Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Genetic Susceptibility to Tardive Dyskinesia and Cognitive Impairments in Chinese Han Schizophrenia: Role of Oxidative Stress-Related and Adenosine Receptor Genes
Authors Jiang Q, Zhang X, Lu X, Li Y, Lu C, Chi J, Ma Y, Shi X, Wang L, Li S
Received 26 June 2023
Accepted for publication 2 November 2023
Published 20 November 2023 Volume 2023:19 Pages 2499—2509
DOI https://doi.org/10.2147/NDT.S427557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Qiaona Jiang,1,* Xiaofei Zhang,1,* Xiaohui Lu,2 Yanzhe Li,3 Chenghao Lu,1 Jinghui Chi,1 Yanyan Ma,1 Xiaomei Shi,1 Lili Wang,1 Shen Li3
1Department of Psychiatry, Tianjin Anding Hospital, Mental Health Center of Tianjin Medical University, Tianjin, People’s Republic of China; 2Department of Psychiatry, Qingdao Mental Health Center, Qingdao, People’s Republic of China; 3Institute of Mental Health, Tianjin Anding Hospital, Mental Health Center of Tianjin Medical University, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lili Wang, Department of Psychiatry, Tianjin Anding Hospital, Mental Health Center of Tianjin Medical University, No. 13, Liulin Road, Hexi District, Tianjin, 300222, People’s Republic of China, Tel +00-86-022-88188796, Email [email protected] Shen Li, Institute of Mental Health, Tianjin Anding Hospital, Mental Health Center of Tianjin Medical University, No. 13, Liulin Road, Hexi District, Tianjin, 300222, People’s Republic of China, Tel +00-86-022-88188875, Email [email protected]
Objective: Tardive dyskinesia (TD) is a severe rhythmic movement disorder caused by long-term antipsychotic medication in chronic patients with schizophrenia (SCZ). We aimed to investigate the association between polymorphisms in oxidative stress-related genes (GSTM1, SOD2, NOS1, and NOS3) and adenosine receptor gene (ADORA2A), as well as their interactions, with the occurrence and severity of TD, and cognitive impairments in a Chinese Han population of SCZ patients.
Methods: Two hundred and sixteen SCZ patients were recruited and divided into TD group (n=157) and non-TD group (n=59). DNA extraction was performed by a high-salt method, followed by SNP genotyping using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The severity of TD, psychopathology and cognitive functioning were assessed using the Abnormal Involuntary Movement Scale (AIMS), the Positive and Negative Syndrome Scale (PANSS) and the Repeated Battery for Assessment of Neuropsychological Status (RBANS), respectively.
Results: The combination of GSTM1-rs738491, NOS1-rs738409 and ADORA2A-rs229883 was identified as the best three-point model to predict TD occurrence (p=0.01). Additionally, GSTM-rs738491 CC or NOS3-rs1800779 AG genotypes may be protective factors for psychiatric symptoms in TD patients. TD patients carrying the NOS1-rs738409 AG or ADORA2A-rs229883 TT genotypes exhibited poorer cognitive performance.
Conclusion: Our findings suggest that the interaction of oxidative stress-related genes and adenosine receptor gene may play a role in the susceptibility and severity of TD in Chinese Han SCZ patient. Furthermore, these genes may also affect the psychiatric symptoms and cognitive function of TD patients.
Keywords: tardive dyskinesia, schizophrenia, oxidative stress, adenosine receptor, polymorphisms, cognitive impairments
Introduction
Tardive Dyskinesia (TD) is a serious rhythmic movement disorder characterized by involuntary, repetitive, purposeless movements of the face, trunk, and extremities.1 The prevalence of TD varies by population, with an average worldwide prevalence of 25.3%,2 and a higher prevalence in the Chinese population, at 36%.3 Long-term use of antipsychotic medications is a well-established risk factor for the development of TD in patients with schizophrenia (SCZ). Age, females, duration of illness, smoking, and drug type have also been identified as risk factors for TD.4 While earlier studies indicated a significantly lower incidence of TD with second-generation antipsychotics (SGAs) compared to first-generation antipsychotics (FGAs), recent years have witnessed a narrowing of the gap between the two due to the widespread use of SGAs. Notably, only a small percentage of patients develop TD, indicating that individual susceptibility and genetic predisposition may play a crucial role in the development of TD. A review summarizing the results of published experimental animal models and clinical studies found a potential correlation between genetic variation and TD development.5
The pathophysiology of TD remains a complex and multifaceted subject with several hypotheses proposed to explain its development. These hypotheses encompass various facets of neurobiology and are essential to understanding this condition, including the dopamine hypersensitivity hypothesis, the gamma-aminobutyric acid (GABA) hypothesis, and the oxidative stress hypothesis.6–8 While the classical dopaminergic pathway has been considered a contributing factor, it is increasingly evident that TD may not solely arise from this mechanism. Notably, in chronic SCZ patients receiving long-term antipsychotic medications, D2 receptor hypersensitivity often manifests within days to weeks. In contrast, the onset of TD occurs months to years later. Furthermore, the capacity for dopamine hypersensitivity tends to diminish with age in chronic SCZ patients, whereas the incidence of TD appears to rise with age.9 Preclinical studies in rodents have also indicated that dopamine hypersensitivity may represent a generalized response to dopamine receptor-blocking drugs, including antipsychotics, yet not all individuals exposed to these drugs develop TD.10 The proposed oxidative stress hypothesis of TD offers a perspective that addresses several limitations of the dopamine hypersensitivity hypothesis outlined above. Antipsychotic drugs are known to induce an upsurge in dopamine synthesis and metabolism by blocking dopamine receptors.11 Within these intricate processes of dopamine transport and metabolism, direct or indirect generation of free radicals may occur. However, the oxidative damage inflicted by free radicals follows a gradual, cumulative trajectory, requiring months or even years to produce clinically observable effects. This temporal progression aligns with the delayed onset of TD symptoms often experienced by patients using antipsychotic medications. Aging further complicates this picture, as oxidative damage tends to increase with age. This phenomenon offers a plausible explanation for the observed rise in delayed-onset dyskinesia with advancing age.12 Additionally, individual variations exist in the capacity to manage heightened free radical production, elucidating why some individuals may develop late-onset dyskinesia while others do not.13
The most extensive data on the mechanisms of oxidative stress come from studies conducted in SCZ, in which evidence can be obtained from different areas of oxidative research, including oxidative marker assays, psychopharmacological studies, and clinical trials of antioxidants.14 In recent years, the oxidative stress hypothesis has also received increasing attention in TD research. For example, neurochemical studies have found increased cerebrospinal fluid free radical activity in TD patients.15 Animal studies have shown that long-term antipsychotic drug use increases membrane lipid peroxidation, impairing antioxidant enzyme activity.16 Pharmacogenetic studies likewise supported a positive relationship between TD and oxidative stress.17 In addition, the oxidative stress-mediated neurotoxic damage hypothesis have been proposed to explain the development of TD.18 Furthermore, studies have demonstrated that TD patients have greater cognitive impairments than non-TD patients,4 and this cognitive decline is related to an imbalance between local reactive oxygen species and inadequate antioxidant capacity. Therefore, oxidative stress not only contributes to the development of TD in SCZ patients but also aggravates cognitive dysfunction.19
The human body has a complex antioxidant defense system, including glutathione s-Transferase M1 (GSTM1),20 manganese superoxide dismutase (MnSOD) encoded by the SOD2 gene,21 neural nitric oxide synthase (NOS1) and endothelial nitric oxide Synthase (NOS3).22 These enzymes play a key role in preventing the formation of reactive oxygen species (ROS). The GSTM1 null genotype has been suggested to be related to the onset of SCZ in the Chinese Han population,23 but its association with TD has not been confirmed. Recently, a meta-analysis by Uludag et al found that SOD2 gene polymorphism may be associated with the risk of TD,24 but another meta-analysis did not support the these findings.25 Studies on the association of NOS1 and NOS3 gene polymorphisms with TD have also yielded different results in different populations.26,27 To date, the relevance of these oxidative stress-related genes to TD in various populations of SCZ patients remains inconclusive. Thus, whether oxidative stress-related genes can be used as genetic markers to predict the occurrence of TD still requires further study.
In addition to oxidative stress-related genes, the adenosine hypothesis of SCZ has gained increasing attention. The interaction of adenosine with dopaminergic and glutamic neurotransmission and its possible role in the neurodevelopment of disease further support the adenosine hypothesis.28 Studies have shown that adenosine receptors (ADORA) play an essential neuromodulatory role in psychotic symptoms and antipsychotic adverse effects in chronic SCZ patients receiving long-term antipsychotic medication.29 However, a study in a Chinese population found no association between ADORA2A gene polymorphism and TD development.30 Therefore, as a potentially attractive candidate gene for TD, further investigation is required to explore the relationship between the ADORA2A gene polymorphism and TD in SCZ.
To our knowledge, no studies have explored whether oxidative stress-related genes and adenosine receptor gene polymorphisms and their interactions contribute to the susceptibility to and development of TD in SCZ patients. In the presents study, we aimed to: 1) explore the relationships between these gene polymorphisms and TD in SCZ patients in a Chinese Han population; 2) investigate the relationship between these gene polymorphisms and psychiatric symptoms and cognitive function in TD patients.
Materials and Methods
Participants
We recruited a total of 216 chronic SCZ patients including 103 males and 113 females. The inclusion criteria were as follows: 1) SCZ diagnosis according to the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5), independently confirmed by two experienced psychiatrists; 2) at least a 2-year history of SCZ; 3) a stable dose of antipsychotic medication for more than 3 months prior to enrollment; 4) Han Chinese. The exclusion criteria included: 1) serious physical illnesses, such as uncontrolled hypertension, severe cardiovascular and cerebrovascular disease, diabetes, and metabolic syndrome; 2) organic brain diseases or brain injury; 3) pregnant or lactating women; 4) substance abuse other than tobacco; 5) meeting DSM-5 other psychiatric diagnoses.
The average duration of the disease was 24.59±14.93 years. All enrolled patients were treated with stable doses of antipsychotics for more than 3 months. Including sulpiride (n = 62), olanzapine (n = 66), quetiapine (n = 8), aripiprazole (n = 10), clozapine (n = 44), ziprasidone (n=1), perospirone (n=1), amisulpride (n=7), lurasidone (n=1), chlorpromazine (n = 3), perphenazine (n = 10), sulpiride (n = 1), blonanserin (n=1), haloperidol (n=1). Since the patients were on different types of antipsychotics, we converted the dosage to chlorpromazine equivalents uniformly according to international standards.31 The average dose was 283.26 ± 169.57mg/day.
All subjects were inpatients from Tianjin Anding Hospital. The study protocol was approved by the Ethical Review Committee of Tianjin Anding Hospital (IRB No.2021–07). After a detailed description provided by the research assistant, all participants signed an informed consent form.
Clinical Assessments
A self-designed questionnaire was used to obtain general information, demographic characteristics, psychological status, and medical history for each patient, along with information obtained from medical records. Schooler and Kane’s criteria and the Abnormal Involuntary Movement Scale (AIMS) were used to diagnose Tardive dyskinesia (TD), with a diagnose of TD given when the score was at least 3 for any body part or at least 2 for two or more body parts.32 The total AIMS score was calculated by summing items 1 through 7, with items 1–4 assessing mainly involuntary movements of the mouth and face, while items 5–7 assess involuntary movements of the patient’s trunk and extremities to ensure the accuracy of the TD diagnosis. Patients diagnosed with TD were assessed again using AIMS after at least 1 month, and TD was identified only if both assessments indicated its presence. The Positive and Negative Syndrome Scale (PANSS)33 was utilized to evaluate the psychiatric symptoms of subjects. Three psychiatrists attended a training course at the same time, and after training, the intraclass correlation coefficient (ICC) within the total PANSS subgroup was 0.8 or higher to ensure reliability and consistency of the assessment.
Cognitive Assessment
The cognitive functioning of all participants was assessed using the Repeatable Battery for the Assessment of Neuropsycho-logical Status (RBANS),34 which provides a total score and five subscales of cognitive functioning, including immediate memory, visuospatial/structural, language, attention, and delayed memory. The reliability and clinical validity of the translated and adapted Chinese version of the RBANS was confirmed in the Chinese general population and in SCZ patients.35 Three psychiatrists assessed patients’ cognitive impairment using RBANS. Similarly, the ICC in the RANBS score group remained above 0.8. All assessments were completed prior to the laboratory experiment.
DNA Extraction and Genetic Analysis
Venous blood samples were drawn in early morning after overnight fasting from each patient and stored in −80◦ C refrigerator. DNA extraction was performed using the high salt method at the Molecular Laboratory of Tianjin Mental Health Centre.36 Genotyping was performed using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) SNP genotyping assays. The specific process unfolds as follows: firstly, the DNA sequence containing the target SNP undergoes amplification through PCR, and subsequent removal of unbound dNTPs is achieved through purification. Following this, an extension primer tailored for each target SNP is designed using the purified PCR product. Subsequently, a single-base extension takes place within the system, utilizing ddNTPs as the substrate. Notably, the extension product of each SNP allele differs by just one base at the SNP’s terminal end. Finally, the products were transferred to SpectroCHIP microarray for mass spectrometry detection. Distinguishing between alleles of the same SNP is facilitated by the variance in molecular weights and the formation of distinct detection peaks. To ensure consistency, approximately 10% of the samples were randomly selected and retested for quality control. The concordance between replicate samples was 99.3% in this study. In the Chinese Han population, the mutation frequencies of GSTM1 (rs1056806), SOD2 (rs4880), NOS1 (rs1047735), NOS3 (rs1800779) and ADORA2A (rs2298383) minimal alleles were 0.08, 0.12, 0.49, 0.13 and 0.43, respectively.
Statistical Analyses
Statistical analyses were using the Statistical Package of Social Sciences version 25 for Windows (SPSS, Inc., Chicago, IL, USA). The independent sample t-test was used for continuous variables, and the chi-square test was used for categorical variables to determine the differences between the TD and non-TD groups. The Shesis project was utilized to assess Hardy-Weinberg equilibrium for the 5 SNPs in both groups.37 Allele and genotype frequencies were calculated using this project. Differences between categorical and continuous variables were tested by one-way analysis of variance (ANOVA). If the significance level was <0.05, multivariate analysis of covariance (MANCOVA) was used to analyze and exclude possible confounding factors.
Generalized Multifactor Dimensionality Reduction (GMDR) software was used to assess gene–gene interactions with TD.38 The model with the highest testing balanced accuracy, highest cross-validation consistency score, and p<0.05, derived from the sign test automatically in the GMDR software, was considered to be the best model. Linear regression has been used to evaluate the effect of three different SNP genotype combinations on total AIMS scores. In this analysis, the total AIMS scores of all patients were selected as the dependent variable while the combination of factors was entered as independent variables. The odds ratio (OR) and 95% confidence intervals (CIs) between the two variables were calculated as risk assessment. All the statistical analyses were two-tailed, and the statistical significance level was set at p<0.05.
Results
Demographic Characteristics and Clinical Characteristics Between TD and Non-TD Groups
The demographic characteristics and clinical variables of TD and non-TD patients are shown in Table 1. Significant differences in terms of sex, age, illness duration and smoking were found between TD and non-TD groups (all p<0.05). Compared to non-TD patients, the AIMS total score and the PANSS negative symptom subscore were higher in TD patients, whereas the PANSS positive symptom subscore was lower in TD patients. In addition, TD patients exhibited lower cognitive performance scores in immediate memory, attention, delayed memory, and total score compared to non-TD patients. After controlling for age, gender, duration of illness, and smoking, significant differences remained between TD and non-TD patients in total AIMS scores and visuospatial/structural domain (p<0.05). However, there were no significant differences between TD patients and non-TD patients in PANSS positive symptoms, negative symptoms, total RBANS, immediate memory, and attention scores.
 |
Table 1 Demographic Characteristics, Clinical Data in Chronic Schizophrenia with TD and Non-TD |
The Allele and Genotype Frequency Between TD and Non-TD Groups
The genotype distribution of GSTM1 (rs1056806), SOD2 (rs4880), NOS1 (rs1047735), NOS3 (rs1800779), and ADORA2A (rs2298383) was consistent with Hardy–Weinberg equilibrium in all patients, TD group and non-TD group (Table S1). As shown in Table 2, there were no significant differences were noted in allele SNPs and genotype distribution between the TD group and the non-TD group (p>0.05). Still not statistically significant after Bonferroni correction (p>0.05).
 |
Table 2 Comparison Between the Allele and Genotype Frequencies of Schizophrenic Patients with and without TD |
Analysis of Gene-Gene Interactions
The GMDR analysis revealed a significant three-locus model consisting of the combination of the GSTM1-rs738491, NOS1-rs738409, and ADORA2A-rs2298383 on the risk of TD (p=0.01, testing balanced accuracy=59.35%, CVC=10/10, see Table S2). In Figure 1, We found that this interplay had a significant effect on total scores on the AIMS, and the OR for the high-risk genotype combination (CC-GG-TT) was 3.092 (95% CI: 0.466–5.717, p=0.021, see Table S3).
Differences in TD Severity Between Genotypes
Due to the small number of SOD2- rs4880 GG (n=4) genotypes and NOS3-rs1800779 GG (n=3) genotypes, they were combined with their respective heterozygous genotypes as a group in all analyses. Table 3 shows the differences in TD severity between genotypes. The one-way ANOVA analysis showed that total AIMS scores of TD patients were significantly different between both SOD2 and NOS3 genotype subgroups (p<0.05), the extremity and trunk scores were only significantly different between NOS3 genotype subgroups (p<0.05). In all patients, the oral-facial scores in the ADORA2A genotype group showed a significant difference (p<0.05). After correction, significant differences in TD patients were still observed in the total AIMS scores (p=0.007) between the two SOD2 genotype groups and the extremity and trunk scores (p=0.014) and total AIMS scores (p=0.008) in the two NOS3 genotype groups. However, corrected AIMS scores did not differ between genotypes in non-TD patients or in all patient groups (p>0.05).
 |
Table 3 Comparison Between the AIMS Scores of GSTM1 (rs1056806), SOD2 (rs4880), NOS1 (rs1047735), NOS3 (rs1800779) and ADORA2A (rs2298383) Genotype Groups |
Differences in PANSS Scores Between Genotypes
Table S4 shows the results of ANOVA analysis of PANSS scores versus genotypes, and significant differences were observed in the PANSS positive symptom subscore in the GSTM1 genotype group and in the PANSS general subscore between the NOS3 genotype groups in TD patients (p<0.05). Additionally, there were significant differences in PANSS total scores between GSTM1 genotypes among non-TD patients (p<0.05). After correlations, significant differences remained between GSTM1 genotypes and PANSS positive symptom subscore in TD patients (p=0.012, see Figure 2A). Moreover, the PANSS general subscore differed between the two NOS3 genotype groups (p=0.044, see Figure 2B)
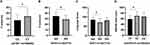 |
Figure 2 Comparison of GSTM1, NOS3, NOS1 and ADORA2A genotypes in TD patients on the P subscore (A), G subscore (B), language score (C) and RBANS total score (D), respectively. *p < 0.05. |
Differences in RANBS Scores Among Different Genotypes
The results of the analysis of the differences between the RANBS scores stratified by genotypes are summarized in Table S5. Among TD patients, significant differences were observed between NOS1 genotype groups for the language score, and between ADORA2A genotype groups for the attention score and RBANS total score (p<0.05). After adjusting for age, sex and course of disease, a significant difference still existed between NOS1 genotype groups for the language score in TD patients, and between ADORA2A genotype groups for the total RBANS score in TD patients too (p<0.05). Post hoc analysis revealed that TD patients with the NOS1-rs738409 AG genotype has lower language scores (70.67±21.042 vs 85±14.401, p=0.019, see in Figure 2C) than TD patients with the NOS1-rs738409 AA genotype. Moreover, TD patients with ADORA2A-rs229883 TT genotype also has a significantly lower RBANS total score (58.55±13.256 vs 69.75±15.548, p =0.012, see in Figure 2D) than those with the ADORA2A-rs229883 TC genotype.
Discussion
The present study is the first to explore the relationship between oxidative stress-related genes and adenosine receptor gene polymorphisms and their interactions with the occurrence and severity of Tardive dyskinesia (TD) in a Chinese population, and also to investigate the relationship between these genetic polymorphisms and psychiatric symptoms and cognitive function in patients. The present study yielded the following results: (1) The best three-locus model was the combination of the GSTM1-rs738491, NOS1-rs738409, and ADORA2A-rs2298383 on the risk of TD. (2) There was a significant relationship between SOD2 and NOS3 genotype polymorphisms and TD severity in the Chinese Han population. (3) GSTM C/C genotype and NOS3 A/G may be a protective factor for psychiatric symptoms in TD patients. (4) Our results also suggest that TD patients with NOS1 A/G or ADORA2A T/T exhibit poor cognitive ability.
The possible role of the GSTM1 (rs1056806), SOD2 (rs4880), NOS1 (rs1047735), NOS3 (rs1800779), and ADORA2A (rs2298383) polymorphisms as candidate risk genes in TD was investigated based on the oxidative stress mechanism and adenosine hypothesis. No significant difference in the allele frequencies and genotype frequencies between the TD and non-TD groups was found. Since consideration of multiple inheritance and insufficient sample size may lead to undetectable small genetic effects. Therefore, it has been suggested that TD-associated SNPs identified by GWAS explain only a small part of disease etiology, as the complexity of the disease and the correlation between multiple genes are ignored. Analysis emphasizing gene-gene interactions has emerged as one of the approaches to better understand the complex etiology of disease.39 Using the GMDR method and conventional statistical analysis, the combination of GSTM1-rs738491, NOS1-rs738409 and ADORA2A-rs2298833 was identified as the best three-locus model for predicting the occurrence of TD. Thus, the synergistic effect between the three SNPs may indicate that the interaction between adenosine and oxidative stress (OS) plays an important role in the occurrence and development of TD. It has been shown that A2A activation accumulates ROS together and play a complex role in the regulation of OS in the SCZ.40 This finding further supports our hypothesis. Despite the use of the GMDR method followed by conventional statistical analysis, the best three-locus model in this study still needs further replication in a larger sample size and in the Chinese Han population.
The TD group did show a relationship between genotype and TD severity compared to the non-TD group. Patients with the A/A genotype of SOD2 had lower AIMS scores than the other two genotype groups, indicating a protective effect. This finding is consistent with the results of Hitzeroth et al in the Xhosa population.41 Furthermore, our study observed reduced severity of TD in homozygous subjects with NOS3 allele, which is in contrast to the results Tiwari et al, who concluded that an increased severity of TD was observed in homozygous subjects with NOS3 allele.18 These results suggest that NOS3 polymorphism may play a role in the severity of TD. However, further studies are needed to investigate this relationship across different races and environmental factors.
Moreover, we investigated the relationship between different genotypes and the severity of psychotic symptoms in TD and non-TD patients. Our study demonstrated that PANSS-positive symptom scores were significantly lower in TD patients with GSTM C/C genotype. While Lim et alfound that the GSTM1 polymorphism may only be associated with susceptibility to SCZ, but not with severity.42 Loss of enzymatic activity due to the presence of GSTM1 deletion genotype leads to reduced detoxification associated with DNA damage and excessive neurotoxic compounds.43 Furthermore, a study by Zhang et al found lower plasma glutathione peroxidase levels in SCZ patients with TD.44 Additionally, we found that TD patients carrying NOS3 A/G had significantly lower PANSS composite scores. Based on these findings, we hypothesized that GSTM1 and NOS3 gene polymorphisms may contribute to the development of TD in SCZ patients after antipsychotic treatment and lead to more severe psychiatric symptoms. Further research is needed to confirm this hypothesis and explore the underlying mechanisms.
It has been shown that chronic SZC patients with TD have greater cognitive deficits than those without TD.4 We investigated the effect of these five genetic polymorphisms on the cognitive functioning in both TD and NTD patients. We found that TD patients carrying NOS1 A/G had poorer cognitive performance in language. McNeill et al implied that the NOS genotype may increase the severity of psychosis by modulating peripheral NOx concentrations,45 Whereas nitric oxide (NO) is not only an important neuronal messenger molecule, but also one of the oxygen radicals,46,47 animal models of TD have found that in nitric oxide synthase (NOS) inhibitors can effectively inhibit vacuolar masticatory movement (VCM) induced by nerve blockers.48 The underlying mechanisms between TD and cognitive deficits are unclear. Some studies have found that cognitive decline is associated with an imbalance between localized reactive oxygen species and insufficient antioxidant capacity. This imbalance, in turn, causes impaired neurotransmitter function and leads to significant clinical consequences of SCZ,49 such as psychotic symptoms, severity of extrapyramidal symptoms, and cognitive function.50 In addition, TD patients with ADORA2A T/T displayed poor cognitive ability. DA alterations play an important role in the cognition of SCZ patients.51 The activation of the ADORA2A receptor reduces the effectiveness of DRD2 signaling.52 These findings suggest a potential mechanism for the effects of adenosine receptors on cognitive function in TD patients.
The findings of this study contribute to a deeper understanding of the underlying molecular mechanisms that drive the onset and progression of TD. They underscore the significance of the oxidative stress hypothesis in shaping our comprehension of TD development. It is widely recognized that individuals possess unique genetic profiles that influence their antioxidant capacity, consequently impacting their vulnerability to oxidative damage, particularly in the context of SCZ.53 Therefore, there arises a need for the establishment of stratification criteria grounded in oxidative stress-related markers. Stratification criteria could be gene-based biomarkers, enabling the identification of individuals at heightened risk due to common oxidative stress-related genetic variations. Such an approach would facilitate the tailored selection of therapeutic strategies, moving us closer to the realization of personalized and precision medicine in the treatment of TD. However, here are several limitations in the present study. First, the sample size was relatively small, and the results need to be replicated in a larger population. Second, the number of female TD patients (n=9) may limit the generality of our findings. Although the data were adjusted for in the statistical analysis. Third, only one SNP was selected in the GSTM1, SOD2, NOS1, NOS3, and ADORA2A genes, which may not capture most of the genetic information conveyed by these five genes. Overall, our findings provide valuable insights into the genetic factors that may contribute to the development of TD in SCZ patients and lay the foundation for future studies in this area.
In conclusion, our findings shed light on the role of the genetic factors in TD induced by long-term use of antipsychotics in chronic SCZ patients. The interaction of GSTM1-rs738491, NOS1-rs738409, and ADORA2A-rs2298383 played a significant role in TD risk. TD patients with NOS1 A/G or ADORA2A T/T exhibit poor cognitive ability. Our findings establish a solid molecular biological foundation for comprehending the oxidative stress hypothesis as a central element in the pathogenesis of TD. Furthermore, they offer potential biological markers that could facilitate the early detection of TD development in SCZ patients. However, the functional implications of the genetic interactions between these SNP genes remain to be elucidated, and further experimental evidence is needed to confirm the underlying pathophysiological mechanisms.
Data Sharing Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, on request to the corresponding author.
Ethics Approval and Informed Consent
This study is in line with the Declaration of Helsinki. Ethical approval for the study was granted by the Ethical Review Committee of Tianjin Anding Hospital. Ethical approval number (IRB No.2021-07).
Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We thank the editor and the reviewers for their useful feedback that improved this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study received fundings from National Natural Science Foundation of China (82371512), Scientific Research Project of Tianjin Health Commission (ZC20135), Tianjin Science and Technology Project (18ZXRHSY00100), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-033A). We thank all the study participants for their cooperation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Freedman DX. Neurological syndromes associated with antipsychotic drug use. A special report. Arch Gen Psychiatry. 1973;28(4):463–467.
2. Carbon M, Hsieh CH, Kane JM, Correll CU. Tardive Dyskinesia Prevalence in the Period of Second-Generation Antipsychotic Use: a Meta-Analysis. J Clin Psychiatry. 2017;78(3):e264–e278.
3. Uludag K, Wang DM, Goodman C, Chen DC, Wang L, Zhang X. Prevalence, clinical correlates and risk factors associated with Tardive Dyskinesia in Chinese patients with schizophrenia. Asian J Psychiatr. 2021;66:102877.
4. Liang Q, Wang D, Zhou H, et al. Tardive dyskinesia in Chinese patients with schizophrenia: prevalence, clinical correlates and relationship with cognitive impairment. J Psychiatr Res. 2022;151:181–187.
5. Tsermpini EE, Redensek S, Dolzan V. Genetic Factors Associated With Tardive Dyskinesia: from Pre-clinical Models to Clinical Studies. Front Pharmacol. 2021;12:834129.
6. Margolese HC, Chouinard G, Kolivakis TT, Beauclair L, Miller R. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatry. 2005;50(9):541–547.
7. Ward KM, Citrome L. Antipsychotic-Related Movement Disorders: drug-Induced Parkinsonism vs. Tardive Dyskinesia-Key Differences in Pathophysiology and Clinical Management. Neurol Ther. 2018;7(2):233–248.
8. Lohr JB, Kuczenski R, Niculescu AB. Oxidative mechanisms and tardive dyskinesia. CNS Drugs. 2003;17(1):47–62.
9. Kruk J, Sachdev P, Singh S. Neuroleptic-induced respiratory dyskinesia. J Neuropsychiatry Clin Neurosci. 1995;7(2):223–229.
10. Sachdev PS. The current status of tardive dyskinesia. Aust N Z J Psychiatry. 2000;34(3):355–369.
11. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549–562.
12. Hensley K, Floyd RA. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys. 2002;397(2):377–383.
13. Cadet JL, Lohr JB. Possible involvement of free radicals in neuroleptic-induced movement disorders. Evidence from treatment of tardive dyskinesia with vitamin E. Ann N Y Acad Sci. 1989;570:176–185.
14. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876.
15. Lohr JB, Kuczenski R, Bracha HS, Moir M, Jeste DV. Increased indices of free radical activity in the cerebrospinal fluid of patients with tardive dyskinesia. Biol Psychiatry. 1990;28(6):535–539.
16. Cadet JL, Perumal AS. Chronic treatment with prolixin causes oxidative stress in rat brain. Biol Psychiatry. 1990;28(8):738–740.
17. Cho CH, Lee HJ. Oxidative stress and tardive dyskinesia: pharmacogenetic evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:207–213.
18. Thelma BK, Tiwari AK, Deshpande SN, Lerer B, Nimgaonkar VL. Genetic susceptibility to Tardive Dyskinesia in chronic schizophrenia subjects: role of oxidative stress pathway genes. Schizophr Res. 2007;92(1–3):278–279.
19. Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. Effects of exercise on brain function: role of free radicals. Appl Physiol Nutr Metab. 2007;32(5):942–946.
20. Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324(Pt 1):25–28.
21. Robinson BH. The role of manganese superoxide dismutase in health and disease. J Inherit Metab Dis. 1998;21(5):598–603.
22. Lipton SA, Choi YB, Pan ZH, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364(6438):626–632.
23. Yan C, Duan L, Fu C, et al. Association Between Glutathione S-Transferase (GST) Polymorphisms and Schizophrenia in a Chinese Han Population. Neuropsychiatr Dis Treat. 2020;16:479–487.
24. Uludag K, Wang DM, Zhang XY. Tardive Dyskinesia Development, Superoxide Dismutase Levels, and Relevant Genetic Polymorphisms. Oxid Med Cell Longev. 2022;2022:5748924.
25. Wang DF, Cao B, Xu MY, et al. Meta-Analyses of Manganese Superoxide Dismutase Activity, Gene Ala-9Val Polymorphism, and the Risk of Schizophrenia. Medicine (Baltimore). 2015;94(36):e1507.
26. Liou YJ, Lai IC, Lin MW, et al. Haplotype analysis of endothelial nitric oxide synthase (NOS3) genetic variants and tardive dyskinesia in patients with schizophrenia. Pharmacogenet Genomics. 2006;16(3):151–157.
27. Wang YC, Liou YJ, Liao DL, et al. Association analysis of a neural nitric oxide synthase gene polymorphism and antipsychotics-induced tardive dyskinesia in Chinese schizophrenic patients. Journal of Neural Transmission. 2004;111(5):623–629.
28. Boison D, Singer P, Shen HY, Feldon J, Yee BK. Adenosine hypothesis of schizophrenia--opportunities for pharmacotherapy. Neuropharmacology. 2012;62(3):1527–1543.
29. Miao J, Liu L, Yan C, et al. Association between ADORA2A gene polymorphisms and schizophrenia in the North Chinese Han population. Neuropsychiatr Dis Treat. 2019;15:2451–2458.
30. Turcin A, Dolzan V, Porcelli S, Serretti A, Plesnicar BK. Adenosine Hypothesis of Antipsychotic Drugs Revisited: pharmacogenomics Variation in Nonacute Schizophrenia. OMICS. 2016;20(5):283–289.
31. Leucht S, Samara M, Heres S, Davis JM. Dose Equivalents for Antipsychotic Drugs: the DDD Method. Schizophr Bull. 2016;42(Suppl 1):S90–94.
32. Johnson GF, Hunt GE, Rey JM. Incidence and severity of tardive dyskinesia increase with age. Arch Gen Psychiatry. 1982;39(4):486.
33. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276.
34. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319.
35. Zhang BH, Tan Y, Zhang W. Repeatable battery for the assessment of neuropsychologicla status (RBANS) as a screening test in Chinese: reliability and validity. J Chin Ment Health. 2009;28:865–869.
36. Lahiri DK, Nurnberger JI Jr. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19(19):5444.
37. Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15(2):97–98.
38. Lou XY, Chen GB, Yan L, et al. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. 2007;80(6):1125–1137.
39. Lim K, Lam M, Zai C, et al. Genome wide study of tardive dyskinesia in schizophrenia. Transl Psychiatry. 2021;11(1):351.
40. Huang L, Tang Y, Sperlagh B. Glial Purinergic Signaling-Mediated Oxidative Stress (GPOS) in Neuropsychiatric Disorders. Oxid Med Cell Longev. 2022;2022:1075440.
41. Hitzeroth A, Niehaus DJ, Koen L, Botes WC, Deleuze JF, Warnich L. Association between the MnSOD Ala-9Val polymorphism and development of schizophrenia and abnormal involuntary movements in the Xhosa population. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):664–672.
42. Zhang X, Yang J, Liu X, Zhao G, Li X, Xun G. Glutathione S-transferase gene polymorphisms (GSTT1 and GSTM1) and risk of schizophrenia. Medicine. 2020;99(36):e54.
43. Gronau S, Koenig-Greger D, Jerg M, Riechelmann H. GSTM1 enzyme concentration and enzyme activity in correlation to the genotype of detoxification enzymes in squamous cell carcinoma of the oral cavity. Oral Dis. 2003;9(2):62–67.
44. Zhang XY, Tan YL, Zhou DF, et al. Disrupted antioxidant enzyme activity and elevated lipid peroxidation products in schizophrenic patients with tardive dyskinesia. J Clin Psychiatry. 2007;68(5):754–760.
45. McNeill RV, Kehrwald C, Brum M, et al. Uncovering associations between mental illness diagnosis, nitric oxide synthase gene variation, and peripheral nitric oxide concentration. Brain Behav Immun. 2022;101:275–283.
46. Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560.
47. Furchgott RF. The 1989 Ulf von Euler lecture. Studies on endothelium-dependent vasodilation and the endothelium-derived relaxing factor. Acta Physiol Scand. 1990;139(2):257–270.
48. Naidu PS, Kulkarni SK. Excitatory mechanisms in neuroleptic-induced vacuous chewing movements (VCMs): possible involvement of calcium and nitric oxide. Behav Pharmacol. 2001;12(3):209–216.
49. Mahadik SP, Evans D, Lal H. Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(3):463–493.
50. Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15(7):2011–2035.
51. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329–336.
52. Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochimica et Biophysica Acta. 2011;1808(5):1380–1399.
53. Ermakov EA, Dmitrieva EM, Parshukova DA, Kazantseva DV, Vasilieva AR, Smirnova LP. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid Med Cell Longev. 2021;2021:8881770.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

