Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Glucarpidase for Treating Adults with Delayed Methotrexate Elimination Due to Impaired Renal Function: An Economic Simulation Analysis
Authors Kala J, Nelson R, Drudge C , Zhou A, Ward S, Bourque M
Received 22 November 2022
Accepted for publication 23 February 2023
Published 8 March 2023 Volume 2023:15 Pages 165—179
DOI https://doi.org/10.2147/CEOR.S397154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Jaya Kala,1,* Rebecca Nelson,2,* Christopher Drudge,3 Allen Zhou,3 Suzanne Ward,4 Megan Bourque3
1University of Texas Health Science Center, Houston, TX, USA; 2Moffitt Cancer Center and Research Institute, Tampa, FL, USA; 3Value and Evidence, EVERSANA, Burlington, ON, Canada; 4BTG International Inc, West Conshohocken, PA, USA
*These authors contributed equally to this work
Correspondence: Christopher Drudge, Value and Evidence, EVERSANA, 204-3228 South Service Road, Burlington, ON, L7N 3H8, Canada, Tel +1 905 637 6231, Fax +1 905 637 5014, Email [email protected]
Background: Glucarpidase is indicated for treating delayed methotrexate (MTX) elimination due to impaired renal function. Although glucarpidase is capable of rapidly eliminating MTX independent of renal clearance, its cost can be perceived as a barrier to use. However, no published economic analyses have evaluated glucarpidase relative to comparable treatments.
Purpose: To assess the economic value of glucarpidase for treating adult patients in the United States (US) who experience delayed MTX elimination due to impaired renal function.
Methods: A decision tree model was developed to assess the economic value of glucarpidase. The short-term inpatient management of patients as well as long-term survival were simulated. Costs associated with the use of glucarpidase were compared against other methods for treating delayed MTX elimination due to impaired renal function under two scenarios: current practice (ie, mix of timely/delayed use of glucarpidase, hemodialysis, or supportive care [SC] alone) as compared with proposed practice (ie, timely glucarpidase administration within 60 hours for all eligible patients). Hypothetical practical scenarios for US institutions were also considered.
Results: For adult patients with delayed MTX elimination, proposed practice as compared to current practice was associated with an increased cost of $20,024 per patient, not considering any incremental reimbursement associated with glucarpidase administration. Importantly, early treatment with glucarpidase, within 60 hours, was shown to be less expensive per patient than delayed glucarpidase treatment or treating with hemodialysis, but more expensive than SC alone. However, proposed practice was associated with multiple clinical benefits, including shorter hospital length of stay. For hypothetical practical scenarios, minimal shifts in treatment patterns had minimal cost impacts.
Conclusion: Treatment of all eligible patients with glucarpidase within 60 hours was associated with an increased cost per patient (relative to current practice) but substantial improvements in clinical outcomes. Timely glucarpidase use was less expensive than delayed glucarpidase or hemodialysis.
Keywords: chemotherapy, toxicity, costs, outcomes
Introduction
Methotrexate (MTX) is an antifolate widely used to treat cancers (eg, lymphomas, acute lymphoblastic leukemia, osteosarcoma) and autoimmune diseases.1 As a cancer therapy, MTX may be given at doses exceeding 500 mg/m2 (high-dose MTX; HDMTX).1 It is primarily eliminated by the kidney through passive glomerular filtration and active tubular reabsorption and secretion; as such, renal function is a major determinant of MTX clearance.2 In a minority of patients, estimated at less than 15%, administration of HDMTX is followed by delayed MTX elimination due to impaired renal function.3–7 The resulting elevated MTX plasma concentration and tissue accumulation can lead to a range of severe toxicities (eg, hepatotoxicity, mucositis, myelosuppression, and nephrotoxicity) and appreciable morbidity and mortality, in part because of cancer treatment disruption.1,8
To reduce the risk of toxicity from delayed MTX elimination due to impaired renal function, HDMTX treatment is typically accompanied by supportive care (SC) measures including fluid hydration, urine alkalinization, and leucovorin (and, in many cases, high-dose leucovorin [HDLV]).1,8 Patients are monitored regularly for signs of delayed MTX elimination, since early intervention is of crucial importance to not only lower the likelihood of toxicity but also minimize the impact to cancer treatment.1 In the event of delayed MTX elimination due to impaired renal function, treatment options include HDLV, hemodialysis, and/or glucarpidase.8 Leucovorin has to compete with MTX to be taken up by cells and thus is less effective when MTX concentrations are too high (>10 µM).8 Whereas leucovorin requires the restoration of normal renal function for MTX elimination to proceed, high-flux hemodialysis can improve MTX clearance. However, hemodialysis typically requires multiple sessions, extends inpatient stay, may lead to severe complications, and can be followed by a rebound in MTX levels.1,9
Glucarpidase (Voraxaze®, BTG International Inc.) is a recombinant carboxypeptidase that rapidly reduces plasma MTX concentration by hydrolyzing MTX into two inactive metabolites.8 Administered intravenously, glucarpidase can substantially lower the plasma MTX concentration by 97% within 15 minutes, independent of renal clearance, with 91% of patients sustaining MTX reduction for up to 8 days.10 Adverse events (AEs) are minimal with glucarpidase, with the most common reactions generally being grade 1 or 2.11 In 2012, the United States (US) Food and Drug Administration (FDA) approved glucarpidase for lowering toxic plasma MTX levels (>1 μmol/L) in adult and pediatric patients with delayed MTX clearance (plasma MTX concentrations greater than 2 standard deviations of the mean MTX excretion curve specific for the dose of MTX administered) due to impaired renal function.11 Notably, the FDA label for MTX explicitly recommends glucarpidase use for this patient population.12
A consensus guideline identified optimal glucarpidase administration as that occurring within 48–60 hours from the start of HDMTX infusion (hereafter referred to as “timely glucarpidase”).8 Although glucarpidase can still effectively reduce plasma MTX concentrations if given beyond 60 hours (“delayed glucarpidase”), delayed glucarpidase administration may impact the prevention of life-threatening toxicities, as MTX toxicity is both concentration and time dependent.8 A study of 76 patients treated with leucovorin and glucarpidase demonstrated that treatment with glucarpidase within 96 hours of HDMTX administration without pre-existing grade 4 adverse events resulted in only 14% experiencing grade 4 toxicity compared to 55% of patients treated with glucarpidase after 96 hours.13 Similarly, in a study of 476 patients, timely administration of glucarpidase reduced mortality rates from 22% to 10.9%.14
Although there are no head-to-head randomized controlled trials comparing glucarpidase with other interventions (reflecting its infrequent use, heterogeneous target population, and ethical considerations), several key observational studies have demonstrated superior glucarpidase efficacy. A pooled analysis of four multicenter single-arm compassionate-use clinical trials demonstrated that (1) glucarpidase rapidly and consistently reduced plasma MTX concentrations in patients with HDMTX-induced renal toxicity and (2) 64% of patients with Common Terminology Criteria for AEs grade 2 or higher renal impairment recovered to grade 0 or 1 after a median of 12.5 days following glucarpidase administration.10 A retrospective study using Medicare claims data (2010–2017) found that older adult cancer patients treated with glucarpidase had lower inpatient mortality and 90-day mortality compared with non-glucarpidase patients (including a subset of patients who received hemodialysis), and patients who received glucarpidase also had both shorter overall hospital length of stay (LOS) and shorter intensive care unit (ICU) LOS.15 Based on a systematic literature review and modified Delphi panel, the EXTRIP (EXtracorporeal TReatments In Poisoning) workgroup recommended against hemodialysis instead of administering glucarpidase for patients with severe MTX toxicity receiving standard care.16 The clinical value associated with the administration of glucarpidase for patients with delayed MTX clearance is well accepted, and its use is widely recommended.5,8,12,16,17
Although the clinical value of glucarpidase in patients with delayed MTX elimination due to impaired renal function is well accepted, this medication may be underutilized in practice. A recent consensus guideline for the stocking of antidotes in emergency departments recommended that hospitals should stock glucarpidase,18 but this is not routinely done by many institutions.9 There may be hesitancy, or funding restrictions, around acquiring and prescribing glucarpidase due to its cost.9,19 However, there have been no published economic analyses to evaluate the cost-effectiveness or budget impact of glucarpidase. There are other costs (eg, hospitalization, SC, hemodialysis, monitoring) associated with delayed MTX elimination treatment that are important to consider when evaluating the impact of glucarpidase use.
The objective of this study was to conduct an economic analysis for the use of glucarpidase in adults in the US who experience delayed MTX elimination due to impaired renal function after HDMTX, to (1) compare outcomes associated with current practice versus proposed practice (ie, timely access to glucarpidase within 60 hours for all patients who are eligible) and (2) determine the clinical and cost impact of timely glucarpidase use versus delayed glucarpidase use, hemodialysis, or SC alone.
Methods
Model Overview
Economic modelling seeks to estimate costs and clinical outcomes as accurately as possible, informing the simulation with simplifying assumptions to reflect complex disease and treatment paradigms. A decision tree model was developed in Microsoft Excel (Microsoft 365) from the US hospital institution perspective to simulate clinical outcomes and costs associated with the treatment of adult patients with delayed MTX elimination due to impaired renal function, as defined in the FDA label for glucarpidase,11 who received one of four treatments: SC alone or in conjunction with timely glucarpidase, delayed glucarpidase, or hemodialysis. The model compared two hypothetical scenarios: current practice (ie, fewer patients on glucarpidase than indicated based on the FDA label, with delayed or no administration in many patients) versus proposed practice (ie, timely glucarpidase administration for all eligible patients). The analysis was selected to evaluate the most extreme cost impact associated with shifting practice patterns, that is, if all eligible patients were to receive glucarpidase. The core model considered inpatient care and used a two-month time horizon; a short time horizon prevented the need for extrapolation of clinical outcomes over the long-term. A longer (3-year) time horizon was considered to estimate cancer survival associated with ability to resume chemotherapy after treatment for delayed MTX elimination.
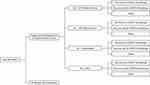
Model Design
The model structure is illustrated in Figure 1. Adult patients (18 years of age or older) who received HDMTX and experienced delayed MTX elimination due to impaired renal function in the simulation received one of four treatments (SC alone, or SC in conjunction with timely glucarpidase, delayed glucarpidase, or hemodialysis). Patients receiving SC alone may have also received HDLV. Patients were modelled to have experienced one of three outcomes: (1) recovery and rechallenge with HDMTX, (2) recovery but no rechallenge with HDMTX, or (3) death. Patients who received HDMTX but did not experience delayed MTX elimination due to impaired renal function were not considered.
Clinical outcomes and costs were modeled to calculate the difference between current practice patterns and proposed practice. Only costs incurred during inpatient stay were considered. Costs for cancer treatment (eg, chemotherapy) were not considered because it was assumed that those would not differ by treatment arm. Costs associated with readmissions related to delayed MTX elimination (eg, acute kidney injury, mucositis) were also not considered, which was expected to underestimate the benefit associated with glucarpidase use as timely glucarpidase would likely reduce long-term damage associated with slow recovery from high MTX levels.
Incremental total costs (ie, the difference in all healthcare costs when shifting from current practice to proposed practice) and estimated inpatient survival for current practice versus proposed practice were used to calculate the cost per additional inpatient survival under the proposed practice scenario.
Model Parameters
Model inputs and their sources are shown in Table 1. A series of targeted literature searches were used to identify model inputs. The starting patient population was chosen to reflect an average number of patients treated with HDMTX at a large US institution in one year. It was estimated that 6.4% of adult patients treated with HDMTX may experience delayed MTX elimination due to impaired renal function, using the proportion of patients experiencing acute renal failure after treatment with HDMTX as a proxy to inform this.3 Market research data were used to inform the mix of treatments used in current practice, which estimated that 10.8% of patients receive timely glucarpidase, 7.2% received delayed glucarpidase, 30% received dialysis, and the rest received supportive care, and it was assumed in the proposed practice scenario that 100% of eligible patients receive glucarpidase in a timely manner. To enable the evaluation of whole patients in the analysis (ie, one patient receiving glucarpidase in the current scenario), and optimize interpretability of the results, a starting population of 78 patients was assumed. Drug costs, SC costs, and monitoring costs used in the model were current at the time of analysis (June 2022). Where possible, a single reference was used to inform a given input across treatment arms, to reduce the bias associated with comparing divergent study designs. For example, many clinical inputs, including length of stay and inpatient mortality, were derived from a retrospective analysis of patients with delayed clearance of MTX who were treated with glucarpidase. This dataset was a 2010–2017 Medicare claims retrospective study that captured real-world evidence on the clinical experience of patients with MTX toxicity, reflecting the variability in patient experience (eg, different cancer types, different MTX dosing regimens, different supportive care procedures) that is inherent in clinical practice.15 In this study, there was a small number of patients (13%) who received glucarpidase as well as dialysis, so the cost of dialysis for those patients was included in the present model so as to not bias in favor of glucarpidase. It was assumed that adult patients only require one round of glucarpidase treatment. Further, it was assumed that adult patients receive 4 vials of glucarpidase, based on the recommended weight-based dose in the FDA label and the average adult weight in the US,11 and to align with efficacy estimates available for glucarpidase. Additional assumptions for individual model inputs are described in Table 1.
 |
Table 1 Model Inputs for the Base Case Analysis |
Reimbursement was not considered in the model, due to the large variability in reimbursement rates across patients and facilities. It is expected that glucarpidase use is associated with incremental reimbursement rates, and therefore the economic impact to the institution associated with glucarpidase is expected to be less in real world practice than reported in the model.
Sensitivity Analyses
A sensitivity analysis using model inputs for pediatric patients (where available) was conducted to understand any differences in model results compared with adults. Pediatric patients were not included in the base case analysis because key model inputs (eg, inpatient mortality rates, average LOS) specific to this age group were not available.
Additional sensitivity analyses were conducted to assess the impact of changing the following model inputs: proportion of patients who experienced delayed MTX elimination due to impaired renal function following HDMTX, inpatient mortality rates, and hospitalization costs. All included sensitivity analyses and model inputs specific to these analyses are provided in Table 2.
 |
Table 2 Changes to Model Inputs for Sensitivity Analyses |
Hypothetical Practical Scenarios
Two hypothetical practical scenarios were tested in the model to consider the real-world implications of changes in practice at US institutions. The first scenario involves an institution that sees three adult patients with delayed MTX elimination due to impaired renal function (of 50 treated with HDMTX) per year and does not stock glucarpidase or use hemodialysis. This scenario compares current practice of one patient treated with delayed glucarpidase to a hypothetical future practice treating either one or two patients with timely glucarpidase.
The second scenario involves an institution that sees five adult patients with delayed MTX elimination due to impaired renal function (of 100 treated with HDMTX) per year, maintains a stock of glucarpidase, and uses hemodialysis. In this scenario, current practice sees two patients treated with timely glucarpidase and one with hemodialysis, and future practice increases the number of patients treated with timely glucarpidase to either four or all five patients. All included hypothetical practical scenarios are provided in Table 3.
 |
Table 3 Hypothetical Practical Scenarios for US Institutions |
Results
For the base case analysis, of the 78 adult patients treated with HDMTX, a cohort of five patients was estimated to experience delayed MTX elimination due to impaired renal function. In the current practice scenario, only one patient received glucarpidase. Average costs per patient in the current practice scenario as compared with the proposed practice scenario are presented in Figure 2. Hospitalization (ICU and non-ICU), SC, and monitoring costs were lower in the proposed practice scenario, whereas glucarpidase drug costs were higher. Across cost components, the largest differences between scenarios were for glucarpidase drug costs and ICU hospitalization costs. Proposed practice was associated with an increase of $20,024 in costs per patient. The average per-patient cost was $168,501 in the current practice scenario and $188,525 in the proposed scenario. Considering all costs, proposed practice was associated with an increased total cost of $99,959 for the cohort of five patients with delayed MTX elimination due to impaired renal function. The cost per patient (considering all cost categories) by treatment received was $188,525 for timely glucarpidase, $262,895 for delayed glucarpidase, $234,487 for hemodialysis, and $113,203 for SC ± HDLV (Figure 3).
 |
Figure 2 Average costs per patient (USD) associated with current practice (ie, fewer patients on GPD than indicated based on FDA label, with delayed or no administration in many patients) and proposed practice (ie, timely GPD administration for all eligible patients) scenarios for adult patients with delayed MTX elimination due to impaired renal function by each component of care. Abbreviations: GPD, glucarpidase; ICU, intensive care unit; MTX, methotrexate; SC, supportive care; USD, United States dollars. Note: ‘All costs’ is a weighted average of costs shown in Figure 3. |
Of patients with delayed MTX elimination due to impaired renal function, 31% required hemodialysis in the current practice scenario, but none required hemodialysis in the proposed practice scenario. The total hospital LOS (ICU and non-ICU) for the cohort was reduced by 80 days in the proposed glucarpidase use scenario (average of 16 fewer days per patient) compared with the current practice scenario. Total ICU LOS was reduced by 48 days (average of 10 fewer days per patient) in the proposed practice scenario. Total hospital (ICU and non-ICU) LOS and total ICU LOS for the cohort were 130 days and 52 days, respectively, in the current practice scenario and 50 days and 4 days, respectively, in the proposed practice scenario. The proportion of patients able to resume and complete HDMTX increased from 18% (current practice) to 25% (proposed practice) of patients. No patients died during inpatient treatment in the proposed practice scenario, whereas inpatient mortality was 27% in the current practice scenario. The proposed practice scenario was associated with an increase in long-term survival. The proportion of patients who survived after three years was 35% in the current practice scenario and 48% in the proposed practice scenario. The proposed practice scenario was associated with an additional cost per life saved of $75,296, based on an incremental total inpatient cost of $99,959 and incremental inpatient survival of 1.3 patients.
For outcomes per patient by treatment received, the average overall (ICU and non-ICU) hospital LOS was 10.0 days for timely glucarpidase, 21.7 days for delayed glucarpidase, 40.2 days for hemodialysis, and 21.9 days for SC ± HDLV. The corresponding ICU LOS was 0.8 days, 8.9 days, 18.2 days, and 8.3 days, respectively. Inpatient survival by treatment received was 100% for timely glucarpidase, 92% for delayed glucarpidase, 49% for hemodialysis, and 79% for SC ± HDLV. The corresponding 3-year survival was 48%, 44%, 24%, and 38%, respectively. After accounting for inpatient mortality, the proportion of patients who were able to rechallenge with HDMTX by treatment received was 25% for timely glucarpidase, 23% for delayed glucarpidase, 12% for hemodialysis, and 20% for SC ± HDLV.
Results of sensitivity analyses are provided in Figure 4. For the analysis considering pediatric patients, total costs for the cohort and average costs per patient were lower compared with adults (ie, the base case analysis), and cost savings of $3444 per patient were achieved with proposed practice compared with current practice. Proposed glucarpidase use was also consistently associated with improved clinical outcomes in this patient group.
 |
Figure 4 Variation in treatment costs (USD) associated with sensitivity analyses described in Table 2. Note: Budget impact refers to the cost difference in switching from current practice to proposed practice. Abbreviations: HD, hemodialysis; ICU, intensive care unit; MTX, methotrexate; SC, supportive care; USD, United States dollars. |
Results of two hypothetical practical scenarios are provided in Table 3. For the first scenario, changing from current practice of one patient treated with delayed glucarpidase to either one or two patients treated with timely glucarpidase was associated with a cost savings of $74,370 and an additional cost of $952, respectively, for the institution. For the second scenario, changing from current practice of two patients treated with timely glucarpidase and one with hemodialysis to either four or all five patients treated with timely glucarpidase was associated with an additional cost of $29,359 and $104,681, respectively, for the institution.
Discussion
This economic analysis considered the estimated clinical and cost outcomes for adult patients in the US who received treatment for delayed MTX elimination due to impaired renal function, for the mix of treatments used in current practice as compared with proposed practice.
Enabling timely access to glucarpidase for all eligible patients was associated with a cost increase per patient of 12% relative to the average per-patient cost in the current practice scenario, while at the same time increasing patient survival, decreasing the number of patients who require hemodialysis, increasing the opportunity for HDMTX rechallenge, and reducing hospital LOS. A reduction in hospital LOS will additionally help to free up resources for other patients, and so is associated with increased economic opportunity. There are many factors to consider in therapeutic decision-making, including, but not limited to, the costs and clinical outcomes discussed herein. For each patient with delayed MTX elimination due to impaired renal function, timely treatment with glucarpidase resulted in cost savings compared with hemodialysis or delayed glucarpidase. Treatment with glucarpidase had an incremental cost versus SC alone but was associated with substantially improved clinical outcomes. Timely treatment with glucarpidase resulted in decreased hospital costs, which helped offset drug acquisition costs.
It was not possible to perform a full analysis of pediatric patients based on currently available data. Nonetheless, a sensitivity analysis was possible using the risk of delayed MTX elimination due to impaired renal function and the likelihood of HDMTX rechallenge and 3-year OS for a pediatric population, using drug doses appropriate for this group, and assuming they are not treated with hemodialysis. Other model inputs were the same as those used for the base case analysis in adult patients. The results of this sensitivity analysis suggest that for pediatric patients, shifting to proposed glucarpidase use not only improves patient outcomes but also results in cost savings.
The impact of minimal shifts in treatment patterns to improve patient outcomes on institution costs was demonstrated for two hypothetical practical scenarios anticipated to be relevant to US institutions. First, moving from one patient treated with delayed glucarpidase to one patient treated with timely glucarpidase resulted in cost savings; moving to two patients being treated with timely glucarpidase was associated with a very minimal additional cost. Second, moving from two patients treated with timely glucarpidase and one with hemodialysis to four or all five patients treated with timely glucarpidase was associated with additional costs that were relatively small compared to the cost for the institution. For example, the $29,359 additional cost for four patients treated with timely glucarpidase represents a cost increase of only 3.5%.
There were a few limitations to note for this simulation. First, limited data were available to inform model inputs, necessitating a variety of assumptions. Where data were limited, similar assumptions were made across treatment arms to not bias the model results. A key source of model inputs was a study of Medicare claims data by Demiralp and colleagues (2019),15 which, while valuable as a reflection of real-world experience (in that it represents the true variability observed in clinical practice, and captures different cancer types, MTX dosing regimens, and supportive care practices), was impacted by challenges shared by all retrospective database analyses, such as missing data and potential inconsistency in reporting diagnosis of MTX-associated kidney injury. Second, the inherent simplicity of decision tree models constrained the scope and complexity of this analysis; simulated results aim to present an average patient and facility experience, but do not capture patient-to-patient nuances. However, the use of this modeling approach was appropriate given the type of data that were available, the short-term time horizon, and the research question. Finally, the model did not include parameters for individual AEs, as AE-associated costs were assumed to be captured within the hospitalization costs. This approach may have resulted in an underestimation of the benefits associated with proposed glucarpidase use given that (1) timely glucarpidase may reduce long-term AEs requiring readmission, such as mucositis, and (2) hemodialysis may lead to specific severe AEs not expected for other treatment approaches (eg, catheter exit site bleeding, electrolyte imbalances, stroke, arrhythmias).20
Study strengths included the use of conservative assumptions expected to bias against the economic value of glucarpidase and the inclusion of sensitivity analyses using published alternative data sources to assess the robustness of model results to changes in key inputs. To our knowledge, this is the first economic analysis to consider the costs and clinical outcomes associated with the use of glucarpidase and other treatments for delayed MTX elimination due to impaired renal function in the US.
Additional analyses would be helpful to build on this analysis and consider other patient populations by age, cancer type, and geographical location, as well as different model perspectives (eg, considering societal impacts/patient-borne costs). However, given the data limitations encountered in this analysis, future analyses would benefit from additional studies aimed at generating relevant data for key model inputs. For example, there was a paucity of mortality and resource use data specific to pediatric patients, which necessitated using data for adult patients in this sensitivity analysis.
Conclusions
For adult patients in the US with delayed MTX elimination due to impaired renal function, shifting from current practice to all eligible patients receiving treatment with glucarpidase within 60 hours (ie, proposed practice) was estimated to be associated with a cost increase per patient of $20,024 and an additional cost per life saved of $75,296, while improving patient outcomes substantially. In the simulation, timely treatment with glucarpidase resulted in a cost savings of $74,370 and $45,962 per patient compared to delayed glucarpidase treatment or treating with hemodialysis, respectively, but was more expensive than SC alone, without considering the additional reimbursement that may be associated with glucarpidase use.
Abbreviations
AE, adverse event; AKI, acute kidney injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; CMS, Centers for Medicare & Medicaid Services; CNS, central nervous system; FDA, Food and Drug Administration; GPD, glucarpidase; HCPCS, Healthcare Common Procedure Coding System; HDLV, high-dose leucovorin; HDMTX, high-dose methotrexate; HPLC, high-performance liquid chromatography; ICU, intensive care unit; KM, Kaplan-Meier; LDLV, low-dose leucovorin; LOS, length of stay; MTX, methotrexate; N/A, not applicable; NDC, National Drug Code; OS, overall survival; SC, supportive care; US, United States; USD, United States dollars; WAC, wholesale acquisition cost.
Ethics Statement
Institutional review board or ethics committee approval was not required for this study because it was a pharmacoeconomic modeling study that did not involve patient contact and used aggregated anonymized data from other studies.
Acknowledgments
The authors thank Heidi Trinkman (Cook Children’s Medical Center) for feedback on model parameters and assumptions; Salvatore Miragliotta, Christon Hill, Brian Hogan, Marie Bodart, and Scott Zimmerman (BTG International) for feedback on the model and the draft version of this manuscript; Stephen Brown (EVERSANA) for assistance with model design; and Coby Martin (EVERSANA) for assistance with model parameterization and review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by BTG International Inc. The funder was involved in the study design; collection, analysis, and interpretation of data; writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosure
J. Kala and R. Nelson have received compensation for serving as a consultant or speaker for BTG International Inc. C. Drudge, A. Zhou, and M. Bourque are employees of EVERSANA, which has provided paid consulting services to BTG International Inc. S. Ward is an employee of BTG International Inc. The authors report no other conflicts of interest in this work.
References
1. Howard SC, McCormick J, Pui C-H, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471–1482. doi:10.1634/theoncologist.2015-0164
2. Joerger M, Huitema ADR, Van Den Bongard HJGD, et al. Determinants of the elimination of methotrexate and 7-hydroxy-methotrexate following high-dose infusional therapy to cancer patients. Br J Clin Pharmacol. 2006;62(1):71–80. doi:10.1111/j.1365-2125.2005.02513.x
3. de Miguel D, García-Suárez J, Martín Y, Gil-Fernández JJ, Burgaleta C. Severe acute renal failure following high-dose methotrexate therapy in adults with haematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23(12):3762–3766. doi:10.1093/ndt/gfn503
4. Drost SA, Wentzell JR, Giguère P, et al. Outcomes associated with reducing the urine alkalinization threshold in patients receiving high-dose methotrexate. Pharmacotherapy. 2017;37(6):684–691. doi:10.1002/phar.1935
5. Gros L, Roldán A, Cabero-Martínez A, et al. Incidence and management of patients with methotrexate delayed elimination in the clinical practice: a Delphi study. J Oncol Pharm Pract. 2022:107815522210795. doi:10.1177/10781552221079568
6. May J, Carson KR, Butler S, Liu W, Bartlett NL, Wagner-Johnston ND. High incidence of methotrexate associated renal toxicity in patients with lymphoma: a retrospective analysis. Leuk Lymphoma. 2014;55(6):1345–1349. doi:10.3109/10428194.2013.840780
7. Steward JS, Bullard HM, O’Rourke TJ, et al. Effect of single agent high-dose methotrexate-related acute kidney injury on length of hospitalization and relative dose intensity in adult patients with central nervous system lymphoma. J Oncol Pharm Pract. 2016;23(7):496–501. doi:10.1177/1078155216665244
8. Ramsey LB, Balis FM, O’Brien MM, et al. Consensus guideline for use of glucarpidase in patients with high‐dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist. 2018;23(1):52–61. doi:10.1634/theoncologist.2017-0243
9. Kitchlu A, Shirali AC. High-flux hemodialysis versus glucarpidase for methotrexate-associated acute kidney injury: what’s best? J Onco-Nephrol. 2019;3(1):11–18. doi:10.1177/2399369319827305
10. Widemann BC, Schwartz S, Jayaprakash N, et al. Efficacy of glucarpidase (carboxypeptidase G2) in patients with acute kidney injury after high-dose methotrexate therapy. Pharmacotherapy. 2014;34(5):427–439. doi:10.1002/phar.1360
11. VORAXAZE (glucarpidase). Package insert. BTG International Inc; 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125327s064lbl.pdf.
12. Methotrexate. Package insert. Pfizer Inc; 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/011719Orig1s131lbl.pdf.
13. Widemann BC, Balis FM, Kim A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28(25):3979–3986. doi:10.1200/JCO.2009.25.4540
14. Ward S, King T, Chauhan N. Pooled analysis of time to administration of glucarpidase for methotrexate toxicity versus mortality. Clin Toxicol. 2013;51(7):577–578. doi:10.3109/15563650.2013.817658
15. Demiralp B, Koenig L, Kala J, et al. Length of stay, mortality, and readmissions among Medicare cancer patients treated with glucarpidase and conventional care: a retrospective study. Clinicoecon Outcomes Res. 2019;11:129–144. doi:10.2147/ceor.S188786
16. Ghannoum M, Roberts DM, Goldfarb DS, et al. Extracorporeal treatment for methotrexate poisoning: systematic review and recommendations from the EXTRIP workgroup. Clin J Am Soc Nephrol. 2022;17(4):602. doi:10.2215/CJN.08030621
17. National Comprehensive Cancer Network. NCCN Guidelines Version 1.2022 Acute Lymphoblastic Leukemia. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1410.
18. Dart RC, Goldfrank LR, Erstad BL, et al. Expert consensus guidelines for stocking of antidotes in hospitals that provide emergency care. Ann Emerg Med. 2018;71(3):314–325.e1. doi:10.1016/j.annemergmed.2017.05.021
19. Truong H, Leung N. Fixed-dose glucarpidase for toxic methotrexate levels and acute kidney injury in adult lymphoma patients: case series. Clin Lymphoma Myeloma Leuk. 2021;21(6):e497–e502. doi:10.1016/j.clml.2021.01.006
20. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694–703. doi:10.1634/theoncologist.11-6-694
21. Singh C, Jain A, Takkar A, et al. Successful use of high dose methotrexate in treatment of primary CNS lymphoma patients without access to serum methotrexate levels monitoring: challenges and outcome. Indian J Hematol Blood Transfus. 2022;38(1):68–77. doi:10.1007/s12288-021-01438-5
22. EVERSANA. NAVLIN price data. Available from: https://navlin.com/.
23. Candrilli S, Bell T, Irish W, Morris E, Goldman S, Cairo MS. A comparison of inpatient length of stay and costs among patients with hematologic malignancies (excluding Hodgkin disease) associated with and without acute renal failure. Clin Lymphoma Myeloma. 2008;8(1):44–51. doi:10.3816/CLM.2008.n.003
24. Sanders KN, Aggarwal J, Stephens JM, et al. Cost impact of hydroxocobalamin in the treatment of patients with known or suspected cyanide poisoning due to smoke inhalation from closed-space fires. Burns. 2021;48(6):1325–1330. doi:10.1016/j.burns.2021.10.017
25. CMS. Clinical laboratory fee schedule, 2nd quarter 2022. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.
26. Flombaum CD, Liu D, Yan SQ, et al. Management of patients with acute methotrexate nephrotoxicity with high-dose leucovorin. Pharmacotherapy. 2018;38(7):714–724. doi:10.1002/phar.2145
27. Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One. 2010;5(1):e8933. doi:10.1371/journal.pone.0008933
28. Drugs.com. Leucovorin dosage.; 2021. Available from: https://www.drugs.com/dosage/leucovorin.html.
29. Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222–2232. doi:10.1002/cncr.20255
30. Svahn T, Mellgren K, Harila-Saari A, et al. Delayed elimination of high-dose methotrexate and use of carboxypeptidase G2 in pediatric patients during treatment for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017;64(7):e26395. doi:10.1002/pbc.26395
31. Howard S, Harry MT, John S, Marada S, Napier N. Management of delayed elimination of high-dose methotrexate in community oncology practices can mitigate its effect on length of hospital stay, treatment dose-density, acute kidney injury, dialysis, and death. Blood. 2019;134(S1):5889. doi:10.1182/blood-2019-132098
32. GlobalRPh. Body surface area multi-calculator; 2022. Available from: https://globalrph.com/medcalcs/body-surface-area-multi-calc-multiple-analysis/.
33. Binder AF, Burdette S, Galanis P, Birchmeier K, Handley N, Piddoubny M. Decreasing cost and decreasing length of stay after implementation of updated high-dose methotrexate discharge criteria. JCO Oncol Pract. 2020;16(8):e791–e796. doi:10.1200/JOP.19.00566
34. Halpern NA, Pastores SM. Critical care medicine beds, use, occupancy, and costs in the United States: a methodological review. Crit Care Med. 2015;43(11):2452–2459. doi:10.1097/CCM.0000000000001227
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Frailty and Cancer: Current Perspectives on Assessment and Monitoring
Goede V
Clinical Interventions in Aging 2023, 18:505-521
Published Date: 28 March 2023
Examining the Burden of Potentially Avoidable Heart Failure Hospitalizations
Zilberberg MD, Nathanson BH, Sulham K, Mohr JF, Goodwin M, Shorr AF
ClinicoEconomics and Outcomes Research 2023, 15:721-731
Published Date: 29 September 2023