Back to Journals » Journal of Inflammation Research » Volume 14
Genistein Attenuates Isoflurane-Induced Neuroinflammation by Inhibiting TLR4-Mediated Microglial-Polarization in vivo and in vitro
Authors Jiang T, Xu S, Shen Y, Xu Y, Li Y
Received 3 February 2021
Accepted for publication 9 March 2021
Published 17 June 2021 Volume 2021:14 Pages 2587—2600
DOI https://doi.org/10.2147/JIR.S304336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Tao Jiang, Shoucai Xu, Yangyang Shen, Yong Xu, Yuwen Li
Shandong Cancer Research Institute, Shandong First Medical University & Shandong Academy of Medical Sciences, Tai’an, People’s Republic of China
Correspondence: Tao Jiang
Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, 440, Jiyan Road, Jinan City, Shandong Province, 250117, People’s Republic of China
Tel +86-15963126851
Email [email protected]
Background: Isoflurane, a widely used anesthetic in surgery, has been found to induce neurotoxicity. In parallel, genistein is thought to attenuate isoflurane-induced neurotoxicity, although underlying molecular mechanisms are still unclear. In this study, we studied the protective effects of genistein on isoflurane-induced neuroinflammation in rats and BV2 cells.
Methods: Sprague-Dawley rat pups were exposed to 0.75% isoflurane for 6 hours at postnatal day 7 (P7), and genistein (20, 40, or 80 mg/kg/day) or saline administered from P3 to P15. Hippocampal single-cell suspensions were prepared and apoptosis analyzed by flow cytometry. mRNA expression was determined by RT-qPCR, while protein expression was assessed using Western blot, immunochemistry and immunofluorescence. TLR4 was knocked-out in BV2 cells through CRISPR-Cas9.
Results: Genistein treatment reduced isoflurane-induced apoptosis and inflammation in rat hippocampus. Importantly, genistein promoted M2 and suppressed M1 microglia polarization in rat hippocampus after stimulation with isoflurane. In addition, genistein reduced isoflurane-induced protein expression levels of TLR4, MyD88, TRAF6, p-TAK1, p-p38, p-ERK, p-IκBα and p-NF-κB in rat hippocampus. In BV2 cells exposed to isoflurane, genistein treatment decreased IL-1β, TNF-α, IL-6 and IL-8 mRNA expressions, promoted M2 and suppressed M1 microglia polarization. Similarly, genistein also decreased TLR4 protein levels in isoflurane-induced BV2 cells. However, genistein did not affect CD16, iNOS, CD206 and Arg1 protein levels in TLR4-KO BV2 cells exposed to isoflurane.
Conclusion: Genistein attenuates isoflurane-induced neurotoxicity by inhibiting TLR4-mediated microglial inflammation in vivo and in vitro.
Keywords: genistein, isoflurane, neurotoxicity, TLR4
Introduction
Inhalation anesthetics, such as isoflurane, are commonly used in clinical practice, especially in infants, therefore there is a concern related to their safety and long-term effects on neurodevelopment.1,2 A large number of animal experiments have shown that anesthetics such as isoflurane and sevoflurane can cause apoptosis of developing neurons.3 Indeed, clinical research shows that general anesthetic exposure in infants and young children can cause serious adverse consequences in nerves, cognition and other neurological factors.4,5 The apoptosis of developing neurons and the impairment of neurocognitive function in adulthood caused by general anesthetic exposure is termed developmental neurotoxicity of general anesthetics.6 However, the molecular mechanisms of developmental neurotoxicity caused by general anesthesia remain controversial.
Neuroinflammation plays an important role in neurodegenerative alterations caused by anesthesia and subsequent cognitive dysfunction.7,8 Previous studies have shown that isoflurane can not only up-regulate the expression of pro-inflammatory factors such as TNF-α, IL-1β and IL-6 in adult animals,9,10 but also increase the expression of IL-1β in the rat cortex.11 Moreover, isoflurane has also been found to cause NF-kB activation of microglia, rather than neurons, so it can be speculated that isoflurane may specifically act on microglia to induce neuroinflammation.12 Microglia, which are myeloid-lineage cells residing in the central nervous system (CNS), are necessary for healthy brain functioning.13,14 As a neuron protector, microglia are sensitive to the microenvironment and readily become activated in response to immunological stimuli, toxins, or injury.13,14 Microglial cells develop roles similar to macrophages in the brain, resting under normal physiological conditions, and becoming activated and polarized to different phenotypes in response to factors of the microenvironment: M1 microglia (pro-inflammatory) and M2 microglia (anti-inflammatory).15 Therefore, the study of microglia is the core of neuroinflammation research.
Genistein is a natural isoflavone compound, mainly found in legumes, with anti-tumor, antioxidant, anti-inflammatory, anti-aging, anti-senile dementia and estrogen-like effects.16 Recent studies have found that genistein can improve neuronal apoptosis induced by Aβ and tau protein through its antioxidant and anti-inflammatory effects.17,18 The underlying mechanisms of the anti-inflammatory effects of genistein have been gradually uncovered. Genistein is able to inhibit the expression of pro-inflammatory factors induced by β-amyloid,19 to reduce the secretion of inflammatory factors, such as TNF-α, IL-1β and IL-4 in hepatic encephalopathy rats, and to improve the cognitive function of rats.20 In addition, a previous study found that genistein suppressed LPS-induced inflammatory response through the inhibition of NF-κB, following AMP kinase activation in RAW 264.7 macrophages. Our previous study has shown that genistein attenuates isoflurane-induced neurotoxicity and improves impaired spatial learning and memory by regulating cAMP/CREB and BDNF-TrkB-PI3K/Akt signaling.21 However, the neuroprotective mechanisms of genistein are still not fully understood. It is also unclear if/how this natural compound can exert neuroprotection by inhibiting neuroinflammation.
In this study, we investigated the protective effect of genistein on isoflurane-induced neuroinflammation in rats and BV2 cells. Our results suggest that genistein attenuates isoflurane-induced neurotoxicity by inhibiting TLR4-mediated inflammation of microglia in vivo and in vitro. Therefore, genistein, as a natural isoflavone compound, can be considered for the prevention and treatment of isoflurane-induced neurotoxicity.
Materials and Methods
Animals Ethics
This study and its experimental design were approved by the animal experimentation ethics committee of Shandong Cancer Research Institute, Shandong First Medical University & Shandong Academy of Medical Sciences. All techniques were performed in compliance with the guidelines issued for the care and use of laboratory animals by the NIH and National Animal Welfare Law of China.
Isoflurane Exposure
Pregnant Sprague-Dawley rats were housed in individual sterile plastic cages under standard animal house conditions (12-h day/night cycle, 23°C±2°C) at 55~65% humidity levels,21 with six rat pups in each group. Animals were monitored carefully for the birth of pups, and the day of birth was designated as postnatal day 0 (P0). Rat pups in ISO+L-Gen, ISO+M-Gen and ISO+H-Gen groups were respectively given 20, 40 or 80 mg/kg/day genistein (G6649, sigma, USA) from P3 to P15,22 and were exposed to 0.75% isoflurane (6 hours) in 30% oxygen or air [~0.3 minimum alveolar concentration (MAC)] at P7. Pups in the control group were given saline from P3 to P15 and exposed to the same concentration of isoflurane at P7.
Preparation of Single-Cell Suspension and Flow Cytometry Analysis
We prepared hippocampal single-cell suspensions as described previously.23 After removing the red blood cells and counting the cells, single-cell suspensions were incubated for 30 min on ice with the following antibodies: anti-Iba1 antibody (Alexa Fluor® 568, ab221003, Abcam, UK), anti-CD16 (PE, 12–0161-82, eBioscience, USA) and anti-arginase 1 (Alexa Fluor 700, 12–0161-82, eBioscience, USA). For the apoptosis assay, we used Annexin V-FITC/PI Apoptosis Detection Kit (40302ES60, YEASEN, China) according to the manufacturer’s protocol. Flow cytometry was used to detect the fluorescence and data analyzed using Flowjo 7.6.2.
Real-Time Quantitative PCR
Real time quantitative PCR (RT-qPCR) was performed to assess the influence of genistein on gene expression in the hippocampal tissue and BV2 cells (CL-0493, Procell, China) stimulated with isoflurane as described previously.21 Briefly, after preparing the complementary DNA using a PrimeScript RT reagent kit with gDNA eraser (RR047A, Takara Bio, Japan), 20 μL of a qPCR mixture were prepared using GoTaq qPCR Master Mix (A6001, Promega, China) according to the manufacturer protocols using the Touch system (CFX384, Biorad, USA). The thermocycling conditions were: 95°C of 2 minutes, (95°C of 5 seconds and 65 °C of 15 seconds) for 40 cycles. qPCR primers were: IL-1β-F: 5ʹ-CGCATGTTCCTGGGGAGATT-3ʹ, IL-1β-R: 5ʹ-TGGGATGCAACATGGCTCTT-3ʹ; TNF-α-F:5ʹ- GCTGCACTTTGGAGTGATCG-3ʹ, TNF-α-R: 5ʹ-GGGAGAGTGGATGAAGGCTG-3ʹ; IL-6-F: 5ʹ-CACTTCACAAGTCGGAGGCT-3ʹ, IL-6-R: 5ʹ-ACATTGGGCCGTCATCACTT-3ʹ; IL-8-F:5ʹ- AAGCCTGAGCAGGAAAGCAT-3ʹ, IL-8-R: 5ʹ-CAGCAGGGCACACAAGACTA-3ʹ; CD16-F: 5ʹ- ACTACCAACGGCACTGCTAC-3ʹ, CD16-R: 5ʹ-CAGCGAGATTCCCTCCCATC-3ʹ; iNOS-F: 5ʹ- GCCGTTGCTATCCTACTCCC-3ʹ, iNOS-R: 5ʹ- ATTTCCTGGCATCAGCGTCA-3ʹ; CD206-F: 5ʹ-AAAACGGCAGCAGGAAATGT-3ʹ, CD206-R: 5ʹ-CCTCGGTCCACGCCTTCCCTC-3ʹ; Arg1-F: 5ʹ- CTTAACAGGCGTTGCCTTGG-3ʹ, Arg1-R: 5ʹ-GCCACACAGTGGTGAAGAGA-3ʹ.
Immunohistochemistry
We detected the protein expression of TLR4 in tissues using immunohistochemistry. Briefly, the slices were blocked with 5% w/v BSA for 1 hour at room temperature, and the anti-TLR4 antibody (ab22048, Abcam, UK) was incubated overnight at 4°C. The secondary antibody was incubated at room temperature for 1 hour. Proteins were visualized with DAB solution (P0202, Beyotime, China).
Western Blot
The total protein was extracted from tissues and cells using a Tissue Protein Extraction Reagent (78,510, ThermoFisher, USA) and RIPA lysis buffer (89,900, ThermoFisher, USA), respectively. Protein concentration was determined using a BCA kit (23,227, ThermoFisher, USA). Next, 40 μg of total protein was analyzed using a 10% SDS-PAGE. After transfer, PVDF membranes (LC2002, ThermoFisher, USA) were first blocked with 5% skimmed milk powder, and then was probed with primary antibodies against TLR4 (ab22048), MyD88 (ab19413), TRAF6 (ab33915), TAK1 (ab109526), p-TAK1 (ab109404), p38 (ab31828), p-p38 (ab4822), p-ERK1 (ab201015), ERK1 (ab184699), p-IκBα (ab133462), IκBα (ab32518), p-NF-κB (ab86299), NF-κB (ab16502), CD16 (ab203883), iNOS (ab15323), CD206 (ab64693) or Arg1 (ab91279) at 4°C overnight. The next morning, the secondary antibody was incubated at room temperature for 1 hour. Proteins were visualized with ECL solution (WBKLS0100, Beijing Xinjingke Biotechnologies Co., Ltd, China), followed by densitometry analysis using ImageJ 3.0 (IBM, USA). Anti-β-actin was used for equal protein loading control. All antibodies used for Western blot detection were purchased from ABCAM.
Cell Viability Assay
Cells (20,000/well) were seeded into a 96-wells cell culture plate and cultured for 24 hours at 37°C. Cells were exposed to different concentrations (0, 0.2, 0.4, 0.8 and 1.6%) of isoflurane for varying time periods (0, 3, 6, 9 and 12 hours), and were incubated with different concentrations (0, 25, 50, 100 and 200 μmol/L) of genistein for varying time periods (0, 12, 2, 48 and 72 hours). Cells were washed and cell culture medium (100 μL/well) and CCK-8 reagent (10 μL/well) were added. After 1 hour, we determined the OD450 value of each well and calculated the relative cell viability as per the manufacturer’s conditions.
Cellular Immunofluorescence
BV2 or TLR4 KO BV2 cells were seeded (1 x 105 cells/well) onto Lab-Tek chambered (Thermo Scientific, USA) and cultured for 24 hours at 37°C with 5% CO2. Cell culture medium was removed and cells fixed with 4% paraformaldehyde for 10 minutes at room temperature. Then cells were blocked with 5% w/v BSA for one hour at room temperature. After fixing and blocking, cells were incubated with a primary antibody against TLR4 (ab22048, Abcam, UK) overnight at 4 °C. Next, cells were incubated with a secondary antibody, namely goat anti-mouse IgG H&L (Alexa Fluor® 488) (ab150113, Abcam, UK). The Nucleus was stained with 5 μg/mL DAPI for 5 minutes at room temperature. Finally, all samples were visualized and analyzed using a Leica TCS SP5 microscope (Leica microsystem) with the LAS AF Lite 4.0 image browser software.
TLR4 Gene-Specific Knockout
We used the CRISPR-Cas9 technique to knockout the TLR4 gene as described previously.24 Briefly, gRNA for TLR4 (Oligo 1: 5′-CACCGCAGCTGGATCTTCCGCTCAA-3′, Oligo 2: 3′-AAACTTGAGCGGAAGATCCAGCTGC-5′) was reorganized with lentiCRISPRv2 vector (52,961, Addgene, USA). Lentiviral particles were prepared by transferring the recombinant plasmid into HEK-293T cells. The cell culture medium containing the lentivirus was added to the BV2 cells. After 72 hours, we added 1 μg/mL puromycin for 7 days, and the surviving BV2 cells were monoclonalized. Finally, TLR4 KO BV2 cells were confirmed using immunoblotting and immunofluorescence and were used in subsequent studies.
Statistical Analysis
We used Graph Prism 8 software (GraphPad Software, USA) to analyze the data contained in the present study. Data were expressed as (mean ± SD). Student’s t-test was used to compare the difference of TLR4 protein expression in two groups and one-way ANOVA with Tukey’s post-test was used to compare the difference between multiples groups. P <0.05 indicates a significant difference.
Results
Genistein Protects Against Isoflurane-Induced Neuroapoptosis in Rat
Our previous study showed that isoflurane induces neuronal apoptosis which is detected by TUNEL assay and Fluoro-Jade B staining in hippocampal regions of rats.21 Here, we prepared a single-cell suspension from rat hippocampus and stained it with Annexin V-FITC/PI double staining reagent to detect the proportion of apoptotic cells with a flow cytometer. We found results consistent with previous findings: isoflurane exposure led to neuronal apoptosis in the hippocampus of rats, and genistein treatment attenuated isoflurane-induced neuronal apoptosis in a dose-dependent manner (Figure 1).
Genistein Reduced Isoflurane-Induced Inflammation in Rat
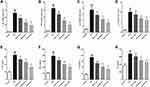
We hypothesized that isoflurane exposure caused neuronal apoptosis by inducing neuronal inflammation in rats. To test this hypothesis, we harvested the tissues of hippocampal regions and detected the expression of inflammatory cytokines (IL-1β, TNF-α, IL-6 and IL-8) using qPCR and ELISA kits. Data from qPCR showed increased mRNA expressions of IL-1β (Figure 2A), TNF-α (Figure 2B), IL-6 (Figure 2C) and IL-8 (Figure 2D) in hippocampal tissues of the ISO group when compared to those from control rats. Similarly, protein detection by ELISA showed that the levels of IL-1β (Figure 2E), TNF-α (Figure 2F), IL-6 (Figure 2G) and IL-8 (Figure 2H) in hippocampal tissues of the ISO group were significantly higher than those from the control group. Importantly, we also found that genistein dose-dependently reduced isoflurane-induced inflammation in hippocampal regions of rats. Thus, genistein may protect against neuronal apoptosis by inhibiting neuroinflammation caused by isoflurane.
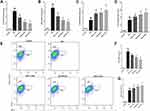
Genistein Promoted M2 and Suppressed M1 Microglia Polarization in Rats
Microglia-mediated neuroinflammation is considered to play an important role in the pathogenesis and progression of anesthetic-induced neurotoxicity.25,26 Here, we first extracted the total RNA from the rat hippocampus to detect M1 (CD16 and iNOS) or M2 microglia (CD206 and Arg1) surface markers. Isoflurane exposure significantly increased the expression of CD16 (Figure 3A), iNOS (Figure 3B), CD206 (Figure 3C) and Arg1 (Figure 3D), indicating that isoflurane exposure activated microglia. Additionally, genistein treatment dose-dependently decreased the mRNA expression of CD16 and iNOS and increased the mRNA expression of CD206 and Arg1 induced by isoflurane. In parallel, we prepared a single-cell suspension from the rat hippocampus to detect M1 and M2 microglia using flow cytometry. We first sorted out Iba-1 positive cells in a single-cell suspension, and then analyzed the ratio of CD16 and Arg1 positive cells among the Iba-1 positive cells. Data showed that isoflurane exposure significantly increased the ratio of M1 (Iba+/CD16+) and M2 microglia (Iba+/Arg1+) (Figure 3E–G). Similarly, genistein dose-dependently reduced M1 microglia (Iba+/CD16+) and increased M2 microglia (Iba+/Arg1+) induced by isoflurane. In summary, these data suggest that genistein promoted M2 and suppressed M1 microglia polarization in the hippocampus of rats exposed to isoflurane.
Genistein Suppressed Isoflurane-Activated TLR4/P38 and TLR4/NF-κB Pathways in Rats
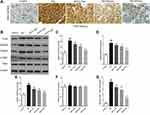
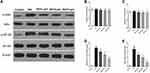
We studied the molecular mechanisms of the neuroprotective effects of genistein in isoflurane-induced neuroinflammation. The TLR4 pathway is regarded as an important candidate signaling pathway, not only because TLR4 mediates neuroinflammation,27,28 but also because previous studies have reported that genistein inhibits TLR4-mediated inflammation.29,30 In the present study, we first detected the expression of TLR4 protein expression using immunohistochemistry. Isoflurane exposure increased TLR4 expression, while genistein reduced isoflurane-induced TLR4 expression in a dose-dependent manner (Figure 4A). Next, we used Western blot to measure the expression levels of key proteins within the TLR4 pathway, namely TLR4, MyD88, TRAF6 and TAK1 (Figure 4B–G). It was evident that isoflurane exposure increased the expression of TLR4, MyD88, TRAF6 and p-TAK1/TAK1 proteins in hippocampal regions, while genistein reduced the levels of the above proteins.
Furthermore, we evaluated two important inflammation-related signaling pathways downstream of TLR4, namely the p38 MAPK signaling pathway and the NF-κB signaling pathway.31,32 These were investigated because p38 MAPK and NF-κB pathways are established signaling pathways related to microglia inflammation, as well as because genistein was shown to inhibit these pathways.33,34 We found that isoflurane exposure not only increased the expression of p-p38/p38 and p-ERK/ERK (Figure 5) but also increased the expression of p-IκBα/IκBα and p-NF-κB/NF-κB (Figure 6). Importantly, genistein reduced the phosphorylation of the abovementioned proteins in hippocampal regions of rats.
Genistein Switched Microglia Polarization in Isoflurane-Induced BV2 Cells
We used BV2 cells in vitro to study the effect of genistein on the polarization of microglia. First, we used the CCK-8 assay to determine the optimal conditions of genistein and isoflurane. We found that BV2 cells exposed to 0.4% isoflurane for 6 hours (Figure S1A and S1B) or 50 μmol/L genistein for 24 hours (Figure S1C and S1D) presented normal cell activity. In addition, BV2 cells pre-treated with 50 μmol/L genistein for 24 hours and then stimulated with 0.4% isoflurane for 6 hours presented normal cell activity (Figure S1E). Therefore, we used these conditions to conduct our experiments.
It was evident that isoflurane not only increased the expression of inflammatory cytokines (IL-1β, TNF-α, IL-6 and IL-8) but also augmented the expression of M1 (CD16 and iNOS) and M2 (CD206 and Arg1) microglia surface markers (Figure 7). More importantly, genistein pre-treatment was able to decrease the levels of IL-1β, TNF-α, IL-6, IL-8, CD16 and iNOS mRNA expression, while it increased CD206 and Arg1 mRNA expression in isoflurane-exposed cells. In addition, we also detected the expression of M1 (CD16 and iNOS) and M2 (CD206 and Arg1) microglia surface markers using Western blot and cell immunofluorescence, and results showed that genistein pre-treatment was able to decrease the levels of CD16 and iNOS protein, and increase the levels of CD206 and Arg1 protein (Figure 8). Altogether, this dataset indicates that genistein promoted M2 and suppressed M1 microglia polarization in isoflurane-induced BV2 cells.
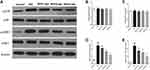
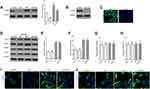
Genistein Switched Microglia Polarization in a TLR4-Dependent Manner
Since genistein promoted M2 and inhibited M1 microglia polarization, we hypothesized that this may be related to the TLR4 signaling pathway in vivo. However, the lack of TLR4 gene knockout animals makes it impossible to confirm whether genistein’s regulation of microglial polarization is TLR4-dependent. In vitro, we found similar results to experiments in vivo, namely that genistein could decrease TLR4 protein expression in BV2 cells induced by isoflurane (Figure 9A). To investigate if the effects of genistein were dependent on TLR4, we established a TLR4 knockout BV2 cell line using CRISPR-Cas9 and verified by immunoblotting (Figure 9B) and immunofluorescence (Figure 9C). TLR4 KO BV2 cells were pre-treated with or without 50 μmol/L genistein for 24 hours and then stimulated with 0.4% isoflurane for 6 hours. We found that genistein did not affect the protein expression levels of CD16, iNOS, CD206 and Arg1 in TLR4 KO BV2 cells induced by isoflurane (Figure 9D–J), which indicated that genistein switched microglia polarization in a TLR4-dependent manner in isoflurane-induced BV2 cells.
Discussion
Isoflurane can cause neuroapoptosis in the developing brain and, in line with previous reports,35,36 the results from flow cytometry experiments showed that isoflurane exposure resulted in neuronal apoptosis in the hippocampus of rats. Genistein treatment attenuated isoflurane-induced neuronal apoptosis in a dose-dependent manner. Although previous studies suggest that isoflurane preconditioning ameliorates electromagnetic pulse-induced neural damage,37,38 this seems to contradict the results herein presented. It should be emphasized that the conditions of isoflurane exposure (time and concentration) may determine its effect,37,38 since low-dose isoflurane pretreatment for a short time plays a neuroprotective role, while high-dose long-term (anesthesia) causes neurotoxicity and neuroinflammation. In this study, we investigated the neurotoxicity of isoflurane as an anesthetic, therefore we used isoflurane at a high dose for a long time (0.75% isoflurane exposed for 6 hours).
In our previous study, we had found that genistein significantly reduced apoptosis in the hippocampus, reduced the expression of proapoptotic factors (Bad, Bax, and cleaved caspase-3), and increased the expression of Bcl-2 and Bcl-xL, which led us to characterize genistein as exerting a “direct neuroprotection” effect. Here, we focused on “indirect neuroprotection” to assess if genistein exerts neuroprotection by inhibiting isoflurane-induced neuroinflammation since it is known that neuroinflammation plays an important role in neurodegenerative changes caused by anesthesia and subsequent cognitive dysfunction.7,8 We found that genistein reduced isoflurane-induced inflammatory cytokines expression in hippocampal regions of rats in a dose-dependent manner. The anti-inflammatory effects of genistein have been reported previously, for example, genistein was found to inhibit Alzheimer’s disease-related neuroinflammation by inhibiting the expression of PPAR.39 It was shown that oral treatment with genistein reduced the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis.40 In addition, a previous study found that genistein attenuated retinal inflammation associated with diabetes by targeting microglial activation.41 This latter study inspired us to assess if the effects of genistein on isoflurane-induced neuroinflammation were related to the polarization of microglia.
Microglia, the only cells derived from the mesoderm in the nerve tissue, are inherent immune effector cells in the central nervous system and play an important role in the physiological regulation of the CNS.15 Microglial cells can be activated and polarized to different phenotypes in response to the microenvironment: M1 (pro-inflammatory) and M2 microglia (anti-inflammatory).15 The activation of microglia and neuroinflammation are the main features of neuropathology since they mediate endogenous immune responses to CNS injury and disease, thereby exerting neuroprotective or neurotoxic effects.42,43 Microglia respond to microenvironmental disturbances by undergoing phenotypic changes and releasing inflammatory mediators. Such acute inflammatory response is usually beneficial to the survival of nerve cells, can reduce secondary damage in the brain, and heal damaged tissues.42,44 However, persistent and excessive inflammation is harmful to the body and can cause nerve tissue damage.43
In the present study, we provide evidence that sustained high-dose isoflurane exposure was neurotoxic by inducing neuroinflammation, while genistein attenuated isoflurane-induced neurotoxicity by inhibiting microglia inflammatory signals. Moreover, we found that genistein promoted M2 and suppressed M1 microglia polarization in the hippocampus of rats exposed to isoflurane. Additionally, we also found that genistein suppressed isoflurane-activated TLR4/p38 and TLR4/NF-κB pathways in rats. TLR4 is a key receptor that regulates the polarization and function of microglia. In the cerebral ischemia-reperfusion injury model, TLR4 is found to be up-regulated, microglia are activated, and inflammatory factors are released, triggering CNS immune inflammatory response, neuronal degeneration, and death.45 In the study of Alzheimer’s disease, it was found that the increased Aβ oligomers could activate the pattern recognition receptor TLR4 on the surface of microglia, causing chronic inflammation, stimulating cascade reactions, and promoting neurodegeneration.46 Furthermore, Fellner et al47 administered α-synuclein to TLR4 gene-deficient mouse microglia and wild-type mouse microglia, and found that compared with the wild-type group, TLR4 deficiency led to decreases in NF-κB Nuclear transfer, cytokine release, and reactive oxygen species production. These data indicate that TLR4 plays an important role in α-synuclein activation of microglia. Therefore, TLR4 plays a key role in the activation of microglia and the inflammation cascade, while genistein inhibits the polarization of microglia to M1 by inhibiting the signaling pathway mediated by TLR4, thereby inhibiting isoflurane-induced neuroinflammation.
The lack of TLR4 gene knockout animals makes it impossible to confirm whether genistein’s regulation of microglial polarization is TLR4-dependent in vivo. Thus, we used BV2 cells to study the effect of genistein on the polarization of microglia, and found the same results as in vivo. Genistein promoted M2 and suppressed M1 microglia polarization in isoflurane-induced BV2 cells and decreased the elevated expression of TLR4 induced by isoflurane in BV2 cells. Importantly, we also found that genistein did not affect CD16, iNOS, CD206 and Arg1 protein expressions in TLR4 KO BV2 cells induced by isoflurane, which indicated that genistein switched microglia polarization in a TLR4-dependent manner in isoflurane-induced BV2 cells.
Altogether, isoflurane can lead to neurotoxicity by causing neuroinflammation, while genistein attenuates isoflurane-induced neuroinflammation by inhibiting TLR4-mediated microglia polarization in vivo and in vitro. In addition, our results provide a potential drug for the prevention and treatment of developmental neurotoxicity caused by isoflurane in general anesthetics. Genistein, as a natural isoflavone compound, can be considered for the prevention and treatment of isoflurane-induced neurotoxicity (Figure 10). However, it should be noted that due to the lack of TLR4 knockout rats, we could not confirm if genistein switching of microglia polarization is dependent on TLR4 in vivo.
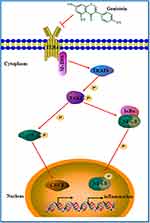 |
Figure 10 Schematic diagram of genistein attenuates isoflurane-induced neurotoxicity by inhibiting TLR4-mediated microglia-inflammatory. |
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Shandong Cancer Research Institute, Shandong First Medical University & Shandong Academy of Medical Sciences.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Li Y, Liu C, Zhao Y, et al. Sevoflurane induces short‐term changes in proteins in the cerebral cortices of developing rats. Acta Anaesthesiol Scand. 2013;57(3):380–390. doi:10.1111/aas.12018
2. Li Y, Wang F, Liu C, et al. JNK pathway may be involved in isoflurane-induced apoptosis in the hippocampi of neonatal rats. Neurosci Lett. 2013;545:17–22. doi:10.1016/j.neulet.2013.04.008
3. Jevtovic-Todorovic V. Anesthesia and the developing brain. Curr Opin Anaesthesiol. 2011;24(4):395–399. doi:10.1097/ACO.0b013e3283487247
4. Yan J, Li YR, Zhang Y, Lu Y, Jiang H. Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective Preliminary Clinical Study. J Child Neurol. 2014;29(10):1333–1338. doi:10.1177/0883073813517508
5. Miller TLK, Park R, Sun LS. Report of the Fourth PANDA Symposium on “anesthesia and neurodevelopment in children”. J Neurosurg Anesthesiol. 2014;26(4):344–348. doi:10.1097/ANA.0000000000000109
6. Perouansky M, Hemmings HC, Riou B. Neurotoxicity of general anestheticscause for concern? Anesthesiology. 2009;111(6):1365–1371. doi:10.1097/ALN.0b013e3181bf1d61
7. Wang Q, Liu Y, Zhou J. Neuroinflammation in parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4(1):19. doi:10.1186/s40035-015-0042-0
8. Baune BT. Inflammation and neurodegenerative disorders: is there still hope for therapeutic intervention? Curr Opin Psychiatry. 2015;28(2):148. doi:10.1097/YCO.0000000000000140
9. Shen X, Dong Y, Xu Z, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118(3):502–515. doi:10.1097/ALN.0b013e3182834d77
10. Wu X, Lu Y, Dong Y, et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging. 2012;33(7):1364–1378. doi:10.1016/j.neurobiolaging.2010.11.002
11. Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z. Anesthetic sevoflurane causes neurotoxicity differently in neonatal naive and alzheimer disease transgenic mice. Anesthesiology. 2010;112(6):1404–1416. doi:10.1097/ALN.0b013e3181d94de1
12. Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth. 2013;110(suppl_1):i82–i91. doi:10.1093/bja/aet115
13. Georg K. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318.
14. Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi:10.1016/j.cell.2010.02.016
15. Xu L, He D, Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol. 2016;53(10):6709–6715. doi:10.1007/s12035-015-9593-4
16. Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698(1–3):31–38. doi:10.1016/j.ejphar.2012.11.013
17. Uddin M, Kabir M. Emerging signal regulating potential of genistein against alzheimer’s disease: a promising molecule of interest. Front Cell Dev Biol. 2019;7:197. doi:10.3389/fcell.2019.00197
18. Uddin MS, Kabir MT, Al Mamun A, et al. Pharmacological approaches to mitigate neuroinflammation in alzheimer’s disease. Int Immunopharmacol. 2020;84:106479. doi:10.1016/j.intimp.2020.106479
19. Chong YH, Sung JH, Shin SA, Chung J-H, Suh Y-H. Effects of the ?-Amyloid and carboxyl-terminal fragment of alzheimer’s amyloid precursor protein on the production of the tumor necrosis factor-? And matrix metalloproteinase-9 by human monocytic THP-1. J Biol Chem. 2001;276(26):23511–23517. doi:10.1074/jbc.M009466200
20. Ganai AA, Husain M. Genistein alleviates neuroinflammation and restores cognitive function in rat model of hepatic encephalopathy: underlying mechanisms. Mol Neurobiol. 2018;55(2):1762–1772. doi:10.1007/s12035-017-0454-1
21. Jiang T, Wang XQ, Ding C, Du XL. Genistein attenuates isoflurane-induced neurotoxicity and improves impaired spatial learning and memory by regulating cAMP/CREB and BDNF-TrkB-PI3K/Akt signaling. Korean J Physiol Pharmacol. 2017;21(6):579–589. doi:10.4196/kjpp.2017.21.6.579
22. Orliaguet G, Vivien BT, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95(3):734–739. doi:10.1097/00000542-200109000-00028
23. Huber SA, Sartini D. Roles of Tumor Necrosis Factor Alpha (TNF-?) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J Virol. 2005;79(5):2659–2665. doi:10.1128/JVI.79.5.2659-2665.2005
24. Jie T, Zhang J, Yun L, Mccall CE, Fu LT. Mitochondrial sirtuin 4 resolves immune tolerance in monocytes by rebalancing glycolysis and glucose oxidation homeostasis. Front Immunol. 2018;9:419. doi:10.3389/fimmu.2018.00419
25. Jackson WM, Gray CDB, Jiang D, Schaefer ML, Connor C, Mintz CD. Molecular mechanisms of anesthetic neurotoxicity: a review of the current literature. J Neurosurg Anesthesiol. 2016;28(4):361. doi:10.1097/ANA.0000000000000348
26. Yang X, Ren H, Wood K, et al. Depletion of microglia augments the dopaminergic neurotoxicity of MPTP. FASEB J. 2018;32(6):3336–3345. doi:10.1096/fj.201700833RR
27. Hollingsworth JW, Whitehead GS, Lin KL, et al. TLR4 signaling attenuates ongoing allergic inflammation. J Immunol. 2006;176(10):5856–5862. doi:10.4049/jimmunol.176.10.5856
28. Yang HZ, Wang JP, Mi S, et al. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012;180(1):275–292. doi:10.1016/j.ajpath.2011.09.019
29. Zhou X, Yuan L, Zhao X, et al. Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-κB. Nutrition. 2014;30(1):90–95. doi:10.1016/j.nut.2013.06.006
30. Byun EB, Sung NY, Yang MS, et al. Anti-inflammatory effect of gamma-irradiated genistein through inhibition of NF-κB and MAPK signaling pathway in lipopolysaccharide-induced macrophages. Food and chemical toxicology. 2014:74:255–264. doi:10.1016/j.fct.2014.08.019
31. Huang X, Chen S, Xu L, Liu Y, Bergan RC. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65(8):3470–3478. doi:10.1158/0008-5472.CAN-04-2807
32. Cui S, Wienhoefer N, Bilitewski U. Genistein induces morphology change and G2/M cell cycle arrest by inducing p38 MAPK activation in macrophages. Int Immunopharmacol. 2014;18(1):142–150. doi:10.1016/j.intimp.2013.11.016
33. Dai H, Jia G, Wang W, et al. Genistein inhibited ammonia induced astrocyte swelling by inhibiting NF-κB activation-mediated nitric oxide formation. Metab Brain Dis. 2017;32(3):841–848. doi:10.1007/s11011-017-9975-6
34. Lim K, Son MY, Seo WH, et al. AP-1 and NF-KB are related to genistein-dependent induction of vimentin gene in HL-60 cells. Eur J Cancer. 2001;37(01):S130–S130. doi:10.1016/S0959-8049(01)80969-2
35. Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112(4):834–841. doi:10.1097/ALN.0b013e3181d049cd
36. Ma D, Williamson P, Januszewski A, et al. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106(4):746–753. doi:10.1097/01.anes.0000264762.48920.80
37. Zhang X, Lv M, Zhu X, et al. Isoflurane preconditioning ameliorates electromagnetic pulse-induced neural damage by shifting microglia polarization toward anti-inflammatory phenotype via upregulation of SOCS1. Int Immunopharmacol. 2019;68:48–57. doi:10.1016/j.intimp.2018.12.064
38. Li J-J, Deng B, Zhang XJ, et al. Isoflurane preconditioning attenuates brain injury induced by electromagnetic pulse via the TLR4/NFκB signaling pathway. Oxid Med Cell Longev. 2019;2019.
39. Valles SL, Dolz-Gaiton P, Gambini J, et al. Estradiol or genistein prevent alzheimer’s disease-associated inflammation correlating with an increase PPAR gamma expression in cultured astrocytes. Brain Res. 2010;1312(none):138–144. doi:10.1016/j.brainres.2009.11.044
40. Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48(4):213. doi:10.1007/s00394-009-0004-3
41. Ibrahim AS, El-Shishtawy MM, Peña A
42. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi:10.1038/nn1472
43. Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587(2):0–256. doi:10.1016/0006-8993(92)91004-X
44. Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27(1):119–145. doi:10.1146/annurev.immunol.021908.132528
45. Gu J, Su S, Guo J, Zhu Y, Zhao M, Duan J. Anti‐inflammatory and anti‐apoptotic effects of the combination of ligusticum chuanxiong and radix paeoniae against focal cerebral ischaemia via TLR 4/MyD88/MAPK/NF‐κB signalling pathway in MCAO rats. J Pharm Pharmacol. 2018;70(2):268–277. doi:10.1111/jphp.12841
46. Zhan X, Boryana S, Sharp FR. Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in alzheimer’s disease brain: a review. Front Aging Neurosci. 2018;10:42. doi:10.3389/fnagi.2018.00042
47. Fellner L, Irschick R, Schanda K, et al. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi:10.1002/glia.22437
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.