatic cancer, deteriorations of HRQOL may usually develop.29,30
Furthermore, it was elaborated that higher radiation doses correlated with an improvement in BPI other than EQ-5D-5L. Amelioration of symptoms, especially abdominal pain, should be given the priority in the treatment for metastatic pancreatic cancer, which was the aim of radiotherapy in addition to local control. Though our previous studies elucidated that BED10 ≥60 Gy was predictive of tumor response,5 and Krishnan et al31 showed that patients with BED10 >70 Gy had a superior OS, no studies have confirmed the correlation between radiation doses and alleviation of pain. However, better response to the treatment resulting from higher BED10 indicated potential eradication of primary lesions, which may contribute to the remission of local symptoms.
Furthermore, it was identified that a superior OS was found in patients with GS. The potential reason may be that the incidences of adverse effects, including hematological and gastrointestinal toxicities, were lower in the GS group. Therefore, chemotherapy-induced toxicities may counteract the survival benefits provided by GT.
Our study has several limitations. Patient numbers in each group were relatively small, resulting in impermissible generalizations to be made to the broader population of patients. Additionally, due to limited life expectancy of patients with metastatic pancreatic cancer, questionnaires of HRQOL were only completed at baseline and right after SBRT and chemotherapy. Hence, no longitudinal data were acquired and long-term effects of GT and GS regimens on HRQOL remained unanswered.
Conclusion
Despite these limitations, this study has confirmed the validity of BPI and EQ-5D-5L in patients with metastatic pancreatic cancer receiving GS or GT. It provided insight into HRQOL following SBRT plus GS or GT, which has not been previously described. These results may be useful during determinations of therapeutic options for patients with metastatic pancreatic cancer and give support to the use of S-1 as a first-line regimen in this setting, especially for Asian patients. Future trials should incorporate HRQOL as an end point.
Acknowledgments
We appreciate Dr Jiuhong Chen for her precise comments and LinkDoc for their constructive advice in the follow-up of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;2016(66):7–30. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;2017(67):7–30. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Zhu X, Ju X, Cao F, et al. Safety and efficacy of stereotactic body radiation therapy combined with S-1 simultaneously followed by sequential S-1 as an initial treatment for locally advanced pancreatic cancer (SILAPANC) trial: study design and rationale of a phase II clinical trial. BMJ Open. 2016;6(12):e013220. | ||
Zhu X, Li F, Ju X, et al. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. 2017;6(10):2263–2270. | ||
Verma V, Lazenby AJ, Zheng D, et al. Dosimetric parameters correlate with duodenal histopathologic damage after stereotactic body radiotherapy for pancreatic cancer: Secondary analysis of a prospective clinical trial. Radiother Oncol. 2017;122(3):464–469. | ||
Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. | ||
Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology. 2005;68(2-3):171–178. | ||
Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31(13):1640–1648. | ||
Morizane C, Okusaka T, Furuse J, et al. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;63(2):313–319. | ||
Sudo K, Yamaguchi T, Nakamura K, et al. Phase II study of S-1 in patients with gemcitabine-resistant advanced pancreatic cancer. Cancer Chemother Pharmacol. 2011;67(2):249–254. | ||
Kharofa J, Tsai S, Kelly T, et al. Neoadjuvant chemoradiation with IMRT in resectable and borderline resectable pancreatic cancer. Radiother Oncol. 2014;113(1):41–46. | ||
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. | ||
Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. | ||
Lee SH, Chung MJ, Kim B, et al. The significance of the prognostic nutritional index for all stages of pancreatic cancer. Nutr Cancer. 2017;69(3):512–519. | ||
Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41(11):1508–1514. | ||
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. | ||
Dias-Santos D, Ferrone CR, Zheng H, Lillemoe KD, Fernández-del Castillo C. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery. 2015;157(5):881–887. | ||
Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-Delcastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24(18):2897–2902. | ||
von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. | ||
Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152(11):1048–1056. | ||
Wang XS, Mendoza TR, Gao SZ, Cleeland CS. The Chinese version of the Brief Pain Inventory (BPI-C): its development and use in a study of cancer pain. Pain. 1996;67(2-3):407–416. | ||
Luo N, Li M, Liu GG, et al. Developing the Chinese version of the new 5-level EQ-5D descriptive system: the response scaling approach. Qual Life Res. 2013;22(4):885–890. | ||
Fischer D, Stewart AL, Bloch DA, et al. Capturing the patient’s view of change as a clinical outcome measure. JAMA. 1999;282(12):1157–1162. | ||
Oettle H. Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev. 2014;40(9):1039–1047. | ||
Kristensen A, Vagnildhaug OM, Grønberg BH, Kaasa S, Laird B, Solheim TS. Does chemotherapy improve health-related quality of life in advanced pancreatic cancer? A systematic review. Crit Rev Oncol Hematol. 2016;99:286–298. | ||
Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006;24(24):3831–3837. | ||
Au HJ, Karapetis CS, O’Callaghan CJ, et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 Trial. J Clin Oncol. 2009;27(11):1822–1828. | ||
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. | ||
Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31(1):23–29. | ||
Krishnan S, Chadha AS, Suh Y, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94(4):755–765. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.