Back to Journals » Infection and Drug Resistance » Volume 15
Gut Microbiota Alternation in Disease Progression of Neurosyphilis
Authors Wang G , Zou D , Lu X , Gu X, Cheng Y , Qi T , Cheng Y , Yu J, Ye M , Zhou P
Received 8 September 2022
Accepted for publication 2 November 2022
Published 14 November 2022 Volume 2022:15 Pages 6603—6612
DOI https://doi.org/10.2147/IDR.S389155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guixuan Wang,1,2 Danyang Zou,2 Xinying Lu,2 Xin Gu,2 Yuanyuan Cheng,2 Tengfei Qi,2 Yanchun Cheng,2 Junjun Yu,2 Meiping Ye,2 Pingyu Zhou1,2
1Shanghai Skin Disease Clinical College of Anhui Medical University, Shanghai Skin Disease Hospital, Shanghai, People’s Republic of China; 2STD Institute, Shanghai Skin Disease Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Pingyu Zhou, Shanghai Skin Disease Clinical College of Anhui Medical University, Shanghai Skin Disease Hospital, 1278 Bao’de Road, Shanghai, 200443, People’s Republic of China, Tel +86-18017336631, Email [email protected]
Background: The gut microbiota plays an important role in the development of neurological disorders such as Parkinson’s disease and Alzheimer’s disease. However, studies on the gut microbiota of patients with neurosyphilis (NS) were rarely reported.
Methods: In this study, we collected fecal samples from 62 syphilis patients, including 39 with NS and 23 with non-NS. Among the NS patients, 18 were general paresis (GP). The white blood cell counts, protein concentrations, and Venereal Disease Research Laboratory test positive rates of cerebrospinal fluid from patients in NS or GP group were significantly higher than those from patients in non-NS group. 16S ribosomal RNA sequencing results revealed that the alpha and beta diversities of the gut microbiota were similar between NS and non-NS patients or GP and non-NS patients.
Results: Linear discriminant analysis with effect size (LEfSe) analysis showed that some taxa, such as Coprobacter, were increased in both NS group and GP group, compared with non-NS group. Besides, the clade of Akkermansia was also overrepresented in GP Patients. Meanwhile, some taxa such as Clostridia_UCG-014 and SC-I-84 were underrepresented in NS patients. The abundances of class Bacilli and genus Alloprevotella were decreased in GP patients. Among them, the abundances of some taxa such as Coprobacter and Akkermansia have been reported to be associated with other neuropsychiatric disorders.
Conclusion: Our findings suggest that the alternation of the gut microbiota in NS patients may contribute to the course of NS, which will deepen our understanding of NS.
Keywords: syphilis, neurosyphilis, general paresis, gut microbiota, 16S ribosomal RNA sequencing
Introduction
There has been a resurgence of syphilis worldwide over the last decades, and the steady increase in incidence proves that syphilis remains a threat to public health.1 According to the World Health Organization, there were 6 million new syphilis cases worldwide in 2016.2 Over the past decade, China has experienced an increasing trend in the prevalence of syphilis with 537,720 new cases reported in 2021.3 During the infection, T. pallidum can propagate through multiple organs of the human host. If left untreated, T. pallidum can invade the central nervous system at any stage,4 resulting in neurosyphilis (NS) in approximately one-third of syphilis patients.5,6 NS can be asymptomatic or manifest with a multitude of psychiatric symptoms, including general paresis (GP), meningovascular syphilis, tabes dorsalis, etc.,7 which greatly affect the patients’ quality of life.8
The human gastrointestinal tract harbours trillions of microorganisms, and gut microbiota has a multifaceted impact on host physiology.9 Over the past few decades, researches on the microbiome-gut-brain axis interactions have flourished and the association studies between neurological disorders and gut microbiota have attracted considerable attention.10,11 Understanding the mechanisms of the microbiome-gut-brain axis provided us with new insights for innovative therapeutic strategies.12 Gut microbiota can influence the development of neurological diseases directly or indirectly through the gut-brain axis.13,14 Studies on neuropsychiatric disorders such as multiple sclerosis, autism spectrum disorder, Parkinson’s disease, stroke, and Alzheimer’s disease have shown that the gut microbiota plays an important role in the development of neurological damage.15–20
NS is a chronic progressive disease involving central nervous system injury. Numerous studies support the association of the microbiome-gut-brain axis with dementia such as Alzheimer’s disease.21 However, to the best of our knowledge, the gut microbiota in NS has not been reported yet and the association between the gut microbiota and NS is also poorly understood. In this study, we aimed to analyze the bacterial community composition of the gut microbiota in syphilis patients and identify the bacterial taxa alternations specific to NS patients. The NS-associated bacterial taxa found in this study further the understanding of the pathogenesis of NS through the gut microbiota view and provide new insights for therapeutic interventions for NS.
Materials and Methods
Ethics Statement and Subjects
The study was conducted at the Sexually Transmitted Disease (STD) clinic of the Shanghai Skin Disease Hospital and was approved by the Ethics Committee of Shanghai Skin Disease Hospital. Eligible patients who were first hospitalized from December 2020 to June 2021 were invited to participate in this study. A total of 62 syphilis patients were included in this study. All patients signed the informed consent and agreed to participate in this study.
Diagnostic and Exclusion Criteria
Syphilis was diagnosed as patients with positive serum rapid plasma reagin (RPR) and Treponema pallidum particle agglutination (TPPA) tests. NS is defined as syphilis patients with 1) reactive cerebrospinal fluid (CSF)-Venereal Disease Research Laboratory (VDRL) and CSF-TPPA tests or 2) nonreactive CSF-VDRL but positive CSF-TPPA with CSF protein concentration >450 mg/L and/or white blood cell (WBC) count ≥8 × 106 cells/L.22,23 Syphilis patients without any neurological or psychiatric symptoms and with negative CSF-TPPA plus with normal CSF protein concentration and WBC count were diagnosed as non-NS. In this study, non-NS group includes latent syphilis, secondary syphilis, and ocular syphilis. NS group included GP, asymptomatic NS, tabes dorsalis, and secondary syphilis. Asymptomatic NS is defined as the absence of neurological or psychiatric manifestations with CSF abnormalities consistent with NS. GP is characterized by dementia and personality changes. Tabes dorsalis presents as sensory loss, ataxia, and lancinating pains.
Exclusion criteria include 1) co-infection with HIV; 2) other inflammatory diseases; 3) a history of metabolic diseases such as diabetes; 4) any other neurological or psychiatric disease; 5) a history of antibiotic use in the past 4 weeks; 6) recent abnormal eating behavior, or special dietary habits such vegetarianism; 7) during pregnancy and lactation.
Fecal Sample Collection
Fecal samples were collected in a sampling cup by patients according to the instructions and immediately put into an incubator at 0–4°C. The samples were then transported into storage tubes and stored at −80 °C within 24 hours. The fecal samples were only subjected to a single free-thaw cycle before sequencing processing.
Bacterial 16S rRNA Gene Sequencing and Analysis
Bacterial genomic DNA was extracted using the FastDNA™ SPIN kit according to the manufacturer protocol (MP Biomedicals, Santa Ana, CA, USA), and the final DNA quality was detected using 1% agarose gel electrophoresis.24,25 The V3-V4 variable region of the bacteria’s 16S ribosomal RNA (rRNA) gene was amplified by PCR system (GeneAmp 9700, ABI, USA) with barcode-indexed primers 341F and 806R, which were adapted from the previous protocol.26 Reactions were electrophoresed by agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). Extracted DNA is quantified using QuantiFluor™-ST (Promega, USA). The sequence processing was performed using the MiSeq platform (Illumina, San Diego, CA, USA) by Genergy Biotechnology Co., Ltd. (Shanghai, China).
Sequencing of the 62 fecal samples from syphilis patients generated 14,824,368 raw reads. Raw reads were processed using Trimmomatic and FLASH and clustered into operational taxonomic units (OTUs) at a 97% similarity using Usearch (v 7.1). Then, the data were deposited into the SILVA 16S rRNA gene reference alignment database to calculate the relative abundances of microbiota at different taxonomic levels.
Data Availability
The genome sequences have been deposited in the DDBJ/ENA/GenBank under the bioproject PRJNA898084.
Statistical Analysis
For clinical and laboratory characteristics of study participants, statistical analyses were performed using SPSS (version 26.0; IBM, Chicago, IL, USA). Baseline characteristic data were given as proportion, median or IQR. t-tests were used for continuous variables. Chi-square tests or Fisher’s exact tests were applied for categorical variables. Mann–Whitney tests were adopted for serum RPR titer.
Alpha diversity, including Coverage, Chao, Ace, Simpson, Shannon, and Sobs diversity indices were calculated using mothur. Beta diversity analysis includes principal component analysis (PCA), principal coordinates analysis (PCoA), and non-metric multidimensional scaling (NMDS). PCA and PCoA were calculated using R, and NMDS was calculated using Qiime and drawn by the R package “vegan”. Wilcoxon rank-sum tests were performed to detect differential abundance at the phylum and genus. Linear discriminant analysis with effect size (LEfSe) was performed to determine taxonomic differences from the phylum to the genus level. A p-value <0.05 (two-sided) was considered to be statistically significant.
Results
Patient Characteristics
A total of 62 syphilis patients were enrolled in this study, with 39 diagnosed as NS and 23 were non-NS (Figure 1). Among the 39 NS patients, 18 patients were with GP, 16 were asymptomatic, 3 had tabes dorsalis, and 2 were diagnosed as secondary syphilis but with CSF abnormalities consistent with NS. While among the non-NS patients, 14 patients were diagnosed as latent syphilis, 7 were secondary syphilis, and 2 were ocular syphilis.
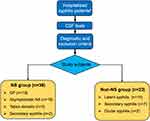 |
Figure 1 Flow chart of the study. aSyphilis patients who were first hospitalized from December 2020 to June 2021. Abbreviations: CSF, cerebrospinal fluid; NS, neurosyphilis; GP, general paresis. |
The clinical and laboratory characteristics of patients are shown in Table 1. The median age of patients in NS group was 60 years old, while the patients in non-NS group were significantly younger (40 years old). The WBC counts, protein concentrations and VDRL positive rates of CSF from patients in NS group or GP group were significantly higher than those from patients in non-NS group. Meanwhile, no significant difference was found in serum RPR titers between NS group and non-NS group or GP group and non-NS group.
 |
Table 1 Clinical and Laboratory Characteristics of Study Subjects |
Gut Microbiota Changes in NS or GP Patients Compared to Non-NS Patients
The 16S rRNA of the gut microbiota was extracted from the fecal samples of patients and sequenced on MiSeq platform with 300bp pair-end reads. The pair-end reads were merged and clustered into operational taxonomic units (OTUs) at 97% similarity. A total of 3504 OTUs were identified in NS group, 2664 OTUs in GP group, and 2778 OTUs in non-NS group. The Venn diagrams showed that 2441 OTUs were shared by NS and non-NS group, and 1929 OTUs were shared by GP and non-NS group (Figure 2). The bacterial community richness and diversity in the fecal samples were assessed by alpha diversity indices including Coverage, Chao, Sobs, ACE, Simpson and Shannon indices. However, no significant difference was observed between samples from NS and non-NS group nor GP and non-NS group (Figure S1). To explore the community structure differences between different groups, beta diversity was analyzed by the PCA, PCoA, and NMDS. The results also showed no significant difference between NS and non-NS group (Figure S2) nor GP and non-NS group (Figure S3).
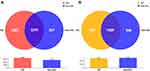 |
Figure 2 Venn diagram of NS versus non-NS group and GP group versus non-NS group. (A) Venn diagram for NS group and non-NS group. (B) Venn diagram for GP group and non-NS group. |
The community composition of each sample at the genus level is shown in Figure 3A. The results showed that the gut microbiota of patients was highly diverse intra and inter groups. At the phylum level, the gut microbiota of all three groups was dominated by Firmicutes, Actinobacteriota, Bacteroidota, Proteobacteria, and Verrucomicrobiota (Figure 3B). Compared with non-NS patients, the relative abundance of Verrucomicrobiota of GP patients was significantly increased (5.3% versus 1.1%, P = 0.02) (Figure 4), while no significant difference between NS and non-NS group at the phylum level could be observed (Figure S4).
 |
Figure 4 Wilcoxon rank-sum test bar plot of GP group and non-NS group at the phylum level. *P value <0.05. |
The gut microbiota was further investigated at the genus level by Wilcoxon rank-sum tests (Figure 5). The results showed that the relative abundances of Erysipelatoclostridiaceae, Coprobacter, Streptococcaceae, and Actinomycetaceae were significantly increased, and the relative abundances of Clostridia_UCG-014, Actinobacteria, Erysipelotrichales, Gardnerella, and SC-I-84 were significantly decreased in NS patients compared to non-NS patients (Figure 5A). Meanwhile, in the GP group, the relative abundances of Akkermansia, CHKCI002, Candidatus Soleaferrea, Holdemania, Coprobacter, Erysipelatoclostridiaceae, Clostridiaceae, and Actinomycetaceae were significantly increased and the relative abundance of Alloprevotella was significantly decreased compared to non-NS group (Figure 5B).
The Disease-Associated Bacterial Taxa in NS or GP Patients
The LEfSe analysis was used to identify the most differentially abundant bacterial taxa in NS or GP patients compared to non-NS patients (Figure 6). In NS group, an increased abundance of Erysipelatoclostridiaceae, Coprobacter, and CHKCI002 was observed (Figure 6A and C). Meanwhile, the underrepresented bacterial taxa include Clostridia_UCG-014 at the order, family, and genus levels, Erysipelotrichales at the family and genus levels, Actinobacteria at the order, family, and genus levels, and SC-I-84 at the family and genus levels.
The differentially abundant bacterial taxa between GP and non-NS group are shown in Figure 6B and D. As the genera in NS group, Erysipelatoclostridiaceae, Coprobacter, and CHKCI002 were also overrepresented in GP group. Besides, the clade of Verrucomicrobiota to Akkermansia and unclassified bacteria were overrepresented, indicating an important role of Akkermansia in GP patients. In addition, the abundances of order Flavobacteriales, genus UBA1819, and Clostridiaceae and Actinomycetaceae at the genus level were also increased in GP patients. The abundances of class Bacilli and genus Alloprevotella were decreased in GP patients.
Discussion
After centuries of research, NS is still poorly understood.7,27 An increasing number of studies have revealed that the microbiota-gut-brain axis contributes to the pathogenesis of numerous neuropsychiatric disorders, including Parkinson’s Disease and Alzheimer’s disease.17,28 In this study, we explored the gut microbiota in non-NS and NS patients and investigated the bacterial community composition difference between the two groups. To our knowledge, the gut microbiota has not yet been studied in NS or GP patients. Overall, we found that the alpha and beta diversities of the gut microbiota were similar in non-NS patients and NS patients. However, several bacterial taxa including Coprobacter, CHKCI002, and Erysipelatoclostridiaceae were overrepresented, and Clostridia_UCG-014, Erysipelotrichales, Actinobacteria, and SC-I-84 were underrepresented in NS patients. We then further analyzed the gut microbiota of NS patients with GP. No significant difference was found in alpha and beta diversities of the gut microbiota between GP and non-NS groups, but compared to non-NS patients, Akkermansia was overrepresented in GP patients. Other taxa such as CHKCI002, Candidatus Soleaferrea, Holdemania, and Coprobacter were also overrepresented, while Bacilli and Alloprevotella were underrepresented.
The Akkermansia genus has long been recognized as a protective bacterium and the decreased abundance of this commensal bacterium has been linked to multiple diseases, such as obesity, diabetes, and liver steatosis.29 However, the abundance of Akkermansia genus was increased in GP patients compared to non-NS patients in our study. Several studies showed that the increased abundance of Akkermansia genus was associated with Alzheimer’s disease and seizure,30–32 and Alzheimer’s disease has similar cognitive impairment manifestations to GP.33,34 Given that caloric restriction or starvation increases the abundance of Akkermansia genus in the human gut,35 the abundance increase of Akkermansia genus in GP patients might due to the limited food intake. It has been reported that Akkermansia could increase the differentiation of Treg cells in the colon and stimulate the production of interleukin-10.36,37 Our previous study revealed that compared to patients without central nervous system involvement, neurosyphilis patients had higher Treg frequency in peripheral blood.38 Other studies demonstrated that interleukin-10 levels were increased in NS patients compared to non-NS patients.39 Therefore, we infer from these that the overrepresented Akkermansia may have a facilitative effect on accelerating the progression of NS. Thus, further study should be investigated in the future.
Several reports showed that the decreased abundance of Alloprevotella genus was associated with cognitive impairment.40 Here, we also found that Alloprevotella genus was underrepresented in GP patients, indicating that Alloprevotella genus may contribute to cognitive impairment. Other taxon changes in NS or GP patients have also been reported in other neuropsychiatric disorders. For example, the Coprobacter genus and Actinomycetaceae genus have been reported enriched in the gut microbiota of autism patients with gastrointestinal disorder.41 The increases in the abundances of Erysipelotrichales genus and Erysipelotrichaceae genus were observed in animal models of Parkinson’s disease or neuropathic pain.42,43 Our findings indicated that the gut microbiota may play important roles in the pathogenesis of NS, especially of NS complicated by GP.
Our study also has several limitations. First, the sample size is not large enough, which may lead to the omission of rare bacteria. Second, the NS patients were older than non-NS patients and the gut microbiota difference might be resulted from different ages. Third, all subjects were Chinese, and dietary habits also contributed to the gut microbiota characteristics. Fourth, it remains to be further investigated whether the gut microbiota alterations change with the progression, aggravation, and treatment of NS or with different NS categories. Our further studies will expand the sample size, exclude age and other confounding factors, and further explore the relationship between altered gut microbiota and the severity of NS.
Conclusion
Based on this small sample study, we have a preliminary understanding of the composition of the gut microbiota in Chinese syphilis patients. We found that the alpha and beta diversities of the gut microbiota were similar between NS and non-NS patients or GP and non-NS patients. However, the abundances of Akkermansia, Coprobacter, Actinomycetaceae, Erysipelotrichales, Erysipelotrichaceae, and Alloprevotella, which have been associated with other neuropsychiatric disorders, were altered in NS or GP patients compared to non-NS patients. Our findings suggest that the alternation of the gut microbiota in NS patients may contribute to the course of NS, which will deepen our understanding of NS.
Acknowledgments
We are grateful for all the participants of this study, and we greatly appreciate the clinical staff who recruited patients.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Shanghai Skin Disease Hospital (approval no 2020-27). We confirm that our study complies with the Declaration of Helsinki.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 82072322, 82172319 and 81471999), Shanghai Science and Technology Commission (grant number: YDZX20193100002868, 17DZ2293300), and the Clinical Research and Cultivation Project of Shanghai Skin Disease Hospital (No. lcfy2020-04).
Disclosure
The authors report no conflicts of interest in this work.
References
1. de Oliveira Narvaez E, de Carvalho Ramos M, Do Amaral LL, Reis F. Neurosyphilis and high-resolution vessel wall imaging: a powerful tool to detect vasculitis and neuritis. Neurol India. 2022;70(1):160–161. doi:10.4103/0028-3886.338673
2. World Health Organization. Report on global sexually transmitted infection surveillance 2018; 2018.
3. CCDC. Reported cases and deaths of national notifiable infectious diseases — China, 2021. China CDC Wkly. 2021;3(9):196–197. doi:10.46234/ccdcw2021.059
4. Feitoza L de M, Stucchi RSB, Reis F. Neurosyphilis vasculitis manifesting as ischemic stroke. Rev Soc Bras Med Trop. 2020;53:e20190546. doi:10.1590/0037-8682-0546-2019
5. Gonzalez H, Koralnik IJ, Marra CM. Neurosyphilis. Semin Neurol. 2019;39(04):448–455. doi:10.1055/s-0039-1688942
6. Berger JR, Dean D. Neurosyphilis. Handb Clin Neurol. 2014;121:1461–1472. doi:10.1016/B978-0-7020-4088-7.00098-5
7. Ropper AH. Neurosyphilis. N Engl J Med. 2019;381(14):1358–1363. doi:10.1056/NEJMra1906228
8. Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS. Syphilis. Nat Rev Dis Primers. 2017;3(1):17073. doi:10.1038/nrdp.2017.73
9. Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473–493. doi:10.1007/s00018-018-2943-4
10. Osadchiy V, Martin CR, Mayer EA. The gut–brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17(2):322–332. doi:10.1016/j.cgh.2018.10.002
11. Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374(6571):1087–1092. doi:10.1126/science.abi6087
12. Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. 2016;158:52–62. doi:10.1016/j.pharmthera.2015.11.012
13. Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–155. doi:10.1038/nn.4476
14. Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179–194. doi:10.1016/S1474-4422(19)30356-4
15. Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. doi:10.1038/ncomms12015
16. Kang DW, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi:10.1186/s40168-016-0225-7
17. Mulak A. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37):10609. doi:10.3748/wjg.v21.i37.10609
18. Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson's Dis. 2017;3(1):3. doi:10.1038/s41531-016-0002-0
19. Tan BYQ, Paliwal P, Sharma V. Gut microbiota and stroke. Ann Indian Acad Neurol. 2019;1(1). doi:10.4103/aian.AIAN_483_19
20. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi:10.1038/s41598-017-13601-y
21. Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;13:1497–1511. doi:10.2147/CIA.S139163
22. Wang Q, Liu Q, Xu J, Zhou P, Su X. Clinical Diagnosis and Treatment of sexually Transmitted Diseases and Prevention Guidelines.
23. Wang C, Zhu L, Gao Z, et al. Increased interleukin-17 in peripheral blood and cerebrospinal fluid of neurosyphilis patients. PLoS Negl Trop Dis. 2014;8(7):e3004. doi:10.1371/journal.pntd.0003004
24. Kennedy NA, Walker AW, Berry SH, et al. The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS One. 2014;9(2):e88982. doi:10.1371/journal.pone.0088982
25. Qbiogene. Rapid isolation of genomic DNA from plant and animal tissue, bacteria, yeast, algae and fungi using the fastprep instrument http://fnkprddata.blob.core.windows.net/domestic/data/datasheet/GEN/6540-600.pdf.
26. Nam YD, Jung MJ, Roh SW, Kim MS, Bae JW. Comparative analysis of Korean human gut microbiota by barcoded pyrosequencing. PLoS One. 2011;6(7):e22109. doi:10.1371/journal.pone.0022109
27. CDC. National overview - sexually transmitted disease surveillance, 2019; 2021.
28. Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–1501. doi:10.1053/j.gastro.2020.10.066
29. Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19(10):682. doi:10.1038/s41575-022-00631-9
30. Zhang L, Wang Y, Xiayu X, et al. Altered gut microbiota in a mouse model of alzheimer’s disease. J Alzheimers Dis. 2017;60(4):1241–1257. doi:10.3233/JAD-170020
31. Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741.e13. doi:10.1016/j.cell.2018.04.027
32. Sun J, Xu J, Ling Y, et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019;9(1):189. doi:10.1038/s41398-019-0525-3
33. Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J Neuroinflammation. 2019;16(1):108. doi:10.1186/s12974-019-1494-4
34. Wang J, Guo Q, Zhou P, Zhang J, Zhao Q, Hong Z. Cognitive impairment in mild general paresis of the insane: AD-like pattern. Dement Geriatr Cogn Disord. 2011;31(4):284–290. doi:10.1159/000326908
35. von Schwartzenberg RJ, Bisanz JE, Lyalina S, et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature. 2021;595(7866):272–277. doi:10.1038/s41586-021-03663-4
36. Liu S, Rezende RM, Moreira TG, et al. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding akkermansia muciniphila. Cell Host Microbe. 2019;26(6):779–794.e8. doi:10.1016/j.chom.2019.10.008
37. Nath N, Khan M, Paintlia MK, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009;182(12):8005–8014. doi:10.4049/jimmunol.0803563
38. Li K, Wang C, Lu H, Gu X, Guan Z, Zhou P. Regulatory T cells in peripheral blood and cerebrospinal fluid of syphilis patients with and without neurological involvement. PLoS Negl Trop Dis. 2013;7(11):e2528. doi:10.1371/journal.pntd.0002528
39. Li W, Wu W, Chang H, et al. Cerebrospinal fluid cytokines in patients with neurosyphilis: the significance of interleukin-10 for the disease. Biomed Res Int. 2020;2020:1–8. doi:10.1155/2020/3812671
40. Hu L, Zhu S, Peng X, et al. High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J Alzheimers Dis. 2020;77(2):629–640. doi:10.3233/JAD-200035
41. Liu S, Li E, Sun Z, et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9(1):287. doi:10.1038/s41598-018-36430-z
42. Shen CL, Wang R, Ji G, et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J Nutr Biochem. 2022;100:108904. doi:10.1016/j.jnutbio.2021.108904
43. Lai F, Jiang R, Xie W, et al. Intestinal pathology and gut microbiota alterations in a methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurochem Res. 2018;43(10):1986–1999. doi:10.1007/s11064-018-2620-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



