Back to Journals » Infection and Drug Resistance » Volume 14
Group B Streptococci Vaginal-Recto Colonization, Vertical Transmission to Newborns, Antimicrobial Susceptibility Profile and Associated Factors in Selected Health Facilities of Bahir Dar City: A Cross-Sectional Study
Authors Leykun Y, Genet C , Mulu W
Received 8 October 2021
Accepted for publication 9 December 2021
Published 17 December 2021 Volume 2021:14 Pages 5457—5472
DOI https://doi.org/10.2147/IDR.S343429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yasabe Leykun,1 Chalachew Genet,1 Wondemagegn Mulu1,2
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 2Department of Biochemistry and Microbiology, Faculty of Sciences, Ghent University, Ghent, Belgium
Correspondence: Wondemagegn Mulu Tel +251918706921
Email [email protected]
Background: Group B streptococci (GBS) is an important pathogen involved in stillbirths, neonatal deaths and neurological defects, and the occurrence of multi-drug resistance (MDR) is an alarming issue. This study determined the prevalence of GBS colonization in pregnant women and newborns, the proportion of vertical transmission, antimicrobial susceptibility profiles of isolates, and the factors associated with colonization and vertical transmission.
Methods: A cross-sectional study was conducted from March 1, 2021 to June 30, 2021, at selected health facilities of Bahir Dar city. Vaginal-recto swabs from 292 pregnant women and pooled ear, nasal and umbilical swabs from 292 newborns were collected. GBS were identified following standard microbiological protocols. Antimicrobial susceptibility testing was performed using modified Kirby–Bauer disk diffusion method and interpreted by the accepted 2020 CLSI M100 guidelines. Logistic regression analysis was computed.
Results: Overall, 54 (18.5%) of pregnant women and 22 (7.5%) of newborns had GBS colonization. The proportion of GBS vertical transmission was 22 (40.7%). Group B Streptococcus isolates scored susceptibility to penicillin, ampicillin and vancomycin with 88.9%, 90.7%, and 96.3% for pregnant women and 86.4%, 90.9% and 95.9% for newborns, respectively. A high percentage of non-susceptibility was found for clindamycin and erythromycin with 33.3% and 25.9% for pregnant women and 31.8% and 22.7% from newborns, respectively. Besides, 19 (35.2%) GBS from pregnant women and 8 (36.4%) from newborns were MDR. Group B streptococci colonization was significantly associated with delivery before 37th week of gestation (AOR=2.77, 95% CI 1.14– 6.68) and history of stillbirth (AOR=3.13, 95% CI 1.13– 8.70).
Conclusion: Pregnant women vaginal-recto GBS colonization and transmission to newborns connected with MDR are a matter of concerns. Although non-susceptible GBS isolates are obtained, penicillin and vancomycin are relatively effective. The use of clindamycin, erythromycin and ceftriaxone should be guided by antimicrobial susceptibility testing. Genetic analysis is recommended to exactly identify the epidemiology of GBS strains, vertical transmission and antimicrobial resistance at the country level.
Keywords: group B streptococci, vaginal-recto colonization, vertical transmission, pregnant women, newborns, MDR, Ethiopia
Introduction
Streptococcus agalactiae has originally been differentiated from other streptococci and named Group B Streptococcus (GBS) by Lancefield in 1930. Group B Streptococcus is Gram-positive cocci and facultative anaerobic that forms β-hemolytic grayish-white mucoid color colonies of 1–3 mm diameter on a blood agar plate.1 It is fastidious, catalase-negative, resistant to bacitracin, and is Christie–Atkins–Munch–Peterson (CAMP) test positive.1,2
Group B streptococci asymptomatically colonize the gastrointestinal and genitourinary tracts of one-third of healthy pregnant women.3 They are predominant cause of meningitis in newborns and infants below 3 months old.4 Group B streptococci cause urinary tract infection (UTI), amnionitis, endometritis, and puerperal sepsis during the gestational and postpartum periods. They also result in stillbirth following endometrium and amniotic sac infection.5 The sialylated capsular polysaccharide (CPS) and β-hemolysin, serine-rich repeat surface glycoproteins, and surface adhesions are the most critical virulence factors of GBS. They contribute to host cell adhesion, colonization, invasion, and progression of invasive diseases.6,7 The type-specific CPS further divides GBS into 10 serotypes. Serotypes Ia, Ib, II, III and V accounted for the majority of worldwide GBS diseases.8
Neonates acquire GBS vertically through ascending infection of the placental membranes and aspiration of infected vaginal fluids during labor.9 Prolonged rupture of membrane, prematurity, chorioamnionitis, and previous early-onset disease (EOD) are the major risk factors for newborn diseases.3 Neonatal diseases can be early-onset and late-onset. Early-onset disease occurs within 7 days of birth and 90% of it in the first 12 hours of birth.10 Therefore, the American College of Obstetricians and Gynecologists (ACOG) recommended universal screening of pregnant women for GBS between 36 0/7 and 37 6/7 weeks of gestation and provision of intrapartum antibiotic prophylaxis (IAP) for vaginal-recto culture positives.11
Earlier reports in the United States of America (USA),12 Asia13–15 and Africa,16–18 archived 20% to 35%, 7.8% to 13.65% and 7.2% to 48.2% of GBS colonization in pregnant women, respectively. Moreover, 11.2%, (6.7% and 7.6%) and 45% to 63.3% of vertical transmission of GBS have been found in German,19 Asia20,21 and Ethiopia,22–25 respectively.
Globally, GBS is implicated in 205,000 cases of EOD, 3.5 million preterm births, 57,000 stillbirths, 90,000 infant deaths, and more than ten thousand new cases of neurodevelopmental defects annually.10,26 Africa shared the highest burden of serious neonatal cases, stillbirths, and infant deaths.26,27 Ethiopia is one of the high burden countries for GBS disease and neonatal deaths.24,28 Moreover, sepsis is the leading and second causes of neonatal admission and neonatal deaths, respectively, in the study area.29
Intrapartum antibiotic prophylaxis is indicated for all GBS carriers before 4 hours of vaginal delivery.11 Penicillin and ampicillin remain effective in preventing EOD during labor. First-generation cephalosporins and clindamycin are recommended for women who have non-severe penicillin allergies and a high risk of anaphylaxis, respectively. The use of vancomycin is also started for clindamycin-resistant GBS from anaphylaxis high-risk women.11 However, there is no clear strategy for the prevention of GBS including practices of routine screening and IAP provision in Ethiopia and most of the African countries.22,30
Over-prescription of antibiotics for various clinical conditions and outpatient care raised antimicrobial resistance. The emergence and increasing trend of GBS resistance to penicillin and macrolides and the increased demand of vancomycin results in multi-drug resistance (MDR).22,30–32 Therefore, studies on maternal GBS colonization, its vertical transmission and susceptibility profile to the commonly used antibiotics and those recommended for prophylaxis or treatment and factors linked with colonization are crucial for the selection of suitable antibiotics and implement preventive strategies. The present study aims to determine the prevalence of vaginal-recto GBS colonization in pregnant women, the proportion of vertical transmission, antimicrobial susceptibility profile of isolates, and to identify factors associated with colonization and vertical transmission.
Materials and Methods
Study Design, Period and Setting
A health facility-based cross-sectional study was conducted from March 1, 2021 to June 30, 2021. This study was conducted in Bahir Dar city, Northwest Ethiopia. Bahir Dar is located 565 kilometers away from Addis Ababa, Capital of Ethiopia. The city has two governmental specialized hospitals (Felege Hiwot Comprehensive Specialized Hospital and Tibebe Ghion Specialized Hospital), a government primary hospital, and ten government health centers. In 2020, a total of 14,612 deliveries were documented in health facilities of Bahir Dar city.33 In this study, Felege Hiwot Comprehensive Specialized Hospital (FHCSH) and Bahir Dar Health Center (BDHC) were included based on their high client flow for Antenatal Care (ANC) and delivery services. All pregnant women attended in FHCSH and BDHC for vaginal delivery service and their newborns were the study population. Amoxicillin, cephalexin, and ceftriaxone are most frequently prescribed drugs for the management of pregnant women with different body site of clinical infections.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board (IRB) of College of Medicine and Health Sciences, Bahir Dar University with the protocol number 163/2021. Informed written consent was obtained from the mothers. GBS colonization positive findings were linked to the attending physician for further follow up and management of mothers and neonates. Confidentiality of results were kept by anonymizing and coding names of the study participants, storing in a locked cabinet, and by documenting data files on a password protected personal computer.
Study Variables
Vaginal-recto GBS colonization and its vertical transmission were the dependent variables, while demographic profiles such as age of the mother, marital status, residence, occupation, level of education, sex and weight of newborns and obstetric and clinical profiles such as Appearance, Pulse, Grimace, Activity, And Respiration (APGAR) score of newborns at 5 minutes, gravidity, gestational age, number of ANC visits, history of hormonal contraceptive use, history of abortion, history of stillbirth, history of UTI, history of sexually transmitted infection and chronic illness and history of antibiotic use at current pregnancy, HIV status, premature rupture of membrane (PROM), duration of labor, meconium stained amniotic fluid were the independent variables.
Inclusion and Exclusion Criteria
Pregnant women admitted to labor and delivery room for vaginal delivery and their newborns were included in the study. Moreover, pregnant women who had vaginal cream or lubricants and were on antibiotics 2 weeks prior to recruitment and cesarean-section delivery were excluded.
Sample Size Determination and Sampling
The sample size was determined based on the proportion of GBS colonization in pregnant women (p = 0.255) found in Gondar, Ethiopia34 using the formula n = (z)2 p (1-p)/d2 and taking z =1.96 for a level of confidence of 95%, and margin of error (d) 5%. It was calculated as n = (1.96)2 × (0.255) (1–0.255)/(0.05)2 = 292. A total of 292 pregnant women and their newborns (n = 292) were included in the study. The sample size was proportionally allocated to FHCSH and BDHC based on their average number of delivery report from March 2020 to June 2020. Accordingly, 192 from FHCSH and 100 from BDHC were taken. Convenient sampling technique was used to include the study participants. Samples were collected consecutively from those pregnant mothers and their newborns that fulfilled the inclusion criteria until the determined sample size was reached.
Data Collection
Data on demographic, obstetrics and clinical related variables were collected with face-to-face interview by the attending midwives using a structured questionnaire and were complemented with medical record review.
Specimen Collection and Transportation
Specimens were collected as per the ACOG committee opinion and American Society for Microbiology (ASM) protocols. A combined vaginal-recto swab was sampled from the mother at the point of labor by trained midwives using a sterile cotton swab. A single swab specimen was collected first from the lower vagina and then from the rectum.11,35 Within 30 minutes of birth and before handling and/or wiping off the surfaces of newborns, ear, nasal and umbilical samples were swabbed with different sterile cotton swabs. Samples placed in Amies Transport Media and were transported to Microbiology laboratory of Bahir Dar University within an hour of collection. Samples were transported in an ice-box at 4°C. All samples were cultured within an hour of arrival in the laboratory following standard bacteriological techniques.11,35
Specimen Processing
All the collected swabs were placed in Todd Hewitt Broth (THB) with gentamicin (8 µg /mL) and nalidixic acid (15 µg/mL) (Oxoid, UK) and incubated for 24 h. at 37 °C under aerobic condition. Subsequently, 10 µL was sub-cultured onto 5% Sheep-Blood Agar (SBA) plates (Oxoid, UK) and incubated for 24 h. at 37 °C in 5% CO2 atmosphere. Pinpointed, with narrow beta-hemolysis colonies were considered as presumptive GBS and subjected to Gram stain and catalase test. All Gram-positive cocci and catalase-negative isolates were transferred to 5% SBA for CAMP factor and bacitracin resistance test as a final identification of GBS.
Christie–Atkins–Munch–Petersen (CAMP) Test
Known colonies of Staphylococcus aureus were streaked onto 5% sheep blood agar down the center of the plate with a wire loop. Then, 2mm apart, single colonies of Group B Streptococcus were streaked in a straight line perpendicular to the S. aureus. Plates were then incubated at 37°C for 24 h. An arrowed-shaped enhanced zone of beta-hemolysis in the area between the test organism and S. aureus with the arrow-point towards the S. aureus streak were considered as CAMP positive and colonies were presumptively considered as GBS. No enhanced zone of beta-hemolysis was observed in a CAMP-negative reaction.11
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) was done for 54 GBS isolates of the mother and 22 from newborns with Kirby–Bauer disk diffusion method. Bacterial inoculum was prepared from four to five freshly grown GBS colonies in 3–5 mL sterile physiological saline, and the turbidity was adjusted using 0.5 McFarland standards. A sterile cotton swab was dipped and rotated several times, and was pressed against the wall of the test tube. It was then streaked over the entire surface of Mueller–Hinton agar (MHA) (Oxoid, UK) with 5% sheep blood. Then, antibiotic impregnated paper disks were placed on the plate and incubated for 24 h at 37°C in 5% CO2 atmosphere.
The antibiotics tested includes penicillin G (P, 10 IU), ampicillin (AMP, 10 µg), clindamycin (DA, 2 µg), erythromycin (E, 15 µg), chloramphenicol (C, 30 µg), ceftriaxone (CRO, 30 µg), vancomycin (VA, 30 µg), and tetracycline (TE, 30 µg) (Oxoid, UK). Zone of inhibition around antibiotic disks were measured by a standard caliper. Raw data (zone of inhibition in mm) were interpreted as susceptible or non-susceptible according to the accepted 2020 Clinical and Laboratory Standard Institute (CLSI) M100 guidelines clinical breakpoints.36 For multi-drug resistance (MDR), the definition from Magi-orakos et al37 was applied and GBS isolates that revealed acquired non-susceptibility to at least one agent in three or more antibiotic categories were considered as MDR.37
Quality Control
The questionnaire was adopted from other similar studies. It was initially prepared in English and translated to local language (Amharic) and then translated back to English. Spot checks on the quality of data collection were made at the study site. Data collectors were trained on the study objectives, method of data and sample collection, and the data collection tools.
Sterility of THB, SBA, and MHA with 5% sheep blood was checked by incubating 5% of the media overnight at 37 º C without specimen inoculation. American Type Culture Collection (ATCC) standard reference strains (E. coli ATCC25922, S. agalactiae ATCC27956, E. faecalis ATCC29212, S. aureus ATCC25923 and S. pyogenes ATCC19615) were used as a quality control for THB, CAMP test, bacitracin test, and AST with known susceptibility to the antimicrobial agents.
The selective performance of THB with gentamicin (8 µg /mL) and nalidixic acid (15 µg/mL) was assessed by inoculating the broth with E. coli ATCC25922, S. agalactiae ATCC27956 and E. faecalis ATCC29212 standard reference strains.
Data Management and Analysis
Descriptive statistics were computed to describe relevant variables. Logistic regression analysis was carried out to assess the association between independent variables and maternal GBS colonization. Majority of the variables were fitted to the bivariable logistic regression. Then, all variables with a p-value of <0.2 in the bivariable analysis were further included in the multivariable logistic regression analysis. A multivariable logistic regression model was fitted to control confounders and to get the independent predictors of maternal GBS colonization. Backward stepwise regression was used. Chi-square and Fisher’s exact tests were calculated to identify variables significantly associated with vertical transmission of GBS. Variables with p-value <0.05 were considered as significant predictors. SPSS 26.0.0 was applied for the above statistical analysis.
Results
Demographic Profile of Pregnant Women and Newborns
A total of 292 pregnant women (median age = 26 years) together with their 292 newborns participated in the study. Majority of the pregnant women (65.8%) were from FHCSH. Most of the pregnant women were married (95.9%) and urban dwellers (77.4%), while 126 (43.2%) were housewives and 99 (33.9%) had a primary level of education. Of the 292 newborns, 153 (52.4%) were females and 5 (1.7%) were stillbirths. The majority of newborns (93.5%) were ≥ 2500 grams at birth, while 14 (4.8%) newborns had APGAR score of <7 at 5 minutes (Table 1).
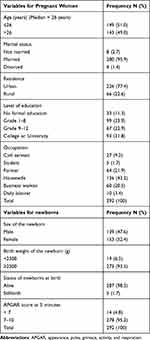 |
Table 1 Demographic Characteristics of Pregnant Women and Their Newborns |
Group B Streptococci Colonization and Vertical Transmission
Overall, 54 (18.5%) (95% CI 14.2–23.4%) of pregnant women and 22 (7.5%) (95% CI 4.8–11.2%) of newborns were colonized with GBS (Figure 1). The proportion of vertical transmission was 40.7% (22/54). All of the newborns colonized with GBS were from GBS colonized mothers (Figure 2).
 |
Figure 1 Prevalence of Group B streptococci colonization among pregnant women and their newborns. |
 |
Figure 2 Proportion of vertical transmission of Group B streptococci from pregnant women to their newborns. |
Obstetrics and Clinical Profiles of Pregnant Women
Of the total 292 pregnant women, 28 (9.6%) delivered before the 37th week of gestation, 24 (8.2%), and 33 (11.3%) had PROM and meconium-stained amniotic fluid, respectively. The majority of pregnant women (66.4%) delivered within 12 hours of labor. Two hundred and eight (71.2%) were multigravida, while 36 (12.3%) and 18 (6.2%) had history of abortion and stillbirth, respectively (Table 2).
 |
Table 2 GBS Colonization and Obstetric and Clinical Factors Among Pregnant Women |
The percentage of GBS colonization was higher among pregnant women who had <37th week of gestation (39.3%), history of abortion (25%) and stillbirth (38.9%), membrane rupture before the start of labor (29%), and meconium-stained amniotic fluid (30.3%) than their counterparts (Table 2).
Antibiotic Susceptibility Profiles of Group B Streptococci Isolates
Among the total 54 GBS isolates of the women, 48 (88.9%) and 49 (90.7%) were susceptible to penicillin, and ampicillin, respectively. From newborns, 19 (86.4%) and 20 (90.9%) exhibited susceptibility to penicillin and ampicillin, respectively. For vancomycin, 96.3% of GBS from pregnant women and 95.5% from newborns were susceptible. Group B streptococci scored the highest non-susceptibility for tetracycline with 79.6% for pregnant women and 86.4% for newborns. It also scored a high percentage of non-susceptibility for clindamycin, erythromycin and ceftriaxone with 33.3%, 25.9%, and 22.2% for pregnant women and 31.8%, 22.7%, and 27.3% for newborn isolates, respectively (Table 3, Table S1).
 |
Table 3 Antibiotic Susceptibility Profiles of Group B Streptococci from Pregnant Women and Newborns |
Multi-Drug Resistance Profile of GBS Isolates
A total of 17 and 11 profiles of antibiotic resistance were detected among GBS isolated from pregnant women and newborns, respectively. Overall, 19 (35.2%) and 8 (36.4%) of GBS isolated from the mother and newborns were found to be MDR, respectively. Of these, 16 (84.2%) isolates from the mother and 7 (87.5%) from newborns exhibited non-susceptibility to 3 antibiotics from different categories. Clindamycin, erythromycin and tetracycline combination was the most frequent non-susceptible category with 18.5% for pregnant women and 18.2% for newborns (Table 4).
 |
Table 4 Multi-Drug Resistance Profiles of Group B Streptococci Isolates of Pregnant Women and Their Newborns |
Multivariable Analysis on Maternal GBS Colonization
From multivariable analysis, delivery before 37 completed weeks of gestation (AOR: 2.77, 95% CI 1.14–6.68) and history of stillbirth (AOR: 3.13, 95% CI 1.13–8.7) were significantly associated with maternal GBS colonization. The likelihood of maternal GBS colonization was 2.8 times higher among mothers who delivered before 37 completed weeks of gestation than those delivered at ≥37 weeks of gestational age at the current pregnancy. The odd of maternal GBS colonization was 3.1 times higher in mothers having history of stillbirth when compared to their counterparts (Table 5).
 |
Table 5 Multivariable Logistic Regression Analysis of Factors Associated with Maternal GBS Colonization |
Univariable Analysis on Vertical Transmission of GBS
The proportion of GBS vertical transmission was higher among pregnant women with multigravida, meconium-stained amniotic fluid, and history of abortion than their counterparts. However, GBS vertical transmission was not significantly associated with any of the associated risk factors assessed (P > 0.05) (Table 6).
 |
Table 6 Chi-Square Test Analysis of Factors Associated with Vertical Transmission of GBS |
Discussion
Group B streptococci colonization in pregnant women and its association with early-onset neonatal disease is a grave public health and clinical concern. It is responsible for an increased level of stillbirths, death and neurological defects. In this study, a total of 292 pregnant women at the time of labor and delivery were participated, with an overall prevalence of 18.5% GBS colonization. This was consistent with the estimated adjusted global prevalence of maternal GBS colonization (18%)30 and previous reports from Africa (15.7–23.6%),22–24,31,38,39 Jordan (19.5%)14 and the USA (21.6%).40 However, it was lower in comparison to reports from South Africa (48.2%),16 Gambia (33.7%),41 the United Kingdom (29.4%)42 and USA (35%).12 But the present finding is higher compared to the 7.2 −13.7% prior reports from other parts of Ethiopia,18,25,43,44 and 6.1% in China.20 The difference in the percentage of GBS colonization could be due to variation in the type of swabs used, sample collection strategy and composition, microbiological protocols used to isolate and identify GBS. In addition, geographical variation and time of screening in pregnancy might contribute to such differences.
In this study, 22 out of 292 newborns (7.5%) were colonized with GBS and all of them were from mothers colonized with GBS. The percentage of newborns colonized with GBS in the present study is consistent with earlier studies in Ethiopia (7.4% and 8.9%),25,38 Tanzania (8.9%),45 and Saudi Arabia (7.4).46 However, it was lower compared to studies from Gondar (16.1%) in Ethiopia,47 Gambia (24.8%),41 Turkey (17.3%)48 and France (11.2%).49 But it was higher in comparison to findings from India (1.0% and 3.2%).21,50 The discrepancies between the studies might be due to variation in the sampled body surface, provision of IAP, geography and prevalence of maternal GBS colonization.
Exact determination of vertical transmission of GBS requires Multilocus sequence typing (MLST) and Pulsed-Field Gel Electrophoresis (PFGE) genetic analysis for isolates of both the mother and newborns. However, collection of swabs within 30 minutes of birth and before any handling and wiping off the surfaces of babies and recovery of GBS only from those born to GBS colonized mothers make vertical transmission more likely in the current study. Hence, the present study found 40.7% proportion of vertical transmission of GBS. This is in agreement with 38% to 49.2% reports elsewhere in Africa and Asia.23,24,50,51 However, the present result is lower compared to the 50–63.3% vertical transmission report from earlier studies in other parts of Ethiopia,22,25,38,41 65% in India21 and 54.2% in Turkey.48 Conversely, a lower percentage of vertical transmission has been reported in Ethiopia52 and China.53
The observed difference between studies for the proportion of GBS vertical transmission is variation in the criteria used to define it, clinical profiles of pregnant women, anatomical surfaces swabbed, time of sample collection, transportation and storage. Moreover, methods employed for GBS detection, IAP practice and density of vaginal-recto GBS colonization might contribute to the variations.
Though isolates non-susceptible to penicillin, ampicillin and vancomycin are obtained, these antibiotics are effective for the management of GBS cases in the present study. The percentage of susceptibility to penicillin (88.9%) among GBS isolates of the pregnant women in the present study is comparable with previous findings from Gondar (89.8%)34 and Addis Ababa (85.5%).54
The percentage of susceptibility to ampicillin (90.7%) among GBS isolates of the pregnant women in the present study is comparable with previous findings in Gondar (90.8%)34 and Addis Ababa (85.4%).54 Moreover, the 96.3% of GBS isolates susceptible to vancomycin obtained in the present study is higher than 83.7% in Gondar34 and 83% in Addis Ababa.54 However, the 2020 CLSI36 guidelines and other related studies elsewhere20,55–57 reported the absence of GBS isolates resistance to penicillin, ampicillin and vancomycin.
Antibiogram of GBS isolates from newborns of the present study showed susceptibility to penicillin (86.4%), ampicillin (90.9%), and chloramphenicol (90.9%) similar to isolates from the mothers. This is correlated with findings from Gondar, Ethiopia,47 where 89.6%, 95.1%, and 85.7% of GBS from newborns were susceptible to penicillin, ampicillin and chloramphenicol, respectively. Moreover, studies in Tanzania45 and Turkey48 documented GBS isolates 100% susceptible to penicillin and ampicillin. Therefore, beta-lactam antibiotics and vancomycin are the recommended drugs for the management of GBS cases.
The percentage of GBS isolates from the mother non-susceptible to ceftriaxone (22.2%) is correlated with those from newborns at 27.3% in the present study. This is comparable with a report from Arbaminch, Ethiopia (29.2%)45 but disagrees with a study from Egypt55 and Brazil57 that documented no resistance to this antibiotic. Non-selective use of ceftriaxone for the treatment of various clinical infections might be linked to the observed GBS resistance in the present study.
In this study, GBS isolates from the mothers showed 33.3% and 25.9% non-susceptibility to clindamycin and erythromycin, respectively. This result is found in the non-susceptibility ranges of 18.2–40.9% for clindamycin and 20.8–50% for erythromycin in other settings of Ethiopia.38,47,52,54 However, 22.6% of non-susceptibility to erythromycin and 52.4% to clindamycin was reported in Egypt55 and China,58 respectively.
The antibiogram of GBS isolates from newborns showed non-susceptibility to clindamycin (31.8%) and erythromycin (22.7%) in the present study. This is higher than the previous 12.5% and 23.9% clindamycin resistance report in other studies of Africa.45,47 Moreover, 18.7% to 29.7% level of erythromycin resistance had been reported in Africa and France.45,47,59 The significant level of non-susceptibility of GBS to clindamycin and erythromycin in the present study might be due to unregulated use of these antibiotics for various infections12 and is an alarm for the need of regular antimicrobial susceptibility testing.
Group B Streptococcus isolates from the mothers and newborns in the current study showed 79.6% and 86.4% of non-susceptible to tetracycline, respectively. This high proportion of non-susceptibility is consistent with a pooled estimate of tetracycline resistance in Africa (82.6%)18 and studies from Gondar (73.4%)34 and Addis Ababa (90.2%).54 Similar to these, a study in Namibia56 and a systematic review by Hayes et al reported 100% and >80% resistance to tetracycline.31 The spreading of GBS non-susceptible to tetracycline in the present study combined with previous findings is an alarm to end the use of tetracycline for both treatment and prophylaxis.
The 35.2% MDR GBS isolates found from the mothers in the present study is in line with a report from Addis Ababa, Ethiopia (43.9%).54 However, it was higher compared to reports from Mekelle (10.5%)43 and Arbaminch (8.3%),44 Ethiopia. Likewise, 36.4% of GBS isolated from newborns were MDR in the present study. This showed that GBS from newborns shared a related and comparable resistance and MDR profile with those from the mothers.
The majority of the MDR profile observed in the present study was shared by non-susceptibility to clindamycin, erythromycin and tetracycline at a time. This can be due to an increasing trend of antimicrobial resistance to these antibiotics over time.57 A study in France in 2019 also showed that 14% of MDR GBS was due to resistance to tetracycline, macrolides and lincosamides.59 This alarms the relevance of culture and antimicrobial susceptibility testing before the use of tetracycline and macrolides for prophylaxis and treatment. In general, GBS colonizing the mothers showed a correlated antibiogram profile to that of isolates from their newborns in the present study. This implicated the greater likelihood of vertical transmission.
Knowledge on risk factors and reducing risks are priorities in prevention of maternal GBS colonization and EOD in Ethiopia and other resource-limited countries, where routine antepartum screening of GBS and provision of IAP are lacked. In the present study, preterm delivery was one of the factors significantly associated with maternal GBS colonization. This is because vaginal GBS colonization results in ascending infection and inflammation, chorioamnionitis and preterm PROM and finally preterm delivery.5 This is in agreement with a study from Jimma, Ethiopia,5 South Africa16 and India.15
The present study finds out that maternal GBS colonization was significantly associated with having history of stillbirth. This is because stillbirth can be resulted from the GBS cytolysis breach of feto-maternal barrier as a result of ascending infection from the vaginal-recto colonization.55 Moreover, the finding is in line with a study from Harar, Ethiopia52 and Gambia.41
Group B streptococci vertical transmission varies according to educational status of the mother, demographic variables, gestational age, PROM, multigravida, prolonged duration of labor and meconium-stained amniotic fluid. However, in the present study, no significant association between vertical transmission and measured risk factors were found (P > 0.05) (Table 6).
Strengths and Limitations
With the accepted protocols, this study addressed the prevalence of GBS colonization in pregnant women and its transmission to newborns at birth that will be an important input for further genetic analysis of GBS and antibiotic resistance in pregnant women and newborns. However, because of the unavailability of reagents and laboratory equipment’s in the country, serotypes were not identified and GBS genotypes from the mother were not compared with those from the newborns. Alive newborns regardless of GBS colonization were not followed for the development of EOD. Furthermore, GBS isolates from newborns were not differentiated for colonization and residual GBS.
Conclusion
This study documented high prevalence of vaginal-recto GBS colonization and vertical transmission. Isolates resistant to clindamycin, erythromycin, ceftriaxone and tetracycline coupled with MDR are becoming a major concern in the study area. Pre-term delivery and history of stillbirth are identified factors associated with maternal GBS colonization. Therefore, screening of all pregnant mothers for GBS colonization between 36 0/7 and 37 6/7 weeks of gestation should be considered. Administration of antibiotics for prophylaxis or treatment of GBS should be guided by antimicrobial susceptibility testing. Further, studies focusing on outcome of newborns colonized with GBS and molecular characterization of genotypes of GBS and their resistance genes are required to clearly define the epidemiology of GBS, vertical transmission and antimicrobial resistance.
Abbreviations
ACOG, American College of Obstetrics and Gynecology; AST, antimicrobial susceptibility test; BAB: blood agar base; BAP, blood agar plate; BDAZHO, Bahir Dar Administrative Zone Health Office; BDHC, Bahir Dar Health Center; CAMP, Christie–Atkins–Munch–Peterson; CLSI, Clinical Laboratory and Standard Institute; EDHS, Ethiopian Demographic and Health survey; EOD, early onset disease; FHCSH, Felege Hiwot Comprehensive Specialized Hospital; GBS, group B Streptococcus; IAP, intrapartum antibiotic prophylaxis; LOD, late onset disease; MHA, Muller–Hinton agar; PROM, premature rupture of membrane; SBA, sheep blood agar; THB, Todd Hewitt broth.
Data Sharing Statement
The finding of this study is generated from the data collected and analyzed based on the stated methods and materials. All the data are already found in the manuscript, and there is a supplementary file.
Acknowledgments
The authors gratefully acknowledge FHCSH, BDHC and College of Medicine and Health Sciences of Bahir Dar University for conducting the study and undertaking the sample analysis in the microbiology laboratory and the contribution of reference strains and supplies from Amhara Public Health Institute and FHCSH, Bahir Dar, Ethiopia. Special thanks given to study participants and data collectors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared that they have no conflicts of interest for this work.
References
1. Rosa-Fraile M, Spellerberg B, Kraft CS. Reliable detection of Group B Streptococcus in the clinical laboratory. J Clin Microbiol. 2017;55(9):2590–2598. doi:10.1128/JCM.00582-17
2. Sastry AS. Review of microbiology and immunology; 2018.
3. Russell NJ, Seale AC, O’Driscoll M, et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(2):S100–11. doi:10.1093/cid/cix658
4. Cheng L, Xu FL, Niu M, et al. Pathogens and clinical features of preterm infants with sepsis. Zhongguo Dang dai er ke za zhi. Chin J Contemp Pediatr. 2019;21(9):881–885.
5. Girma W, Yimer N, Kassa K, Yesuf E. Group B Streptococcus recto-vaginal colonization in near-term pregnant women, Southwest Ethiopia. Ethiop J Health Sci. 2020;30(5):687–695. doi:10.4314/ejhs.v30i5.7
6. Armistead B, Oler E, Waldorf KA, Rajagopal L. The double life of group B Streptococcus: asymptomatic colonizer and potent pathogen. J Mol Biol. 2019;431(16):2914–2931. doi:10.1016/j.jmb.2019.01.035
7. Burcham LR, Spencer BL, Keeler LR, et al. Determinants of group B streptococcal virulence potential amongst vaginal clinical isolates from pregnant women. PLoS One. 2019;14(12):e0226699. doi:10.1371/journal.pone.0226699
8. Furfaro LL, Chang BJ, Payne MS. Perinatal Streptococcus agalactiae epidemiology and surveillance targets. Clin Microbiol Rev. 2018;31(4):e00049–18. doi:10.1128/CMR.00049-18
9. Vornhagen J, Armistead B, Santana-Ufret V, et al. Group B Streptococcus exploits vaginal epithelial exfoliation for ascending infection. J Clin Invest. 2018;128(5):1985–1999. doi:10.1172/JCI97043
10. Vornhagen J, Waldorf KM, Rajagopal L. Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol. 2017;25(11):919–931. doi:10.1016/j.tim.2017.05.013
11. American College of Obstetricians and Gynecologists. Committee on obstetric practice prevention of group b streptococcal early-onset disease in newborns: ACOG Committee Opinion, Number 797. Obstet Gynecol. 2020;135:e51–72. doi:10.1097/AOG.0000000000003668
12. Kum-Nji P, Meloy L, Pierce J, Ritter A, Wheeler R. Group B streptococcal colonization: prevalence and impact of smoking in women delivering term or near term neonates in a large tertiary care hospital in the southern United States. PLoS One. 2020;15(9):e0239294. doi:10.1371/journal.pone.0239294
13. YektaKooshali MH, Hamidi M, Tousi SM, Nikokar I. Prevalence of group B Streptococcus colonization in Iranian pregnant women: a systematic review and meta-analysis. Int J Reprod BioMed. 2018;16(12):
14. Clouse K, Shehabi A, Suleimat AM, et al. High prevalence of Group B Streptococcus colonization among pregnant women in Amman, Jordan. BMC Pregnancy Childbirth. 2019;19(1):1–8. doi:10.1186/s12884-019-2317-4
15. Ashary N, Singh A, Chhabria K, Modi D. Meta-analysis on prevalence of vaginal group B Streptococcus colonization and preterm births in India. J Matern Fetal Neonatal Med. 2020;1:1–9. doi:10.1080/14767058.2020.1813705
16. Lekala LM, Mavenyengwa RT, Moyo SR, et al. Risk factors associated with group B Streptococcus colonization and their effect on pregnancy outcome. J Gynecol Obstet. 2015;3(6):121–128. doi:10.11648/j.jgo.20150306.14
17. Gizachew M, Tiruneh M, Moges F, Tessema B. Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: a meta-analysis. Ann Clin Microbio Antimicrob. 2019;18(1):1–4.
18. Woldu ZL, Teklehaimanot TG, Waji ST, Gebremariam MY. The prevalence of Group B Streptococcus recto-vaginal colonization and antimicrobial susceptibility pattern in pregnant mothers at two hospitals of Addis Ababa, Ethiopia. Reprod Health. 2014;11(1):1–4. doi:10.1186/1742-4755-11-80
19. Kunze M, Ziegler A, Fluegge K, Hentschel R, Proempeler H, Berner R. Colonization, serotypes and transmission rates of group B streptococci in pregnant women and their infants born at a single University Center in Germany. J Perinat Med. 2011;39(4):417–422. doi:10.1515/jpm.2011.037
20. Chen J, Fu J, Du W, et al. Group B streptococcal colonization in mothers and infants in western China: prevalences and risk factors. BMC Infect Dis. 2018;18(1):1–8. doi:10.1186/s12879-018-3216-4
21. Sridhar Santhanam RJ, Sahni RD, Thomas N, Beck MM. Prevalence of group B Streptococcal colonization among pregnant women and neonates in a tertiary hospital in India. J Turk Ger Gynecol Assoc. 2017;18(4):181. doi:10.4274/jtgga.2017.0032
22. Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B. Proportion of Streptococcus agalactiae vertical transmission and associated risk factors among Ethiopian mother-newborn dyads, Northwest Ethiopia. Sci Rep. 2020;10(1):1–8. doi:10.1038/s41598-020-60447-y
23. Fantahun Y, Sebre S, Seman A, Kumbi S. Magnitude of maternal vaginal colonization of Group B Streptococcus and neonatal transmission in pregnant women during labor and delivery at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2020;58:45.
24. Ali MM, Mulate YW, Woldetsadik DA, et al. Group B streptococci carriage rate and serotype distribution among mother newborn dyads attending Tikur Anbessa Specialized Hospital, Ethiopia. Ethiop Med J. 2020;58(02):234.
25. Ali MM, Asrat D, Fenta DA, Chaka TE, Woldeamanuel Y. Group B Streptococcus colonization rate and serotype distribution among pregnant women and their newborns at Adama Hospital Medical College, Ethiopia. Sci Rep. 2020;10(1):1–7. doi:10.1038/s41598-020-66474-z
26. Seale AC, Bianchi-Jassir F, Russell NJ, et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis. 2017;65(Suppl 2):S200–19. doi:10.1093/cid/cix664
27. Sinha A, Russell LB, Tomczyk S, et al. Disease burden of group B Streptococcus among infants in sub-Saharan Africa: a systematic literature review and meta-analysis. J Pediatr Infect Dis. 2016;35(9):933. doi:10.1097/INF.0000000000001233
28. Debelew GT, Afework MF, Yalew AW. Determinants and causes of neonatal mortality in Jimma zone, southwest Ethiopia: a multilevel analysis of prospective follow up study. PLoS One. 2014;9(9):e107184. doi:10.1371/journal.pone.0107184
29. FHCSH. Felege Hiwot Comprehensive Specialized Hospital annual report; 2020.
30. Mengist A, Kannan H, Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B Streptococci isolates among pregnant women in Jimma, Ethiopia. BMC Res Notes. 2016;9(1):1–5. doi:10.1186/s13104-016-2158-4
31. Hayes K, O’Halloran F, Cotter L. A review of antibiotic resistance in Group B Streptococcus: the story so far. Crit Rev Microbiol. 2020;46(3):253–269. doi:10.1080/1040841X.2020.1758626
32. Russell NJ, Seale AC, O’Sullivan C, et al. Risk of early-onset neonatal group B streptococcal disease with maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(Suppl 2):S152–9. doi:10.1093/cid/cix655
33. BDAHO. Bahir Dar Administrative Zone Health Office annual report; 2020.
34. Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B. Streptococcus agalactiae from Ethiopian pregnant women; prevalence, associated factors and antimicrobial resistance: alarming for prophylaxis. Ann Clin Microbiol Antimicrob. 2019;18(1):1–9. doi:10.1186/s12941-019-0303-3
35. Filkins L, Hauser JR, Robinson-Dunn B, Tibbetts R, Boyanton BL, Revell P. American Society for Microbiology provides 2020 Guidelines for detection and identification of group B Streptococcus. J Clin Microbiol. 2020;59(1):e01230–20. doi:10.1128/JCM.01230-20
36. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
37. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi:10.1111/j.1469-0691.2011.03570.x
38. Ali MM, Woldeamanuel Y, Woldetsadik DA, et al. Prevalence of group B Streptococcus among pregnant women and newborns at Hawassa University comprehensive specialized hospital, Hawassa, Ethiopia. BMC Infect Dis. 2019;19(1):1–9. doi:10.1186/s12879-019-3859-9
39. Belard S, Toepfner N, Capan-Melser M, et al. Streptococcus agalactiae serotype distribution and antimicrobial susceptibility in pregnant women in Gabon, Central Africa. Sci Rep. 2015;5(1):1–4. doi:10.1038/srep17281
40. Edwards JM, Wynn C, Watson N, et al. Group B Streptococcus (GBS) colonization and disease among pregnant women: a historical cohort study. Am J Obstet Gynecol. 2017;217(6):731. doi:10.1016/j.ajog.2017.08.073
41. Le Doare K, Jarju S, Darboe S, et al. Risk factors for Group B Streptococcus colonisation and disease in Gambian women and their infants. Int J Infect. 2016;72(3):283–294. doi:10.1016/j.jinf.2015.12.014
42. Rao GG, Nartey G, McAree T, et al. Outcome of a screening programme for the prevention of neonatal invasive early-onset group B Streptococcus infection in a UK maternity unit: an observational study. BMJ Open. 2017;7(4):e014634. doi:10.1136/bmjopen-2016-014634
43. Alemseged G, Niguse S, Hailekiros H, Abdulkadir M, Saravanan M, Asmelash T. Isolation and anti-microbial susceptibility pattern of group B Streptococcus among pregnant women attending antenatal clinics in Ayder Referral Hospital and Mekelle Health Center, Mekelle, Northern Ethiopia. BMC Res Notes. 2015;8(1):1–8. doi:10.1186/s13104-015-1475-3
44. Shiferawu S, Mekonen M, Baza D, Lera T. Prevalence of Group B Streptococcus, Its associated factors and antimicrobial susceptibility pattern among pregnant women attending antenatal care at Arbaminch Hospital, South Ethiopia. Am J Health Res. 2019;7(6):104–115. doi:10.11648/j.ajhr.20190706.12
45. Joachim A, Matee M, Massawe FA, Lyamuya EF. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital indar Es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health. 2009;9:437. doi:10.1186/1471-2458-9-437
46. Al-sunaidi MO, Damole IO, Bello CS. Prevalence of Group B streptococcus colonization in mothers and babies at Abha General Hospital, Kingdom of Saudi Arabia. Med J Cairo Univ. 2011;79(2):45.
47. Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B. Newborn colonization and antibiotic susceptibility patterns of Streptococcus agalactiae at the University of Gondar Referral Hospital, Northwest Ethiopia. BMC Pediatr. 2018;18(1):1. doi:10.1186/s12887-018-1350-1
48. Eren A, Kucukercan M, Oðuzoðlu N, Oðuzoðlu N, Unal N, Karateke A. The carriage of group B streptococci in Turkish pregnant women and its transmission rate in newborns and serotype distribution. Turk J Pediatr. 2005;47:28–33.
49. Jost C, Bercot B, Jacquier H, et al. Xpert GBS assay for rapid detection of group B Streptococcus in gastric fluid samples from newborns.J Clinical Microbiol. 2014;52(2):657–659. doi:10.1128/JCM.02532-13
50. Shah D, Saxena S, Randhawa VS, Nangia S, Dutta R. Prospective analysis of risk factors associated with group B streptococcal colonisation in neonates born at a tertiary care centre in India. Paediatr Int Child Health. 2014;34(3):184–188. doi:10.1179/2046905513Y.0000000112
51. Saha SK, Ahmed ZB, Modak JK, et al. Group B Streptococcus among pregnant women and newborns in Mirzapur, Bangladesh: colonization, vertical transmission, and serotype distribution. J Clin Microbiol. 2017;55(8):2406–2412. doi:10.1128/JCM.00380-17
52. Yadeta TA, Worku A, Egata G, Seyoum B, Marami D, Berhane Y. Maternal group B Streptococcus recto vaginal colonization increases the odds of stillbirth: evidence from Eastern Ethiopia. BMC Pregnancy Childbirth. 2018;18(1):1–7. doi:10.1186/s12884-018-2044-2
53. Chen Z, Wu CA, Cao X, et al. Risk factors for neonatal group B Streptococcus vertical transmission: a prospective cohort study of 1815 mother–baby pairs. J Perinatol. 2018;38(10):1309–1317. doi:10.1038/s41372-018-0182-z
54. Assefa S Prevalence of Group B streptococci colonization and susceptibility pattern among pregnant women attending antenatal care Clinics of Health Institutions, Addis Ababa, Ethiopia [Doctoral dissertation]. Addis Ababa University. 2014.
55. Sadaka SM, Aly HA, Meheissen MA, Orief YI, Arafa BM. Group B streptococcal carriage, antimicrobial susceptibility, and virulence related genes among pregnant women in Alexandria, Egypt. Alex J Med. 2018;54(1):69–76.
56. Haimbodi EL, Mukesi M, Moyo SR. Prevalence and molecular characterization of group B streptococcus in pregnant women from hospitals in Ohangwena and Oshikoto regions of Namibia. BMC Microbiol. 2021;21:224. doi:10.1186/s12866-021-02283-2
57. Melo SC, Santos NC, Oliveiria MD, et al. Antimicrobial susceptibility of Streptococcus agalactiae isolated from pregnant women. Revista Do Instituto de Medicina Tropical de São Paulo;2016. 58. doi10.1590/S1678-9946201658058
58. Gao K, Guan X, Zeng L, et al. An increasing trend of neonatal invasive multidrug-resistant group B Streptococcus infections in southern China, 2011–2017. Infect Drug Resist. 2018;11:2561.
59. Plainvert C, Hays C, Touak G, et al. Multidrug-resistant hypervirulent Group B streptococcus in neonatal invasive infections, France, 2007–2019. Emerging Infect Dis. 2020;26(11):2721. doi:10.3201/eid2611.201669
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
