Back to Journals » Journal of Pain Research » Volume 16
Genetic Factors Associated with Morphine Consumption in Women Undergoing Laparoscopic Cholecystectomy: A Prospective Cohort Study
Authors Elgendy HM , Ibrahim SM, Bader L, Mohammad RA, Ali ZO, Bejaoui MBA, Hilani M, Ismail H, Elewa HF
Received 10 May 2023
Accepted for publication 28 June 2023
Published 15 July 2023 Volume 2023:16 Pages 2407—2417
DOI https://doi.org/10.2147/JPR.S420447
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Timothy Atkinson
Hamed M Elgendy,1,2 Sami M Ibrahim,1,3 Loulia Bader,4 Rudaina A Mohammad,5 Zainab O Ali,5 Mohamed Ben Allala Bejaoui,5 Mohamad Hilani,1 Hesham Ismail,1 Hazem F Elewa4
1Department of Anaesthesia, Hamad Medical Corporation, Doha, Qatar; 2Department of Anaesthesia, Assiut University Hospitals, Assiut, Egypt; 3Department of Anaesthesia and ICU, Faculty of Medicine, Zagazig University, Zagazig, Egypt; 4College of Pharmacy, QU Health, Qatar University, Doha, Qatar; 5Hamad Medical Corporation, Medical Research Center, Doha, Qatar
Correspondence: Hazem F Elewa, College of Pharmacy, Qatar University, P.O. Box 2713, Doha, Qatar, Tel + 974 4403 5616, Fax +974 4403 5551, Email [email protected]
Introduction: Morphine has been a crucial analgesic agent used perioperatively in various surgical procedures. Genetic factors can lead to morphine dose requirement interpatient variability. Our objective was to determine the contribution of genetic polymorphisms in human μ-opioid receptor gene (OPRM1), ATP binding cassette gene (ABCB1) and rs2952768 to the variation of the perioperative morphine consumption in women undergoing laparoscopic cholecystectomy.
Methods: This is a prospective cohort study that included 102 adult Arab females undergoing laparoscopic cholecystectomy. The exposures were carrying the genetic variants of OPRM1, ABCB1 and rs2952768. Our primary outcome was total morphine or morphine equivalent dose required perioperatively. The secondary outcomes were pain score during the first 24 hours and adverse drug reactions. A standardized, general anaesthesia was used for all subjects. In addition to the genetic factors, we also investigated non-genetic factors influencing post-operative pain sensitivity and morphine consumption.
Results: Both (rs1799971, A>G) in OPRM1 and (rs2952768, T>C) showed statistically significant association with intra-operative total morphine dose requirements. Patients carrying the “G” allele in OPRM1 had a significantly higher total morphine mean rank dose compared to the AA genotype [62.9 vs 47.1, p=0.008]. Furthermore, patients homozygous for the rs2952768 (T>C) minor allele “CC” had a higher mean rank compared to the other genotypes [72.7 vs 50.1, p=0.046].
Conclusion: OPRM1 (rs1799971) and rs2952768 are associated with variation of intra-operative morphine consumption in laparoscopic cholecystectomy.
Clinical Trial Identifier: This study was registered at ClinicalTrials.gov, NCT04621864. https://clinicaltrials.gov/ct2/show/NCT04621864.
Keywords: opioid, OPRM1, ABCB1, genetic polymorphisms, post-operative pain, women, Arabs
Introduction
For decades, morphine has been a crucial analgesic agent used in various clinical settings including periprocedural analgesic management. It induces its effects through the µ-opioid receptor (MOR). Clinical analgesic response to morphine varies from patient to patient leading to morphine dose requirement interpatient variability. Such variations have been owed to several factors including genetic polymorphisms in multiple genes. Among those is theOPRM1 gene which encodes the human MOR.1 OPRM1 plays a major role in mediating the effects of opioids through both endogenous opioid peptides and exogenous ligands.2 One of the most common single nucleotide polymorphisms (SNP) in the OPRM1 gene is (rs1799971) which involves a substitution on nucleotide 118 A/G on exon 1 leading to an amino acid change from Asparagine to Aspartic acid. This SNP leads to an enhanced binding affinity of β-endorphin at MOR, which ultimately leads to increased potency at the receptor.3,4 Additionally, variants in the ATP-binding cassette sub-family B member-1 (ABCB1) gene which codes for P-glycoprotein could influence morphine analgesic activity by affecting its absorption from the intestine and its transport via the blood–brain barrier.5 Among other important SNPs shown to be associated with analgesic dose requirement is the rs2952768, which is an intergenic SNP located near the Methyltransferase Like 21A (METTL21A) gene. There has been numerous amount of variants that studied morphine clinical variability and it would be extremely difficult to test them all in this research. Based on our review, these 3 SNPs were among the most well-studied variants that showed some consistency in their effect on morphine outcomes.5,6
Our group has observed clinical variations in analgesic requirements among Arab patients. Only few reports have previously investigated the analgesic inter-individual variability in the Middle Eastern ethnicity. Most of them have focused on the influence of genetic variants on morphine dose requirements in cancer care and opioid dependence cohorts.7–9 To our knowledge, no previous studies have evaluated the influence of morphine genetic polymorphism in the perioperative setting after laparoscopic cholecystectomy in Arabs. Therefore, we decided in this study to investigate whether the genetic polymorphism of human μ-opioid receptor gene (OPRM1), ATP binding cassette gene (ABCB1) and rs2952768 are contributing to the variation of intra-operative morphine consumption in Arab women undergoing laparoscopic cholecystectomy. We decided to include only women in the study to eliminate the gender confounding effect on pain threshold and analgesic requirements. In addition to studying the association between genetic and non-genetic factors on the total perioperative morphine dose, we also assessed the effect of these factors on pain score, analgesic dosage requirements, and complications of morphine use in these patients during the first post-operative day as a secondary objective.
Methods
Research Design and Ethics
This was a prospective observational cohort study targeting Arab female adults undergoing laparoscopic cholecystectomy. Prior to the initiation of patient enrolment, the study was approved by the Qatar University’s Institutional Review Board (QU-IRB 1297-FBA/20) and Hamad Medical Corporation’s IRB (MRC# 01-18-270). Written informed consent was obtained from all subjects participating in the trial. The trial was registered at ClinicalTrials.gov (NCT04621864). The study complies with the Declaration of Helsinki.
Study Setting and Timeline
Patients were recruited at the anaesthesia clinic, Al Wakra Hospital, Hamad Medical Corporation (HMC), Qatar prior to their surgery. Recruitment started on October 20, 2019, and held during COVID-19 pandemic, and was completed on March 30, 2021. DNA samples were processed and analysed at the College of Pharmacy, Qatar University (QU), between August 2020 and April 2021.
Study Population and Sampling
The flowchart for inclusion, follow-up, and genetic analysis is shown in Figure 1. A total of 102 adult female patients with American Society of Anaesthesiologists physical status of I or II (class III cases were included if obesity is the only criteria) and in whom planned post-operative pain management by morphine was requested after laparoscopic cholecystectomy were included in this study. Patients were considered eligible if they were female Arabs (being of any of the League of the Arab States), above 18 years old, undergoing a laparoscopic cholecystectomy and agreed to participate in the study and signed an informed consent form. Patients were excluded if they had any obvious signs or symptoms of respiratory distress, cardiovascular disease, renal impairment, abnormal liver function with Child–Pugh classification other than A, were uncontrolled diabetics, had a history of allergy to morphine or experienced major side effects to morphine. Patients were also excluded if they had chronic pain that required long-term treatment with psychotropic or opioid medications. For the Arab ethnicity criterion, patients reported their nationality verbally, and it was confirmed through the patient’s electronic health record (EHR).
 |
Figure 1 The flowchart for inclusion, follow-up and genetic analysis. |
Data Collection and Outcome Measures
The primary outcome of this study was total morphine or morphine equivalent dose required during the surgery. The total equivalent intra-operative (IO) morphine dose was calculated as below:
The secondary outcomes were pain score during the first 24 hours using a Visual Analogue Score (VAS) and adverse drug reactions such as nausea, vomiting or respiratory depression. Main exposure was carrying genetic variants in OPRM1, ABCB1, rs2952768. Covariate factors such as age, comorbidities and BMI were also tested for their effect on the primary outcome (total equivalent IO morphine dose). After signing a written informed consent form, patients were asked to provide a blood sample. Blood samples were collected prior to surgery by the anaesthesiologist/anaesthesia technologist administering anaesthesia using BD Vacutainer® K3 EDTA 12.15 mg (15% Sol, 0.081 mL) plastic collection tubes (REF368861). Genetic laboratory tests were paid by the study.
Clinical data and demographics of the patients were collected primarily from the medical records and included: weight, height, BMI, age, patient past medical history, current medications, duration of surgery, duration of anaesthesia, dosages of anaesthetics using IO as standard anaesthesia protocol, total morphine administered IO and post-operative, other analgesic dosages (either intra-operative or post-operative), multimodal analgesia protocol in our institute, first time for rescue analgesics and post-operative possible complications of opioids (nausea, vomiting, itching, hypotension or respiratory depression). Pain-scoring using the Visual Analog Scale (VAS) was also documented.
Anaesthesia and Procedure Protocol
A standardized, general anaesthesia protocol was used for all patients. For induction of anaesthesia, 2 µg/kg fentanyl, 2 mg/kg propofol and 0.15 mg/kg cisatracurium were used. After induction of anaesthesia, cisatracurium and the inhaled anaesthetic sevoflurane at a low flow rate of 0.5 L/min were used to maintain the anaesthesia. Thirty minutes before the end of surgery, a 0.08 mg/kg loading dose of morphine was given intravenously (IV) and titrated by adding 2–3 mg as needed. At the end of the procedure, residual neuromuscular block was antagonized with neostigmine in 0.05 mg/kg and glycopyrrolate in 0.01 mg/kg, and patients were extubated. Post-operative nausea and vomiting (PONV) prophylaxis was given as part of our protocol, IV ondansetron, and IV dexamethasone intra-operatively.
After tracheal extubation, patients were transferred to the post anaesthesia care unit (PACU). Patients were assessed for pain score by anaesthetists on charge in PACU; by assessing the VAS score every 10–15 min after arrival in the PACU. Whenever the pain score increased to more than 3, incremental IV morphine titration was administered every 5 min in 2–3 mg IV increments until pain relief (VAS ≤ 3). All IV morphine doses were recorded. Study investigators did not intervene with the pain assessment, narcotic requirements, or ordering during the intra-operative or post-operative time; total administered analgesics during the post-operative 24-hour period were recorded and calculated. The respiratory rate and levels of consciousness were assessed at regular intervals. Average time for patient monitoring in PACU was one hour and then patients were discharged to the ward.
The modified Aldrete score is a widely used, objective method for evaluating post-anaesthetic patients. Each of the following measures received a score of 0, 1, or 2: activity (defined as moving all four limbs), respiratory efficiency, circulation (measured as arterial blood pressure at the pre-anaesthetic level), consciousness (defined as complete alertness), and oxygen saturation is maintained on room air or requires supplemental oxygen. The numbers assigned to each sign were added at the conclusion of each evaluation. In the best case scenario, a patient received a score of 10.10
In the surgical ward, a post-operative regimen was prescribed for rescue pain control as a standard of care: morphine 5 mg subcutaneous (SC) every 8 hours as needed (PRN); paracetamol 1 g IV every 6 hours PRN for nausea and vomiting; metoclopramide 10 mg IV every 8 hours PRN. These patients did not receive any type of regional blocks for pain management. One of our study’s limitations was the implementation of multimodal analgesia utilizing paracetamol, non-steroidal anti-inflammatory (ketorolac), diclophenate and post-operative tramadol PRN as per institutional policy. Pain score was assessed in the ward every 6 h by a research team member.
Patients were monitored closely to prevent morphine overdose. Morphine adverse effects were reported nausea, vomiting, itching and respiratory depression. Respiratory rate (˂10 breaths/min), arterial carbon dioxide level (≥ 50 mmHg) and level of consciousness (progression to somnolence) were assessed at regular intervals. An IV infusion of 100–200 µg/h naloxone was started, to prevent morphine overmedication. When a patient requested treatment for nausea and/or pruritus concomitant with a VAS score greater than 8, naloxone was also administered to reverse the effect of morphine. All patients treated with naloxone because of the above-mentioned causes were excluded from the study analysis.
DNA Extraction, Quantification and Genotyping
Genomic DNA was extracted from fresh frozen whole blood samples using the PureLink® Genomic DNA mini kits, Invitrogen™, as previously described by our group.11
The Nanodrop 2000c Spectrophotometer (Thermo Fisher Scientific™) was used to quantify and assess the quality of the extracted DNA. For single nucleotide polymorphism (SNP) detection and genotyping, we used the QuantStudio™ 5 Real-Time PCR System for Human Identification, 96-well, 0.2 mL, desktop with TaqMan Drug Metabolizing Enzyme (DME) genotyping assay (Applied Biosystems™, Life Technologies). All probes were purchased at ThermoFisher Scientific; their context sequences are listed in Table S1.
Statistical Analysis and Sample Size Calculation
We planned to study 100 patients. We calculated the sample size as follows: if a genetic abnormality was found in 15% of patients and the average total morphine use in this subgroup was 40% lower/higher than in the remainder of the patients, then we would have 80% power to detect that difference significantly with alpha error ≤ 0.05. Descriptive statistics and normality tests were used to analyse baseline demographics. Chi-square Goodness of Fit was used to make sure that all allele frequencies fit the Hardy–Weinberg Equilibrium (HWE). Since continuous variables were not normally distributed, we used non-parametric tests in most of our analysis. Mann–Whitney U or Kruskal–Wallis tests were used to estimate the difference in morphine requirements and difference in VAS score, when appropriate. Spearman’s rho coefficient was used to test for association between total morphine dose and VAS score. The Chi-square test for independence was used to test for association between the patient’s genotype and the need for post-operative morphine. Univariate regression analysis was used to estimate the effect of each genetic and clinical factor studied on total morphine dose. Furthermore, any factor with a p-value of 0.2 or below in the results of univariate analysis was tested in the multiple linear regression model. A two-tailed P-value <0.05 was considered significant. IBM Statistical Package for Social Science (IBM SPSS 27 software; IBM, New York, USA) was used to carry out the statistical analysis.
Results
Patient Recruitment and Study Population Characteristics
A total of 102 females undergoing laparoscopic cholecystectomy were recruited in the study. The majority of the patients were Qataris (49%) with a median (IQR) age of 38 (16) years and a median (IQR) weight of 77.5 (16.2) kg. About two thirds of the patients had an American Society of Anaesthesiologists physical (ASA) status of II. Table 1 shows detailed demographic data of the study subjects.
 |
Table 1 Basic Characteristics of Patients Subjected to Laparoscopic Cholecystectomy |
Intra-Operative and Post-Operative Information
On average, the surgery duration was one hour with a median of 65 (32) minutes and a median of 85 (35) minutes of anaesthesia. All intra-operative and post-operative data are represented in Tables 2 and S2 and show that four cases developed nausea and only two cases had vomiting.
 |
Table 2 Intra-Operative Data of Patients Subjected to Laparoscopic Cholecystectomy |
Prevalence of OPRM1 (A>G), ABCB1 (G>A) and rs2952768
To estimate the prevalence of the studied genetic variants, we calculated their minor allele frequencies (MAF) in this cohort of Arab patients, and they were as follow: 0.15 for OPRM1 (A>G); 0.38 for ABCB1 (G>A); and 0.26 for rs2952768. Table S3 shows the MAF in comparison to other Arab nationalities. The genotype frequencies are shown in Table 3. No deviations from Hardy–Weinberg equilibrium were observed for any of the genotype frequencies.
 |
Table 3 Genotype Frequencies of the Studied Genetic Variants |
Association of Genetic and Non-Genetic Factors with Total Intra-Operative Morphine Dose
Both OPRM1 (rs1799971, A>G), and rs2952768 (T>C) showed statistically significant association with IO total morphine dose requirements. Patients carrying OPRM1 minor allele (GG) and (AG) genotypes had a significantly higher total morphine mean rank compared to the AA genotype [62.9 vs 47.1, p=0.008] (Figure 2). Furthermore, patients homozygous for the rs2952768 (T>C) minor allele (CC) had a higher mean rank compared to the other genotypes [72.7 vs 50.1, p=0.046] (Figure 3). However, no significant association was found between the ABCB1 genotypes and total IO morphine dose.
Multiple linear regression showed that weight, hypertension, using ketorolac during operation, carrying the rs2952768 minor allele (CC) and carrying the OPRM1 minor allele (GG) are all predictors of IO total morphine dose with an adjusted R2 of 0.19 and a p-value less than 0.001 (Table 4).
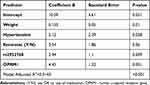 |
Table 4 Multiple Linear Regression Showing the Association of Genetic and Non-Genetic Factors with Intra-Operative Total Morphine Dose |
Association of the Genetic Variants with VAS Score and Post-Operative Analgesic Requirements
There was no statistically significant association between OPRM1 (rs1799971, A>G), rs2952768 (T>C) or ABCB1 (rs1045642, G>A) and the VAS score (overall and at each time point).
A significant correlation was found between post-operative morphine and VAS score at 0.5 and 1 hour (r=0.27, p=0.006 and r=0.24, p=0.015, respectively). The correlation was still significant when we considered the mean VAS score (r = 0.2, p=0.038).
Discussion
In this study, we attempted to investigate the effect of genetic polymorphism of OPRM1, ABCB1 and rs2952768 on the variation of morphine consumption and pain score in women undergoing laparoscopic cholecystectomy. We observed that OPRM1 (rs1799971, A>G) and the intergenic SNP-rs2952768 (T>C) are associated with IO total morphine dose requirements. This was confirmed in multiple linear regressions along with other clinical factors which included weight, use of ketorolac and ketamine during operation. However, there were no significant association with VAS score except for post-operative morphine use.
Genetic variability effect on the response to opioids for the treatment of pain post-surgery has been previously investigated.12,13 Most of these studies have found that carriers of (OPRM1 A118G) are more sensitive to pain. A Swedish study suggested a possible contribution of SNP within the ABCB1 gene after laparoscopic cholecystectomy when comparing the pain sensitivity and post-operative pain intensity in a cohort of patients.14 Regarding analgesic requirements, several studies have shown that carriers of OPRM1 A118G require a higher dose of morphine in cancer and in different surgical settings. For instance, Klepstad et al found that patients with cancer usually require higher doses of morphine during their long-term therapy which was associated with the OPRM1 118G variant.13
In obstetric settings, Sia et al in 2008 demonstrated that A118G polymorphism was associated with inter-individual differences in IV morphine consumption post-operatively following a single intrathecal dose of morphine for post-operative analgesia after caesarean section.15
A Japanese study16 investigated fentanyl sensitivity and polymorphism in the OPRM1 gene in a cohort of patients with painful orofacial surgery. Patients with the G allele of OPRM1 were less sensitive to fentanyl and consumed more fentanyl post-operatively.16 Similar fentanyl sensitivity study was conducted in Chinese gynaecology patients, where they concluded variation in intravenous fentanyl consumption and pain score in subjects with different OPRM1 genotypes.17
rs2952768 (T>C) is an intergenic variant located between the METTL21A and CREB1 genes. rs2952768 and other neighbour SNPs included in that LD (linkage disequilibrium) block have been associated with post-operative opioid requirements in patients undergoing major abdominal surgery in a genome wide association study (GWAS).6 Patients with CC genotype required significantly higher opioid dose compared to TT and T/C genotypes (t110=−2.340, P=0.021). The same study tested the contribution of this SNP to the vulnerability to substance abuse. They observed fewer poly-drug abusers that are homozygous for the C variant compared to mono-drug users. Similarly, a group in Japan has attempted to construct a prediction formula for opioid analgesic requirement post surgery and they found that pain perception latency, weight and 4 SNPs (rs2952768; OPRM1A118G; GIRK2rs2835859; ADRB2 rs11959113) are the significant predictors for 24-hour post-operative fentanyl requirement (R2=0.145, P=5.66×10−10).18 Our study which was performed in laparoscopic cholecystectomy Arab female patients aligns with these previous findings.
Despite the high prevalence of ABCB1 (G>A), rs1045642 variant, its association with opioid dose requirement has not been always consistent. A study on Italian patients showed that rs1045642 strongly affects morphine responsiveness and those patients homozygous for the ABCB1 3435T allele and the OPRM1 118A allele were the best responders to morphine.19 Another research group from China found that patients managed on morphine for pain associated with undergoing a caesarean section and who were homozygous for the ABCB1 3435T allele tended to have persistent pain for three months after surgery compared to the CT and CC genotypes (P=0.07).20 There were also previous reports that found an association between ABCB1 variants and opioids adverse drug reactions such as fatigue and vomiting.5
Other than the association with the efficacy outcomes, we could not detect any adverse drug reaction within our studied sample. We also did not find any association between variants in ABCB1 and morphine dose requirements.
Strengths
Our study is the first to investigate the association between genetic polymorphism and perioperative opioid consumption in the Middle East ethnicity. This study contributes new information to the field of genetic association and post-operative pain management after laparoscopic cholecystectomy. Few reports found OPRM1 gene variants with susceptibility to opioid9 and heroin8 dependence, but there are no studies that discussed this association in the perioperative setting for this ethnic group. Therefore, our study could be a foundation for future research in this area. Moreover, the investigators performing all genotyping related experiments were blinded to the phenotypes, and all samples were managed and handled in a standard and systematic fashion to avoid any bias. Our study uses a critical approach to provide evidence of managing acute IO or perioperative pain and strengthens the future of personalized medicine.
Weaknesses
This study is not without limitation. First, there could be other genetic and non-genetic factors associated with morphine dose requirement that were not tested or collected. Second, our study sample size was small, yet we recruited all eligible patients undergoing laparoscopic cholecystectomy for a specified duration as a pilot study to explore the role of genetic polymorphism association with opioid consumption in our ethnic group. Additionally, intravenous patient-controlled analgesia infusion pumps were not available at our institution during the study period. Therefore, we used incremental IV morphine intra-operatively, in the PACU, continued with PRN SC morphine. Another limitation was the implementation of multimodal analgesia utilizing paracetamol, non-steroidal anti-inflammatory (ketorolac) and post-operative tramadol PRN as per institutional policy. Lastly, including female gender only in our study may have limited our generalizability. Further research could incorporate the addition of pharmacodynamic and pharmacokinetic genes: CYP2D6, CYP2C9, CYP3A4, CYP3A5 to identify male and female patients at risk for pain and other complications.
Conclusion
In this study, both OPRM1 (rs1799971, A>G), and rs2952768 (T>C) showed a significant association with IO total morphine dose requirements in Arab female patients undergoing laparoscopic cholecystectomy. The same variants along with weight, having hypertension and using ketorolac remained as the main factors associated with total intra-operative morphine requirements.
Abbreviations
ABCB1, ATP binding cassette gene; ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; OPRM1, human μ-opioid receptor gene; IRB, institutional review board; SD, 1 standard deviation.
Data Sharing Statement
The authors of this manuscript have no intention to share individual deidentified participant data.
Acknowledgment
Ms Lakshmi PILLAJ, laboratory senior technologist, for her technical and logistic preservation of the blood samples prior to the genetic analysis at QU. Anaesthesia department staff at HMC & Medical Research Centre who supported this research.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: this research was funded by Hamad Medical Corporation, Doha, Qatar (grant number -MRC# 01-18-270). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure
The authors declare no conflicts of interest.
References
1. Lotsch J, Geisslinger G. Are mu-opioid receptor polymorphisms important for clinical opioid therapy? Trends Mol Med. 2005;11(2):82–89. doi:10.1016/j.molmed.2004.12.006
2. LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur J Pharmacol. 2000;410(2–3):249–268. doi:10.1016/S0014-2999(00)00819-0
3. Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89(3):553–560. doi:10.1111/j.1471-4159.2004.02340.x
4. Kosarac B, Fox AA, Collard CD. Effect of genetic factors on opioid action. Curr Opin Anaesthesiol. 2009;22(4):476–482. doi:10.1097/ACO.0b013e32832e34c9
5. Ofoegbu A, Ettienne B. Pharmacogenomics and Morphine. J Clin Pharmacol. 2021;61(9):1149–1155. doi:10.1002/jcph.1873
6. Nishizawa D, Fukuda K, Kasai S, et al. Genome-wide association study identifies a potent locus associated with human opioid sensitivity. Mol Psychiatry. 2014;19(1):55–62. doi:10.1038/mp.2012.164
7. Hajj A, Halepian L, Osta NE, Chahine G, Kattan J, Rabbaa Khabbaz L. OPRM1 c.118A>G polymorphism and duration of morphine treatment associated with morphine doses and quality-of-life in palliative cancer pain settings. Int J Mol Sci. 2017;18(4):669. doi:10.3390/ijms18040669
8. Qasemian-Talgard A, Saadat M. Association between three common genetic polymorphisms of XPC and susceptibility to heroin dependency. Gene. 2020;724:144153. doi:10.1016/j.gene.2019.144153
9. Tolami HF, Sharafshah A, Tolami LF, Keshavarz P. Haplotype-based association and in silico studies of OPRM1 gene variants with susceptibility to opioid dependence among addicted Iranians undergoing methadone treatment. J Mol Neurosci. 2020;70(4):504–513. doi:10.1007/s12031-019-01443-4
10. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7(1):89–91. doi:10.1016/0952-8180(94)00001-K
11. Eljilany I, Elarref M, Shallik N, et al. Genetic and non-genetic factors impact on INR normalization in preprocedural warfarin management. Pharmgenomics Pers Med. 2021;14:1069–1080. doi:10.2147/PGPM.S322743
12. Skarke C, Darimont J, Schmidt H, Geisslinger G, Lötsch J. Analgesic effects of morphine and morphine‐6‐glucuronide in a transcutaneous electrical pain model in healthy volunteers. Clin Pharmacol Ther. 2003;73(1):107–121. doi:10.1067/mcp.2003.5
13. Klepstad P, Rakvåg T, Kaasa S, et al. The 118 A> G polymorphism in the human µ‐opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48(10):1232–1239. doi:10.1111/j.1399-6576.2004.00517.x
14. Persson AKM, Pettersson FD, Akeson J. Single nucleotide polymorphisms associated with pain sensitivity after laparoscopic cholecystectomy. Pain Med. 2018;19(6):1271–1279. doi:10.1093/pm/pnx164
15. Sia AT, Lim Y, Lim EC, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109(3):520–526. doi:10.1097/ALN.0b013e318182af21
16. Fukuda K, Hayashida M, Ide S, et al. Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain. 2009;147(1–3):194–201. doi:10.1016/j.pain.2009.09.004
17. Zhang W, Chang YZ, Kan QC, et al. Association of human micro-opioid receptor gene polymorphism A118G with fentanyl analgesia consumption in Chinese gynaecological patients. Anaesthesia. 2010;65(2):130–135. doi:10.1111/j.1365-2044.2009.06193.x
18. Yoshida K, Nishizawa D, Ichinomiya T, et al. Prediction formulas for individual opioid analgesic requirements based on genetic polymorphism analyses. PLoS One. 2015;10(1):e0116885. doi:10.1371/journal.pone.0116885
19. Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83(4):559–566. doi:10.1038/sj.clpt.6100385
20. Sia AT, Sng BL, Lim EC, Law H, Tan EC. The influence of ATP-binding cassette sub-family B member −1 (ABCB1) genetic polymorphisms on acute and chronic pain after intrathecal morphine for caesarean section: a prospective cohort study. Int J Obstet Anesth. 2010;19(3):254–260. doi:10.1016/j.ijoa.2010.03.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



